INTRODUCTION
Measles is a significant vaccine-preventable disease which ranked fifth among the causes of death in children aged <5 years globally in 2000, and first among the vaccine-preventable diseases [Reference Lopez1]. Although the global mortality was reduced by 50% during 1999–2004, half a million measles-attributed deaths still occur annually worldwide, most of them young children in developing countries [2]. The World Health Organization adopted a targeted policy towards measles elimination, based on three strategic actions: immunization, surveillance and laboratory support [3]. Measles vaccination at 12–15 months is part of routine childhood immunization programmes in developed countries. Routine measles immunization was introduced in Israel in 1967 and a two-dose MMR schedule (at the ages of 12 months and 6 years) was implemented in 1994 [Reference Slater4, Reference Slater, Anis and Leventhal5].
In 2002 the average national immunization coverage of MMR vaccine in children was reported as 94–95% for the first dose and 95–97%, for the second dose of vaccine [Reference Slater, Anis and Leventhal5, 6].
The World Health Organization's detailed strategy includes achieving high immunization coverage (⩾90%) in every district and ensuring that all children received a second opportunity for measles immunization [2]. The average immunization coverage of the first dose of MMR vaccine in children aged 2 years was reported as above 90% in all the districts in Israel in 1999–2002. However, a high overall MMR vaccine coverage does not necessarily reflect certain specific population groups with variable compliance. Consequently, the prevention and control of measles outbreaks remain a major public health problem, even in developed countries [7]. Sporadic outbreaks still evolve in specific communities following spread from an index case of measles that is often imported [Reference Yip, Papania and Redd8, Reference Muscat, Glismann and Bang9], as supported by genotypic and phylogenetic analysis of the virus [Reference Riddell, Rota and Rota10, 11].
During 2003–2004, two measles outbreaks emerged in defined Jewish ultra-orthodox communities in Jerusalem. Intensive epidemiological investigation and control measures were initiated. The specific social and cultural nature of the populations involved posed unusual challenges to the public health services, requiring an innovative approach.
METHODS
Epidemiological investigation
Measles is a notifiable disease in Israel by law and cases are reported to the district health office. The case investigation included: demographic characteristics, clinical and laboratory data and vaccination status. Household, school/kindergarten and community contacts were also investigated and the attack rate in each family was calculated (by dividing the number of measles cases in children by the number of all members aged <21 years in the family). An active surveillance process was employed using daily telephone calls to paediatric departments, primary-care physicians, nurses and teachers and public-health nurse home visits.
Case definition
A clinical case was defined as having: generalized rash for ⩾3 days, temperature ⩾38·3°C and cough, coryza or conjunctivitis. A confirmed case was a clinical case with either laboratory confirmation or an epidemiological link to another case (two epidemiologically linked clinical cases were considered confirmed). A clinical case without laboratory confirmation or an epidemiological link to another case was classified as probable. A case of a febrile illness with exanthem was defined as suspected [12].
Laboratory investigation
Laboratory confirmation was defined as a positive measles IgM antibody test. Virus was isolated from urine; RT–PCR was used for virus detection in urine and throat swabs and genotyping was carried out according to WHO standardized protocols [13, 14].
Measles incidence in the city's neighbourhoods
The Jewish population of Jerusalem in 2003 was 465 600 comprising essentially two sub-populations – ultra-orthodox (37·7%) and traditional/secular (62·3%); children aged 0–14 years comprised 30·8% of the total population [Reference Choshen15].
Within the city's various neighbourhoods the population tends to be homogenous in terms of socio-economic and religious background. Families approach the local well baby clinic in the neighbourhood for preventive health services.
The city's neighbourhoods were divided into two categories according to the number of measles cases: (i) 0–1 cases, (ii) ⩾2 (outbreak neighbourhoods). The specific incidence rate and 95% CI were calculated in both categories for the population of children aged 0–14 years residing in these neighbourhoods.
