Flavan-3-ol and proanthocyanidins are the most abundant flavonoid subgroups in the human diet(Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventós1), with a total flavonoid intake of 313 mg/d for the Spanish population (proanthocyanidins: 189 mg/d; flavonoids without proanthocyanidins: 124 mg/d), where proanthocyanidins represent 60 % of the total phenolic intake(Reference Zamora-Ros, Knaze and Luján-Barroso2). These compounds are mainly found in apples, legumes, grapes, nuts, red wine, tea and cocoa(Reference Wang, Chung and Song3). Epidemiological research and intervention studies have supported an association between the intake of foods containing flavanol and proanthocyanidin and a decreased risk of diseases, in particular CVD(Reference Grassi, Desideri and Necozione4–Reference McCullough, Peterson and Patel6) and cancer(Reference Cutler, Nettleton and Ross7). Moreover, the consumption of flavanol- and proanthocyanidin-rich foods improves dyslipidaemia(Reference Mellor, Sathyapalan and Kilpatrick8, Reference Bladé, Arola and Salvadó9), insulin sensitivity(Reference Hooper, Kay and Abdelhamid10) and obesity(Reference Basu, Sanchez and Leyva11) in humans.
Grape seed proanthocyanidin extract (GSPE) improves the atherosclerotic risk index and reduces postprandial triacylglycerolaemia, in both healthy(Reference Del Bas, Fernández-Larrea and Blay12, Reference Quesada, Díaz and Pajuelo13) and dyslipidaemic rats(Reference Quesada, Del Bas and Pajuelo14). Moreover, GSPE improves glycaemia in rats with altered glucose homeostasis(Reference Pinent, Blay and Bladé15, Reference Pinent, Cedó and Montagut16). In addition, GSPE modulates mitochondrial function, increasing their oxidative capacity in the muscle, white adipose tissue (WAT) and brown adipose tissue (BAT) of rats(Reference Pajuelo, Díaz and Quesada17). The action of the bioactive compounds contained in GSPE could be linked to the modulation of signalling pathways, as occurs with the activation of the insulin receptor and key targets of insulin signalling pathways(Reference Montagut, Onnockx and Vaqué18). Moreover, the hypolipidaemic action of GSPE is mediated by the nuclear receptors farnesoid-X-receptor and small heterodimer partner, a signalling pathway leading to lowered lipogenesis and secretion of VLDL in the liver, as demonstrated in previous experiments(Reference Del Bas, Ricketts and Baiges19, Reference Del Bas, Ricketts and Vaqué20).
Over recent years, great effort has been made to determine flavanol and proanthocyanidin bioavailability with a low grade of polymerisation, confirming dimer and trimer absorption(Reference Serra, Macià and Romero21–Reference Zhu, Holt and Lazarus24). Furthermore, plasma kinetics of parental molecules and metabolites have been defined(Reference Okushio, Suzuki and Matsumoto25, Reference Natsume, Osakabe and Oyama26). However, less is known about the capacity of organs to accumulate flavanols and/or their metabolites. Previous experiments have demonstrated the tissue distribution of flavanol metabolites practically throughout the body, even crossing the blood–brain barrier, after an acute intake of a flavanol-rich extract(Reference Serra, Macià and Romero27), and a similar profile of tissue disposition was detected after an acute intake of a dietary dose of flavanols and proanthocyanidins with a low grade of polymerisation, using cocoa cream as a source of polyphenols(Reference Serra, Macià and Rubió28). Flavanol metabolites and phenolic acids have been detected at nanomolar levels in tissues, such as the heart, lung and liver. However, information derived from an acute intake experiment is not sufficient to evaluate correctly the tissue distribution of flavanols and proanthocyanidins with a low grade of polymerisation and/or their metabolites in tissues.
To gain insight into the molecular mechanisms used by flavonoids to modify lipid, glucose and energy metabolism, it is essential to determine which flavanol and/or metabolite reaches and accumulates in the liver and adipose tissues. As mentioned above, the concentrations of flavonoid metabolites in organs have been measured with acute and high doses of flavanols(Reference Serra, Macià and Romero27, Reference Serra, Macià and Rubió28). Thus, the aim of the present study was to determine flavanol metabolites in rat liver, muscle and adipose tissues after a long-term consumption of lower doses of GSPE than previously studied, this being a situation closer to that of the real human consumption of proanthocyanidins. The experiment was performed with three doses of GSPE (5, 25 or 50 mg/kg body weight) for 21 d in order to determine whether there is a dose–response metabolite distribution in the liver, muscle, WAT (mesenteric and perirenal) and BAT.
