Consumption of sugar-sweetened beverages (SSB) remains high in the USA, with Hispanics and non-Hispanic Blacks consuming the greatest total SSB amounts followed by non-Hispanic Whites( Reference Han and Powell 1 , Reference Bleich, Vercammen and Koma 2 ). Consumption of SSB is associated with higher energy intake( Reference Vartanian, Schwartz and Brownell 3 ), weight gain( Reference Dhingra, Sullivan and Jacques 4 ), fatty liver disease( Reference Ma, Fox and Jacques 5 ), type 2 diabetes mellitus( Reference de Koning, Malik and Kellogg 6 ) and heart disease( Reference Fung, Malik and Rexrode 7 ). Despite Hispanics being disproportionately affected by obesity and obesity-related co-morbidities relative to other racial/ethnic subgroups( Reference Go, Mozaffarian and Roger 8 , Reference Go, Mozaffarian and Roger 9 ), few studies have aimed to reduce energy intake and/or improve cardiometabolic health in this high-risk group( Reference Ceballos 10 – Reference Vargas-Garcia, Evans and Prestwich 12 ). Efforts to identify modifiable risk factors beyond weight loss, which is known to be effective but difficult to sustain( Reference Montesi, El Ghoch and Brodosi 13 ), and to improve cardiometabolic health in this vulnerable population are lacking. One method that has been proposed is to reduce energy and refined carbohydrate intakes through replacement of SSB consumption with alternative, non-sweetened beverages( Reference Mekonnen, Odden and Coxson 14 ).
This strategy may be especially important for Mexican-origin populations, who comprise 64·3 % of the Hispanic population in the USA( Reference Passel and Cohn 15 ). For example, it has been estimated that a 10 % reduction in SSB consumption would reduce the incidence of type 2 diabetes mellitus twofold for Mexican-origin individuals living in California compared with all Californians( Reference Mekonnen, Odden and Coxson 14 ). There are limited interventions that have examined the effects of replacing SSB with healthier alternatives for Mexican-origin adults( Reference Vargas-Garcia, Evans and Prestwich 12 , Reference Hernandez-Cordero, Barquera and Rodriguez-Ramirez 16 , Reference Zoellner, Hedrick and You 17 ). In a study by Hernandez-Cordero et al.( Reference Hernandez-Cordero, Barquera and Rodriguez-Ramirez 16 ), 240 Mexican women were randomized to a water and education intervention, where water was home-delivered, or education only. There were no significant differences in plasma TAG, cholesterol and other cardiometabolic risks between groups at the end of the 9-month intervention( Reference Hernandez-Cordero, Barquera and Rodriguez-Ramirez 16 ). In a secondary analysis, the authors found that the water and nutrition education intervention led to significant reductions in SSB and improved overall diet quality( Reference Rodriguez-Ramirez, Gonzalez de Cosio and Mendez 18 ). This highlights the need for further exploration into alternative beverage choices as a strategy to improve cardiometabolic health/reduce risk in this understudied population.
Several alternative beverages have been studied for their potential to improve cardiometabolic health. These include, but are not limited to, green tea and water( Reference Vargas-Garcia, Evans and Prestwich 12 , Reference Stendell-Hollis, Thomson and Thompson 19 – Reference Basu, Betts and Mulugeta 22 ). Green tea has been reported to have acute( Reference Tsuneki, Ishizuka and Terasawa 23 ) and long-term( Reference Chacko, Thambi and Kuttan 24 ) effects on blood glucose and may modulate lipid metabolism( Reference Yuan, Dong and Fang 25 ). In addition to green tea and water, citrus fruit consumption has been investigated as an alternative beverage to improve cardiometabolic health( Reference Silver, Dietrich and Niswender 26 – Reference Miller, Thompson and Hakim 28 ). Specifically, our research group has examined the effects of Mediterranean lemonade (a beverage rich in d-limonene, a bioactive compound found in the citrus peel) in terms of physiological mechanisms, safety and bioavailability( Reference Miller, Thompson and Hakim 28 – Reference Miller, Hakim and Chew 30 ).
Evidence suggests citrus and d-limonene play a role in cholesterol and glucose regulation. In adipocytes, d-limonene had a positive effect on glucose metabolism and reduced lipid accumulation in the cell( Reference Tan, Chua and Ravishankar Ram 31 ). Further, d-limonene protected against dyslipidaemia and hyperglycaemia in mice fed a high-fat diet( Reference Jing, Zhang and Fan 32 ) and reduced glucose levels of diabetic rats back to normal( Reference Murali and Saravanan 33 ). In our clinical trial with breast cancer patients, d-limonene (given as pure citrus oil) modulated plasma metabolomic profiles; specific changes in metabolites were related to energy metabolism and tighter glucose control( Reference Miller, Pappan and Thompson 34 ). Despite this evidence, no studies have tested the effects of Mediterranean lemonade or green tea and their constitutive bioactive compounds on cardiometabolic regulation for Hispanic adults. Building upon our previous work and the published literature, the primary objective of the present study was to assess the feasibility and acceptability of a beverage intervention (green tea, Mediterranean lemonade or water) in obese Hispanic adults. Secondary outcomes included change in selected biomarkers associated with cardiometabolic disease risk.