Immunization coverage
Data on immunization coverage of the first dose of MMR vaccine were collected from the municipal well baby clinics located in each neighbourhood. The first-dose immunization coverage data were obtained for children of the birth cohorts 1999–2003 at the age of 2 years. Data on immunization coverage of the second dose of MMR vaccine in first-grade students were collected from the school health services provided by the district health office for the years 2000–2005.
We evaluated immunization coverage in the neighbourhoods by rate difference and coverage in the years before and after the outbreaks, by χ2 for trend.
Statistical analysis
The data were analysed using the SPSS software, version 13 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using the Student's t test; dichotomous variables were analysed using the Pearson χ2 test, with a P value of <0·05 being considered significant.
RESULTS
First outbreak
The first outbreak of measles began in mid-March 2003. The index case was a 2-year-old unvaccinated child from Switzerland (a member of an ultra-orthodox Jewish community in that country) who arrived as a visitor to Israel while in the incubation phase of measles, and attended a crowded wedding ceremony. The disease spread rapidly among young children in contact with the index case, and later in the families and friends of infected children. Within 7 weeks 43 cases occurred and over 5 months 107 cases – some 50% of whom were identified by active investigation (Table). All the patients were from Jewish ultra-orthodox groups in Jerusalem, residing mainly in three crowded neighbourhoods of about 28 000 inhabitants, which were well defined geographically and culturally. Most cases (104, 97·2%) were confirmed: 18 (16·8%) were laboratory confirmed and 86 (80·4%) presented clinically and had an epidemiological link to another case. Three cases (2·8%) were defined as probable. The genotype analysis of positive samples from two patients was found to be most closely related to genotype D8 (1·5% difference). Six patients were hospitalized, mostly due to dehydration and pneumonia, and all recovered without major complications. Most of the patients were unvaccinated and none of them had received two doses of MMR vaccine (Table). Most cases were clustered within families; in 78·5% two or more patients within the one family were infected. The attack rate in the 43 families which accounted for all 107 cases was 62·1±27·4%.
Table. Patient characteristics: first and second outbreaks
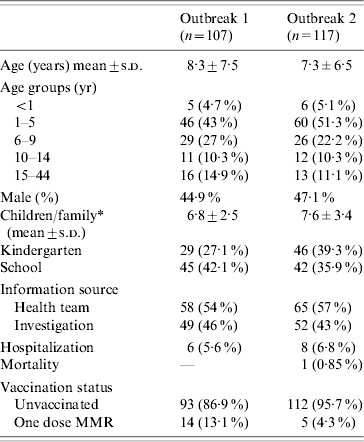
* P<0·05.
Second outbreak
Just over a year later, a second outbreak occurred in a different defined group of the Jewish ultra-orthodox population. This particular sub-population was considered one of the fringe groups of the ultra-orthodox population. Within this community great emphasis was placed on prayer and mysticism, to some extent involving renunciation of what are considered spiritually inferior values, which can include preventive health measures such as immunization. Many adherents of this group are newly religious, and tend to be uncompromising and more extreme in their beliefs and observances. The first cases of measles were in three girls aged 4–5 years in a single kindergarten. Within 5 weeks 59 further cases emerged and in 5 months, 117 cases, with 43% of them traced through active case-finding (Table). Most patients lived in extremely overcrowded conditions in two adjacent streets in an inner Jerusalem neighbourhood of 6500 inhabitants. About a quarter of the sick children attended the community's childcare facilities; 13 children (11·2%) attended the kindergarten in which the outbreak had started and 17 (14·6%) attended a single adjacent school. Most cases (111, 95%) were confirmed: 14 (12%) by laboratory confirmation and the remainder based on a combination of clinical presentation and epidemiological link to another patient. Six cases (5·1%) were defined as probable. Genotype analysis of positive samples from four patients was found to be most closely related to genotype D4 (1·7% difference). Eight patients were hospitalized, including one child with an underlying lung disease who died of pneumonitis. Most cases were unvaccinated. In 78·6% two or more patients within the one family were infected. The attack rate in the 53 families which accounted for all 117 cases was 52·5±29·9%. Seven interrelated households provided 37 (32%) of the cases.