Materials and methods
Chemicals and reagents
GSPE was kindly provided by Les Dérives Résiniques et Terpéniques. This proanthocyanidin extract contained monomers (58 μmol catechin/g extract, 52 μmol epicatechin/g extract, 5·5 μmol epigallocatechin/g extract, 89 μmol epicatechin gallate/g extract, 1·4 μmol epigallocatechin gallate/g extract), dimers (250 μmol/g extract), trimers (1568 μmol/g extract), tetramers (8·8 μmol/g extract), pentamers (0·73 μmol/g extract) and hexamers (0·38 μmol/g extract).
The internal standard catechol, and the standards of ( − )-epicatechin, (+)-catechin, ( − )-epigallocatechin and ( − )-epigallocatechin-3-O-gallate were purchased from Sigma Aldrich and proanthocyanidin dimer B2 (epicatechin-(4β-8)-epicatechin) from Fluka Company. Acetonitrile (HPLC grade), methanol (HPLC grade), acetone (HPLC grade) and glacial acetic acid ( ≥ 99·8 %) were of analytical grade (Scharlab). Ortho-phosphoric acid (85 %) was purchased from MontPlet & Esteban S. A. Formic acid and l-(+)-ascorbic acid (reagent grade) were all provided by Scharlau Chemie. Ultrapure water was obtained from a Milli-Q water purification system (Millipore Corporation).
Treatment of animals and plasma and tissue collection
A total of twenty male Wistar rats (5–6 weeks) weighing 150–175 g were purchased from Charles River. The animals were housed in animal quarters at 22°C with a 12 h light–12 h dark cycle (lights on from 08.00 to 20.00 hours) and were fed ad libitum with a standard chow diet (Panlab) and tap water. After 1 week of adaptation, the animals were trained to lick condensed milk (1 ml), which was used as a vehicle for administering GSPE, for an additional week. After this period, the animals were randomly divided into four groups (n 5 each group), including the control group. Each group was treated with 5, 25 or 50 mg GSPE/kg body weight per d dispersed in condensed milk. The control group was treated with condensed milk. GSPE was administered every day at 09.00 hours for 21 d. On day 21, 5 h after the GSPE treatment, rats were anaesthetised with ketamine/xylazine and killed by exsanguination from the abdominal aorta using syringes, with heparin as the anticoagulant. Plasma was obtained by centrifugation and stored at − 80°C until analysis. Liver, muscle, BAT and mesenteric and perirenal WAT were excised and frozen immediately in liquid N2 and stored at − 80°C until the analysis of phenolic metabolites. All experimental procedures were performed according to the current national and institutional guidelines for animal care and in place at Universitat Rovira i Virgili (Spain). The Animal Ethics Committee of the Universitat Rovira i Virgili approved all the procedures.
Extraction of flavanols and proanthocyanidins with a low grade of polymerisation from plasma and tissues
The method used to extract flavanols and proanthocyanidins with a low grade of polymerisation and their metabolites from plasma and tissues was based on the methodologies described in our previous papers(Reference Serra, Macià and Romero29, Reference Martí, Pantaleón and Rozek30). In order to clean up the biological matrix and to preconcentrate the phenolic compounds, plasma samples were pretreated by microelution solid-phase extraction, and rat tissue samples were pretreated by a combination of liquid–solid extraction and microelution solid-phase extraction. Briefly, the extraction was done with 60 mg of freeze-dried tissue to which 50 μl of ascorbic acid (1 %), 50 μl of catechol (20 mg/l, dissolved in 4 % phosphoric acid) as an internal standard and 100 μl of phosphoric acid (4 %) were added. The sample was extracted four times with 400 μl of 4 % water–methanol–phosphoric acid (94:4:1, by vol.). Then, 400 μl of the extraction solution were added to each extraction. The sample was sonicated during 30 s, maintained in a freeze water-bath to avoid heating and then centrifuged for 15 min at 14 000 rpm, 20°C. The supernatants were collected, and then the extracts were treated by microelution solid-phase extraction before the chromatographic analysis of flavanols and proanthocyanidins with a low grade of polymerisation and their metabolites. OASIS HLB μElution Plates (30 μm; Waters) were used. Briefly, these were conditioned sequentially with 250 μl of methanol and 250 μl of 0·2 % acetic acid. Then, 350 μl of 4 % phosphoric acid were added to 350 μl of the tissue extract or plasma, and then this mixture was loaded onto the plate. The loaded plates were washed with 200 μl of Milli-Q water and 200 μl of 0·2 % acetic acid. Then, the retained molecules (flavanols and proanthocyanidins with a low grade of polymerisation and their metabolites) were eluted with 2 × 50 μl of acetone–Milli-Q water–acetic acid solution (70:29·5:0·5, by vol.). The eluted solution was directly injected into the chromatographic system, and the sample volume was 2·5 μl.
Analysis of flavanols and proanthocyanidins with a low grade of polymerisation and their metabolites by ultra-performance liquid chromatography-electrospray ionisation-MS/MS
Flavanols and proanthocyanidins with a low grade of polymerisation were analysed by Acquity ultra-performance-liquid chromatography from Waters and tandem MS, as reported in our previous studies(Reference Martí, Pantaleón and Rozek30, Reference Serra, Macià and Romero31). Briefly, the column was Acquity high strength silica T3 (100 mm × 2·1 mm inner diameter, 1·8 μm particle size) with 100 % silica particles (Waters). The mobile phase was 0·2 % acetic acid as eluent A, and acetonitrile as eluent B. The flow rate was 0·4 ml/min and the analysis time 12·5 min. Tandem MS analyses were carried out on a triple quadrupole detector mass spectrometer (Waters) equipped with a Z-spray electrospray interface. The ionisation technique was electrospray ionisation. Flavanols and proanthocyanidins with a low grade of polymerisation and their metabolites were analysed in the negative ion mode and data were acquired through selected reaction monitoring.
For each analyte, two selected reaction monitoring transitions were studied, the most sensitive transition being selected for quantification and a second one for confirmation purposes (see the Supplementary material, available online). The dwell time established for each transition was 30 ms. Data acquisition was carried out with MassLynx v 4.1 software (Waters). Considering the ultra-performance liquid chromatography elution order of catechin and epicatechin and the elution order of their metabolites, together with the MS fragmentation pattern, the earlier eluting peaks were assigned to catechin metabolites, while the peaks eluting later were assigned to epicatechin metabolites. Catechin and epicatechin metabolites were tentatively quantified using the calibration curve of (+)-catechin and ( − )-epicatechin, respectively. Dimers, which corresponded to the sum of four peaks of the extracted ion chromatogram at the transition of 577>289(Reference Serra, Macià and Romero31), were quantified using the calibration curve of the commercial standard dimer B2 (epicatechin-(4β-8)-epicatechin). In the present study, quality parameters of the method, such as the limit of detection (LOD) and the limit of quantification (LOQ), are reported. For the determination of catechin in the liver and adipose tissue, LOD were 0·4 and 1·8 nmol/g, and LOQ were 1·0 and 6·1 nmol/g, respectively. On the other hand, for the determination of epicatechin in the liver and adipose tissue, LOD were 0·6 and 1·6 nmol/g, and LOQ were 1·0 and 4·5 nmol/g, respectively(Reference Serra, Macià and Romero29). As a consequence, in the present study, when the concentration of the generated metabolite was lower than the LOD, it was considered not detected, and when its concentration was below the LOQ, it was considered not quantified.
Statistical analysis
Data are expressed as means and standard deviations. Statistical analysis was performed using two-way ANOVA followed by the Tukey–Kramer honestly significant difference multiple comparison test. A P value < 0·05 was considered as significant. All statistical analyses were performed using JMP software (version 8.0.2; SAS Institute, Inc.).