Outbreak control activities
Isolation of patients was practically impossible due to the overcrowded living conditions. Outbreak control was rendered even more difficult by the fact that many patients and families had limited previous contact with preventive health services. Access to this population required a culturally sensitive approach, including communication in the community's language (Yiddish), meetings with religious leaders and cooperation with an ultra-orthodox voluntary organization, which provided access to institutions that were unwilling to obtain services from governmental agencies. Measles control measures included prompt vaccination of susceptible household and childcare-facility contacts. Initially, priority was given to providing the first dose of MMR vaccine to children aged >12 months, followed by a vaccination campaign. In the first outbreak the immunization campaign was initiated in May 2003, and over a period of 3 days, 2250 infants and toddlers aged 1–5 years within the specific community were vaccinated with MMR vaccine (Priorix®, SmithKline Beecham, Brentford, UK) at the local well baby clinics. Within 2 months 6784 children were vaccinated in 71 ultra-orthodox schools (of whom 1130 were vaccinated in private homes). The number of measles cases decreased sharply after the campaign and the last case was reported in August 2003 (Fig.). It should be noted that the second community did not comply with the religious leaders' appeal to vaccinate their children during the first campaign and was reluctant to cooperate during the outbreak. In the second campaign during July–August 2004, 830 infants and toddlers aged 1–5 years and 709 children in ultra-orthodox schools in the community were given MMR vaccine. Since November 2004 there have been no cases of measles in the Jerusalem district (Fig.).
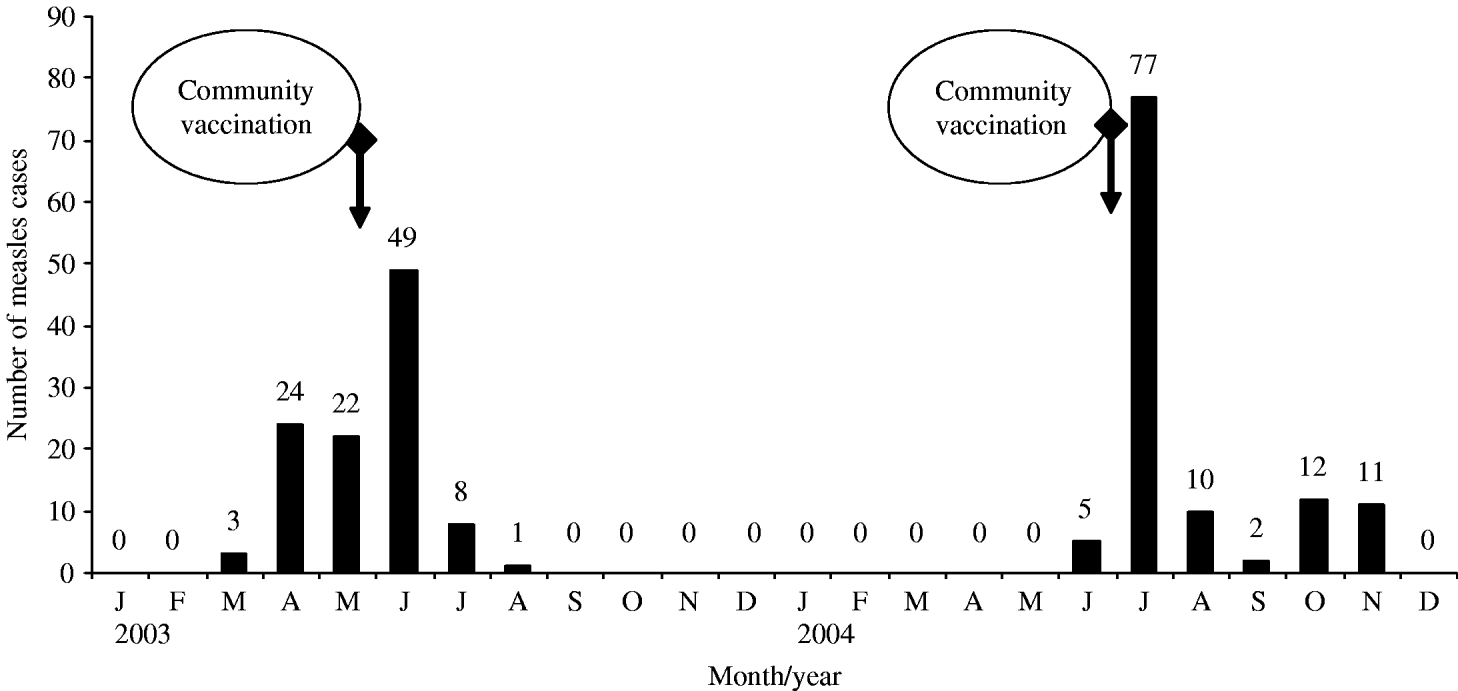
Fig. Measles cases in Jerusalem District January 2003–December 2004 and the two vaccination campaigns. The first campaign was started on 1 May 2003 and the second on 1 July 2004.