Results
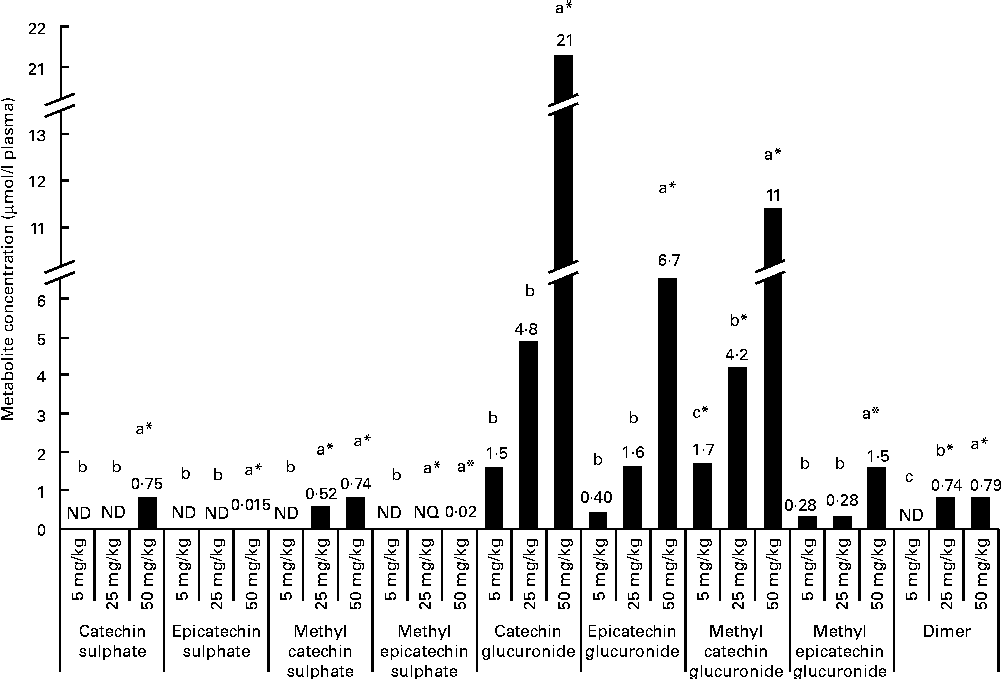
After a regular 21 d consumption of GSPE at different doses (5, 25 and 50 mg/kg body weight), several flavanol metabolites were identified in the plasma (Fig. 1). Glucuronidated conjugates followed by methyl glucuronidated conjugates were the main flavanol metabolites quantified in the plasma, and methyl catechin sulphate was quantified at lower concentrations. In contrast, methyl epicatechin sulphate was only quantified at the trace level (0·02 μmol/l plasma) after the higher tested long-term dose (50 mg/kg). Additionally, dimer was also quantified in plasma samples. Significant differences (P< 0·05) were observed for flavanol plasma concentrations between the doses. For plasma glucuronidated forms, significant differences were only detected with the highest tested dose (CCat_50mg/kg 21 μmol/l plasma and CEpi_50mg/kg 11 μmol/l for catechin glucuronide (P< 0·001) and epicatechin glucuronide (P< 0·001), respectively) compared with the lowest doses, although glucuronidated forms of catechin and epicatechin were quantified in the plasma 21 d after all the tested long-term intakes. A similar behaviour, with significant differences at 50 mg/kg, was observed for methyl epicatechin glucuronide. On the other hand, dimer was quantified at similar concentrations (approximately 0·75 μmol/l plasma) after the 25 and 50 mg/kg of long-term doses. However, dimer was not detected after the 5 mg/kg dose. Methyl catechin sulphate was quantified at a lower concentration in the plasma than glucuronide (P< 0·001) and methyl glucuronide (P< 0·001) conjugates after the 25 and 50 mg/kg long-term doses, and catechin sulphate was only quantified with the 50 mg/kg dose.

Fig. 1 Flavanol metabolites and procyanidins with a low grade of polymerisation concentrations, expressed as μmol/l, quantified in plasma after a 21 d long-term intake of grape seed proanthocyanidin extract (GSPE) at different doses (5, 25 and 50 mg/kg body weight). a,b,cMean values with unlike letters within the same metabolite concentration were significantly different between the GSPE doses (P< 0·05). * Mean values were significantly different between the tested dose and the control group of rats (without GSPE intake) (P< 0·05). ND, not detected; NQ, not quantified.
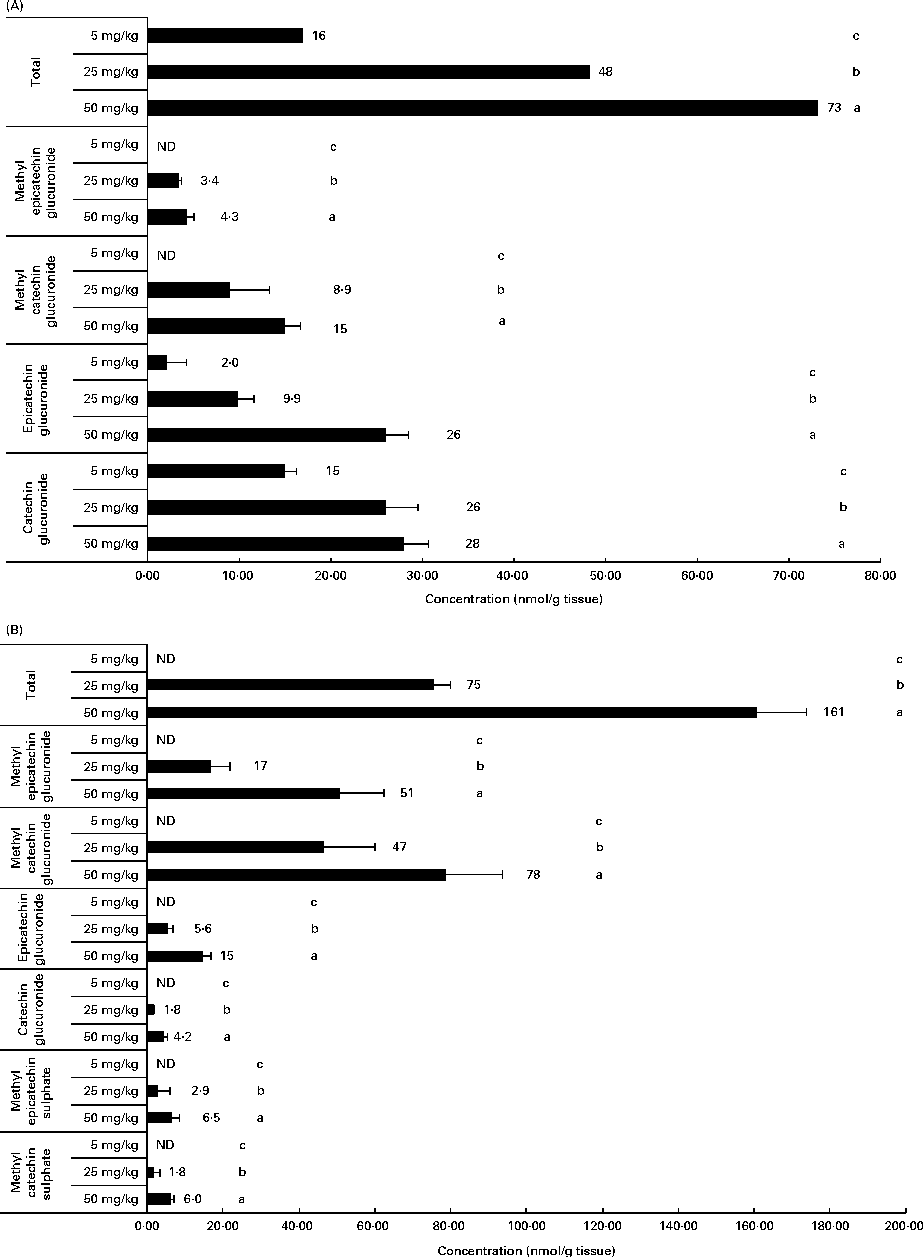
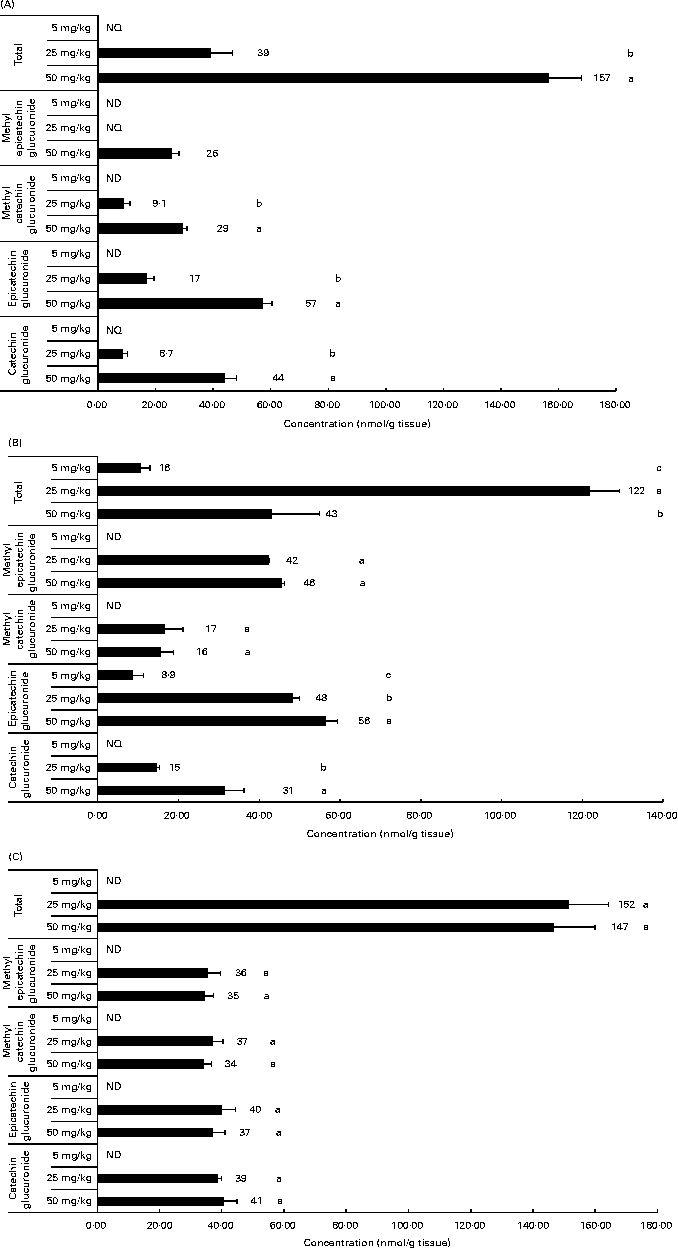
Related to the distribution of flavanol metabolites in tissues, as shown in Fig. 2, the liver and muscle showed a direct relationship between the accumulation and the administered doses of GSPE for all the quantified flavanol metabolites. No flavanol metabolites were quantified in the liver after the lower long-term dose (5 mg/kg). In addition, high concentration levels were detected at 25 and 50 mg/kg. Methyl glucuronide conjugates were the most abundant flavanol metabolites quantified in the liver. Glucuronidated conjugates were the most abundant metabolites detected in the muscle, followed by methyl glucuronidated conjugates. As regards the adipose tissues (Fig. 3), a significant dose-dependent accumulation was observed in BAT for the total flavanol metabolites. Significant differences were detected for all the quantified metabolites 21 d after the 50 mg/kg dose compared with the lower doses (P< 0·001). Glucuronidated and methyl glucuronidated conjugates of catechin and epicatechin were determined in BAT. On the other hand, in the mesenteric and perirenal adipose tissues, no dose-dependent accumulation was observed. Also glucuronidated and methyl glucuronidated conjugates of catechin and epicatechin were quantified in both adipose tissues. Nonetheless, with the 5 mg/kg dose, practically, no metabolites were detected and significant differences for epicatechin and catechin glucuronide were only detected in the brown and mesenteric adipose tissues (P< 0·05) between 25 and 50 mg/kg. It should be noted that dimer was not detected in any of the studied tissues (data not shown).

Fig. 2 Flavanol metabolites quantified in (A) muscle and (B) liver after a 21 d long-term intake of grape seed proanthocyanidin extract at different doses (5, 25 and 50 mg/kg body weight). Values are means (nmol/g tissue), with standard deviations represented by horizontal bars. a,b,cMean values with unlike letters within the same metabolite concentration were significantly different between the doses (P< 0·05). ND, not detected.

Fig. 3 Flavanol metabolites quantified in white adipose tissues (mesenteric (A) and perirenal (B)) and brown adipose tissues (C) after a 21 d long-term intake of grape seed proanthocyanidin extract at different doses (5, 25 and 50 mg/kg body weight). Values are means (nmol/g tissue), with standard deviations represented by horizontal bars. a,b,cMean values with unlike letters within the same metabolite concentration were significantly different between the doses (P< 0·05). ND, not detected; NQ, not quantified.