Measles incidence in the city's various neighbourhoods
Both outbreaks occurred in dense ultra-orthodox neighbourhoods in which about 45% of the population were children aged 0–14 years. Most cases (87%, 195/224) were within that age group. Measles incidence rate was significantly higher in the outbreak vs. non-outbreak neighbourhoods; 272·9 vs. 6·7/100 000 children aged 0–14 years (OR 40·9, 95% CI 16·9–99·4, P<0·0001).
Immunization coverage
The pre-outbreak immunization coverage of the first dose of MMR vaccine at age 2 years for children who were born in 2000 (n=11 263) in Jerusalem, was significantly lower in outbreak neighbourhoods, 88·3% (5233/5925) than in non-outbreak neighbourhoods, 90·3% (4822/5338) (95% CI difference in coverage, 0·84–3·16, P=0·001). The MMR immunization coverage for the first and second dose of vaccine increased gradually. The average immunization coverages for the first dose of MMR vaccine, at the age of 2 years for children in Jerusalem who were born in 1999, 2000, 2001, 2002 and 2003 (follow-up on 2001–2005) were 90·3, 89·3, 92·2, 95·8 and 95·2% respectively (χ2 for trend=531·6, P=0·0001). The average immunization coverages for the second dose of MMR vaccine, at the age of 6 years for first-grade schoolchildren in Jerusalem in 2000–2005 were 87, 85·6, 88, 88·7, 91·9 and 93·3% respectively (χ2 for trend=582·1, P=0·001). Intensive outreach activities by the public health teams, monitoring and follow-up were continuously performed in these communities.
DISCUSSION
Following the introduction of an effective measles vaccine the global incidence of measles has been substantially reduced. In spite of this remarkable public health achievement, measles outbreaks still occur frequently, mainly within unimmunized sub-populations [2, 3, 7]. Such outbreaks, often caused by imported cases, emerge in countries with overall high immunization coverage in Europe [Reference McBrien16–Reference Muscat22] and North America [23–Reference Parker27] and generally remain confined to specific ethnic communities.
Molecular surveillance of the measles virus has an important role in assisting epidemiological investigations of outbreaks [Reference Riddell, Rota and Rota10]. The two Jerusalem outbreaks were each caused by different measles virus genotypes: D8 and D4. Since measles cases were not reported in Israel between the two outbreaks, we can assume that they resulted from two independent instances of virus importation. The index patient in the first outbreak (D8) was an unvaccinated toddler who arrived from Switzerland in March 2003. Richard & Zimmermann reported 317 cases of measles in Switzerland in early 2003 involving genotypes D8 and D5 [Reference Richard and Zimmermann28]. In the second outbreak (D4) no index case was identified. Many members of this ultra-orthodox group travel frequently to Eastern Europe, mainly to the Ukraine and at times through Romania. The measles outbreak in the Ukraine in 2005–2006 was attributed to genotype D6 [Reference Spika20]. The D4 genotype was detected in 2004–2005 in Germany possibly imported from Romania [Reference Siedler19], and also in an outbreak in 2005 in the United States in which the index case was from Romania [Reference Parker27].