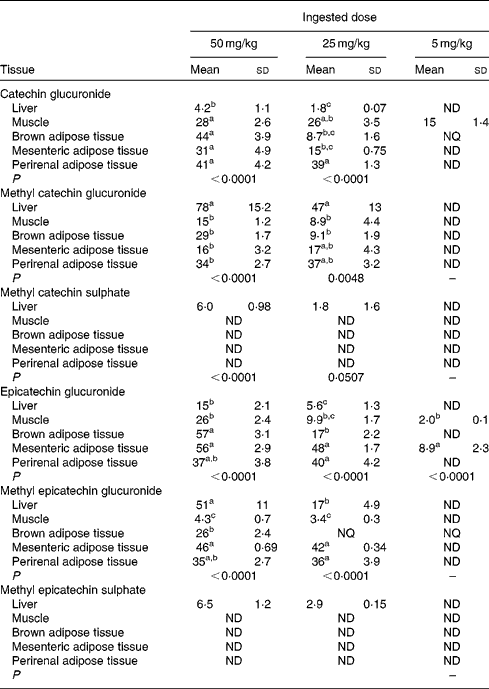
Table 1 allows the detection of significant differences between the tissues for each metabolite differentiating between the ingested doses of GSPE. For catechin glucuronide, at the 5 mg/kg dose, muscle was the tissue with the greatest accumulation. In contrast, tissue distribution was more homogeneous at higher doses, with no significant differences between the muscle, BAT, mesenteric adipose tissue and perirenal adipose tissue, except for the liver, in which the concentration of metabolites remained low. The opposite situation was observed for methyl catechin glucuronide that at low dose (5 mg/kg) was not detected in any tissue. Nevertheless, with increasing doses, accumulation was concentrated in the liver. Regarding methyl sulphated conjugates, tissue deposition was very low. A low accumulation of metabolites after the treatment with doses of GSPE at 25 and 50 mg/kg was only observed in the liver. The epicatechin glucuronidated conjugate was accumulated mainly in the mesenteric adipose tissue during the 5 mg/kg dose. At 25 and 50 mg/kg, accumulation was concentrated in the three adipose tissues (brown, perirenal and mesenteric) with no significant difference between them. Methyl epicatechin glucuronide, at the 5 mg/kg dose, was only accumulated in BAT, showing a value of concentration between the LOD (1·8 nmol/g tissue) and the LOQ (6·1 nmol/g tissue) determined for epicatechin in the adipose tissue(Reference Serra, Macià and Romero27). At a higher dose of GSPE (25 mg/kg), mesenteric and perirenal adipose tissues showed the highest accumulations. In return, at 50 mg/kg of GSPE, liver, mesenteric and perirenal adipose tissues showed the highest concentration. No phenolic metabolites were detected in plasma and tissues in the control group of rats (data not shown).
Table 1 Flavanol metabolite concentrations in the rat liver, muscle, brown adipose tissue and white adipose tissues (mesenteric and perirenal) after a 21 d chronic intake of grape seed proanthocyanidin extract at different doses (5, 25 and 50 mg/kg body weight), with n 5 for each dose (Mean values (nmol/g tissues) and standard deviations)

ND, not detected; NQ, not quantified.
a,b,cMean values within a column with unlike superscript letters were significantly different between the tissues.
Discussion
Liver, muscle and adipose tissues are crucial in the homeostasis of TAG and glucose. Moreover, obesity depends on fat accumulation in WAT and on energy wasted in BAT(Reference Cinti32). Therefore, in order to understand the role of flavanols in improving hypertriacylglycerolaemia(Reference Quesada, Díaz and Pajuelo13, Reference Quesada, Del Bas and Pajuelo14, Reference Ruzaidi, Amin and Nawalyah33, Reference Ruzaidi, Abbe and Amin34), hyperglycaemia(Reference Grassi, Desideri and Necozione4,Reference Landrault, Poucheret and Azay35–Reference Caimi, Carollo and Lo Presti38) and obesity(Reference Basu, Sanchez and Leyva11), it is essential to know whether flavanols reach these organs and which metabolites could be responsible for their effects. The present study shows the plasma bioavailability and tissue distribution of flavanol metabolites in the WAT (mesenteric and perirenal), BAT, muscle and liver after a regular 21 d intake of GSPE. The results obtained show that each of the organs studied has a specific pattern of metabolite accumulation and response to the assayed GSPE doses.
The presence of several catechin and epicatechin metabolites in the plasma reinforces the fact that procyanidins are intensely metabolised by the small intestine and liver. Plasma catechin conjugates are more abundant than epicatechin conjugates, a fact directly related to the proportion of catechin and epicatechin in GSPE (6·3 (sd 0·54) μmol/g of GSPE and 2·4 (sd 0·13) μmol/g of GSPE, respectively, data shown in a previous work(Reference Serra, Macià and Romero21)). Glucuronidated conjugates, followed by methyl glucuronidated conjugates, were the main flavanol metabolites detected in the plasma, and this agrees with the existing literature(Reference Serra, Macià and Romero27, Reference Ottaviani, Momma and Kuhnle39–Reference Tsang, Auger and Mullen42).
Previous studies have shown a high number of phase II metabolites in the liver after a long-term intake of catechin(Reference Urpi-Sarda, Ramiro-Puig and Khan43). By contrast, after an acute intake of a proanthocyanidin-rich extract, these phenolic metabolites were not detected in the liver(Reference Serra, Macià and Romero27). However, the results of the present study show that the sulphate conjugates of catechin and epicatechin were only deposited in the liver, showing a dose–response accumulation and indicating sulphation capacity of rat hepatocytes. Nonetheless, these sulphated conjugates were not detected in the liver after the treatment with 5 mg GSPE/kg body weight. Moreover, their presence in the plasma at low concentrations could reflect the possible sulphotransferase activity of the platelets, as was observed by Anderson et al. (Reference Anderson, Garcia and Liebentritt44) who incubated hydroxytyrosol, a phenyl alcohol typical of virgin olive oil, in whole blood.