The occurrence of 224 cases of measles, including many secondary cases, within a short period of time in confined communities attests to the high transmissibility of the virus in exposed susceptible individuals. This is especially apparent among young unvaccinated children from large families in overcrowded living conditions. Most patients (94·6%) were aged <21 years, 87% were <15 years and 47% were toddlers (1–5 years). Few cases occurred in infants aged <1 year, probably reflecting the effect of maternal immunity. A considerable proportion of the young adult contacts (siblings and parents) were also unvaccinated. Altogether, 96 households accounted for both outbreaks, with two or more patients per family in 79% of cases. Fourteen children (6·3%) were hospitalized and one child died from major complications of the disease. The disease did not spread outside these particular groups and the outbreaks were effectively contained.
The outbreaks described occurred in populations with unique demographic and social characteristics that created unusual problems and challenges for the public health authorities. The ultra-orthodox population comprises about 38% of the Jewish population of Jerusalem. This population tends to have large families, often live in overcrowded conditions, and in general has a socio-economic level lower than the national average [Reference Choshen15]. Frequent large social gatherings are part of their lifestyle. They are not a homogeneous population, but contain many subgroups that differ in specific cultural and religious practices, each tending to follow their specific spiritual and rabbinic authorities. The fact that one sector of the population cooperated with the public health recommendations after the first outbreak did not mean that another group would do likewise. In general the ultra-orthodox community is characterized by its desire to lead their lives in a closed community, with minimal contact with outside influences, which are seen as posing a threat to their religious standards. As a result, they usually live in well-defined areas; most do not have television or read the general press, but rely on printed news media specific to each community. They generally establish their own educational, religious and social institutions, discouraging outside intervention. A few ultra-orthodox subgroups do not recognize the sovereignty of the State of Israel, and on principle spurn any contact with government or state institutions or authorities.
Rabbis or religious leaders enjoy extreme respect and almost blind subservience from their followers, who will refer to them for guidance and advice on matters not necessarily related to religious practice or observance.
When it became obvious that an outbreak of measles was occurring within the ultra-orthodox population, the public health authorities were faced with several unusual and unique problems. Because of the socio-demographic and cultural characteristics outlined above, it was important to establish lines of communication in order to to advise the population of the need for immunization. To this end urgent meetings were held between senior public health officials and rabbis and other religious community leaders to impress upon them the importance and urgency of the situation. Because of the difficulty of propagating information through the usual written and electronic media, contact was established with a voluntary health services organization (Ezer Mitzion) that is conducted by ultra-orthodox personnel. With their cooperation loudspeaker vans were used in the specific areas of the city to advise the population to attend the well baby clinics for immunization.
Since many of the population had never attended well baby clinics, it was important to extend the immunization campaign to the educational institutions. Here also, the voluntary ultra-orthodox organization cooperated fully with the public health authorities by providing vehicles, personnel and even ‘disguising’ the public health nurses and doctors so they could gain access to institutions that did not wish to be seen as obtaining services from official state bodies. In some cases public health officials who spoke Yiddish were utilized to communicate with the leaders of the community.
Control and prevention of an infectious disease such as measles requires a wide immunization coverage within the community, active and ongoing case surveillance and prompt and comprehensive management of outbreaks [7]. The average immunization coverage within these communities was sub-optimal (88·3%), even compared to the less than ideal overall Jerusalem average (90·3%). It emerged that not only were the children with measles unimmunized, but they had not attended preventive services at all and therefore could not have been included in the immunization coverage data. Hence, the true immunization coverage is lower than that based on the health records. In the United Kingdom, Wright & Polack found that areas with the lowest coverage tended to be those with a high population density and high levels of deprivation [Reference Wright and Polack29]. This finding is of concern since children from deprived populations may be at higher risk of disease complications.
Once the measles virus is introduced into a community, it is essential to set the target level of measles immunization coverage to one which is capable of preventing measles transmission. A threshold level of 92–95% immunization coverage is estimated as being necessary to prevent measles outbreaks from occurring in a given population through ‘herd immunity’ [Reference Wright and Polack29]. Our aim was to try to achieve the national MMR immunization coverage of 95% by rapidly increasing the immunization coverage of all susceptible children aged 1–15 years.