Glucuronide conjugates were detected in all the organs studied, but their distribution and concentration may depend on methylation. Non-methylated glucuronide derivatives were mainly accumulated in BAT and in the two white adipose depots studied, mesenteric and perirenal. On the other hand, methyl catechin glucuronide was accumulated mainly in the liver, whereas methyl epicatechin glucuronide was accumulated mainly in BAT and WAT. It is important to highlight that the accumulation of glucuronide derivatives in white adipose depots was not dose responsive. In both the WAT studied (perirenal and mesenteric), the levels of glucuronide derivatives were similar, at 25 and 50 mg of GSPE/kg body weight. This could be related to the storage limit in white adipose cells. However, this might be more related to the saturation of uridine 5′-diphospho-glucuronosyltransferase enzymes responsible for the formation of glucuronidated conjugates. These enzymes are located in the endoplasmic reticulum in many tissues and catalyse the transfer of glucuronic acid from uridine 5′-diphospho-glucuronic acid to steroids, bile acids, polyphenols and xenobiotics(Reference Manach, Scalbert and Morand45). Moreover, the concentration of some of these metabolites was even higher than that in the other tissues studied. Taken together, it can be suggested that visceral WAT could be a store for flavanol metabolites in the body. Nonetheless, further studies are needed to understand whether flavanols accumulate in adipocytes or other cell types in the adipose tissue.
BAT could represent a very important target for flavanol metabolites, due to, for example, their improvement in mitochondrial function related to energy homeostasis of the BAT(Reference Pajuelo, Díaz and Quesada17). This tissue is the only tissue with detectable levels of all the glucuronidated derivatives at the lowest GSPE dose (5 mg/kg body weight). Moreover, BAT showed a dose–response in the concentration of all the conjugate metabolites of catechin and epicatechin. Moreover, some of these metabolites reached high concentrations, probably related to the high irrigation of this type of adipose tissue by blood vessels(Reference Ravussin and Galgani46). These results, together with the possible enhancement of thermogenic capacity and the improvement in mitochondrial function exerted by a long-term supplementation of proanthocyanidins in BAT(Reference Pajuelo, Díaz and Quesada17, Reference Pajuelo, Quesada and Díaz47), it can be suggested that proanthocyanidins may play an important role in reducing or preventing obesity by modulating the functionality of BAT at low doses.
The dose-dependent disposition of phase II metabolites of procyanidins detected in the muscle may also be related to an improvement in mitochondrial function in skeletal muscle detected in a previous experiment(Reference Pajuelo, Quesada and Díaz47), suggesting an improvement in the activity of enzymes involved in the oxidation and metabolism of pyruvate, and a shift in priority to glycosidic metabolism rather than lipid metabolism.
Conclusions
In the present study, after a long-term intake of flavanol-rich extract at different doses, plasma bioavailability, distribution and accumulation of flavanols and their metabolites in the adipose tissues, muscle and liver were studied. Each of the studied organs has a specific behaviour of accumulation and response to the assayed GSPE doses, with a clear dose–response in BAT, in which flavanols could play an important role in reducing or preventing obesity by modulating the functionality of that tissue. The results of the present experiment could be useful for future in vitro research with adipose cell culture giving information about the physiological concentrations reached in specific adipose tissues after a long-term intake of flavanols.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513000706
Acknowledgements
The present study was supported by grant no. AGL 2008-00387/ALI and AGL2009-13517-C03-02 from the Spanish Government. It was also supported by the Catalan Government (Interdepartmental Commission for Research and Technological Innovation) through the A. Serra grant. The authors' contributions are as follows: M.-J. M., L. A. and C. B. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; M.-J. M., L. A. and C. B. contributed to the study concept and design; A. S. and A. M. were responsible for the acquisition of the data on grape seed procyanidin extract; L. A. and C. B. were involved in the treatment of the animals and plasma and tissue collection; A. S., A. M. and M.-J. M. were responsible for the acquisition of the data on procyanidin metabolites in plasma and tissues; A. S., A. M., M.-J. M., C. B. and L. A. conducted the analyses and interpreted the data; A. S., M.-J. M., L. A. and C. B. drafted the manuscript; A. S., A. M., M.-J. M., L. A. and C. B. critically revised the manuscript for important intellectual content; A. M. and C. B. provided administrative, technical and logistic support. The authors declare that there is no conflict of interest.