Hutchins et al. evaluated the vaccine coverage and measles incidence in the United States and concluded that a minimum coverage of 80% at age 2 years is required to prevent transmission in preschool-aged children if the population immunity is ⩾93% among persons aged ⩾6 years [Reference Hutchins30]. They noted that their findings may differ according to the situation in a specific country, including such factors as immunity in schoolchildren, vaccination requirements in day-care and contact rates in young children. In Israel the coverage in schoolchildren is 95–97% and no vaccination requirements exist in day-care settings.
The potential for global measles eradication was evaluated by Meissner et al. [Reference Meissner, Strebel and Orenstein31] based on the decline in the annual number of United States cases from 3–4 million in the pre-vaccine era to fewer than 100 cases. Sustaining the highest immunization rates in history is crucial to achieving this goal. Recently, Parker et al. [Reference Parker27] studied the implications of a measles outbreak of 34 cases in a unique community in Indiana (with an average state immunization coverage of 92% and 98% in pre-school and schoolchildren respectively) and suggested special attention and communication strategies in these groups. Mulholland [Reference Mulholland26] reflected that the global eradication of measles is still far off, due to circulation of virus in several parts of the world, virus importation and potential of outbreaks in unvaccinated communities. Unsubstantiated claims suggesting an association between measles vaccine and neurological and other disorders have led to reduced vaccine use and a resurgence of measles in European countries where immunization rates have declined below the level of herd immunity [Reference Coughlan17–Reference Siedler19, Reference Nagaraj32]. This was not our experience; in interviews with the families, concerns about vaccine safety were very rarely raised and hence the basis of the low immunization coverage lies more in the social and cultural spheres, resulting in under-utilization of preventive health services. Subsequently, the overall national immunization coverage rate does not reflect the state of these particular population groups.
A similar phenomenon had been observed previously during measles outbreaks in the 1990s in the Bedouin population in southern Israel, followed by implementation of a successful targeted public health intervention programme [Reference Agur33–Reference Belmaker35]. The Bedouin population differed in their overall immunization coverage from the general Arab Israeli population, which is 96% for the first dose of MMR vaccine, compared to 94% in the Jewish population [6]. Hanratty et al. reported a measles outbreak in unimmunized anthroposophic communities, concluding that the virus can selectively target such groups even in countries with an overall high vaccine uptake. Attaining a uniform high coverage across Europe is essential to interrupt importation and spread of measles, which defers measles elimination [Reference Hanratty36].
Upon recognition of a measles outbreak it is essential to start vaccination as early as possible, thus preventing further spread of the virus. We believe that the rapid rise in immunization coverage achieved in the children in the community contributed to control of the outbreak and probably prevented spread to other population groups. Grais et al. [Reference Grais37] suggested an option of performing gradual mass vaccination in the situation of semi-epidemics in urban areas of resource-poor countries with low vaccine coverage.
As a result of the receptive approach to the population at risk, a significant increase in immunization coverage of MMR vaccine was achieved; intensive follow-up of compliance with routine childhood immunizations continues. Lines of communication were established between the ultra-orthodox community and the public health services, which may help dispel the hesitation and suspicion that characterized the interaction between these groups in the past. The experience gained from these and similar outbreaks is invaluable, and can be applied to other minority groups. A culture-sensitive, non-judgemental approach is essential, and a continuous, ongoing educational and outreach programme tailored to specific populations must be maintained. It is hoped that better utilization of preventive health services will improve the health status of children in these communities. In spite of high immunization coverage countrywide, close surveillance of susceptible populations is essential. The social derivatives will hopefully assist the implementation of public health measures in the future.
ACKNOWLEDGEMENTS
Thanks are due to the Jerusalem District Health Office and Jerusalem Municipality public health teams, the ‘Ezer Mitzion’ organization and volunteers, and the physicians in the community clinics and in the paediatric wards in Jerusalem hospitals.
DECLARATION OF INTEREST
None.