Methods
Study outcomes and hypotheses
The Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist was used as an evidence-based set of recommendations for what information to include when reporting a pilot or feasibility trial. Primary feasibility outcomes were recruitment, retention and acceptability. Beverage intake was assessed through weekly beverage logs. The preliminary efficacy of the beverage intervention was assessed through examining changes in total cholesterol, HDL-cholesterol and LDL-cholesterol over 8 weeks. Secondary outcomes included changes in fasting glucose and glycated Hb (HbA1c). Outcomes were examined to test the following hypotheses: (i) recruitment and retention of obese Hispanic participants in an 8-week beverage intervention study is feasible; (ii) the consumption of green tea and Mediterranean lemonade will be well tolerated with high adherence; and (iii) green tea and Mediterranean lemonade intake will result in an improved cardiometabolic profile at the end of 8 weeks.
Design
The present study was a pilot randomized controlled trial where participants were randomized to one of three beverage groups: green tea (GT), Mediterranean lemonade (ML) or a flavoured water control (FW). Details on the study’s methods have been described elsewhere( Reference Morrill, Aceves and Valdez 35 ). Briefly, we proposed to consent 150 individuals to randomize fifty participants into our 8-week beverage intervention study. Randomization was performed by a computer system where participants, stratified by gender, were randomized 2:2:1 to GT (n 19), ML (n 21) or FW (n 10) using block randomization, respectively.
Blinding
Study personnel performing the assessments and statisticians analysing the data were blinded to participant randomization status. Investigators, intervention staff (e.g. staff performing beverage pick-ups and phone call reminders) and participants were not blinded to randomization status.
Study population
We recruited fifty Hispanic adults living in the Tucson area, AZ, USA. Individuals were considered eligible if they self-identified as Hispanic, were 18–64 years of age, had a BMI between 30·0 and 50·0 kg/m2, were able to provide informed consent, and were able to speak, read and write in either English and/or Spanish. Individuals were excluded if they reported recent weight loss, history of diabetes, reported engaging in regular physical activity (e.g. ≥3 d/week for ≥20 min/d over the past 3 months) and were taking any medications that would influence cardiometabolic measures (e.g. anti-inflammatory medications, medications for diabetes, steroids, etc.). Individuals reporting ≥1 cup of green tea and/or citrus fruit daily also had to be willing to complete a 2-week washout period prior to randomization into a study group. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Arizona Institutional Review Board. Written informed consent was obtained from all subjects.
Study recruitment and informed consent
Recruitment efforts primarily included local, community-based settings such as health clinics, health fairs and outdoor marketplaces frequently attended by the Hispanic community living in Tucson, AZ, USA. Additional recruitment strategies included the use of social media (e.g. Facebook and Craigslist) and health provider-initiated approaches (e.g. patient referral). Interested individuals engaged in-person or over the telephone were provided a detailed description of the study and its potential risks and benefits. After providing verbal agreement, study staff asked questions regarding medical history and other relevant questions related to exclusion/inclusion criteria. Interested participants provided written informed consent to study personnel using consent forms that were available in the participants’ preferred language (Spanish or English). Research activities took place at the University of Arizona Collaboratory for Metabolic Disease Prevention and Treatment in Tucson, AZ.
Beverage preparation
Study personnel prepared all study beverages. Lipton® Decaffeinated Green Tea was prepared using a ratio of four tea bags per 32 oz (946 ml; 1 US fl.oz = 29·57 ml) water. Upon reaching a boil, tea bags were steeped into water for 3–5 min. When slightly cooled, three drops of Liquid No Calorie Stevia™ were added per 32 oz of tea. For Mediterranean lemonade, two full lemons were de-seeded and blended with 32 oz of water and three drops of Liquid No Calorie Stevia™ were added. The control beverage was prepared using three drops of Crystal Light® Liquid Drink Mix (strawberry lemonade) per 32 oz of water. All beverages were prepared two days prior to distribution to participants. Importantly, mass spectrometry was performed by the study team to inform the preparation protocol of the green tea and Mediterranean lemonade to optimize epigallocatechin gallate and d-limonene concentrations. Green tea beverages were kept frozen to minimize degradation of polyphenols. Mediterranean lemonade and flavoured water were refrigerated for storage and distribution to study participants. Beverages were provided to participants in seven 32 oz plastic cartons and participants were instructed to consume one carton per day.
Run-in period
Participants completed a 2-week run-in period before beginning the 6-week intervention where they were asked to stop all consumption of tea and citrus fruit while limiting consumption of other beverages except water. This 2-week period was considered sufficient to metabolically clear the bioactive compounds under study. During the run-in, participants were provided with a 32 oz Hydro Flask® to support regular intake of liquids. This run-in period served to determine participant adherence to a beverage intervention more generally.
The 6-week intervention
Upon successful completion of the 2-week run-in, participants were randomized to a beverage group. All participants were instructed to consume the entire beverage (i.e. 32 oz) assigned on a daily basis, rather than save up and consume large amounts on fewer days. At the start of the intervention, participants were asked to continue avoiding all other sources of tea, lemonade and other citrus, milk and limit coffee consumption to 2 cups/d. In addition, participants were asked to avoid all sweetened beverages (e.g. agua frescas and horchata) and alcohol in excess of 1 drink/d for women and 2 drinks/d for men. Water could be consumed ad libitum. This was done to minimize confounding. In addition, participants were asked to complete weekly beverage journals, specific to study beverages, to self-monitor their consumption behaviours and assist in habitually regulating beverage intake. Once per week, participants were responsible for picking up one week’s worth of beverages from the study clinic. During this time, participants were greeted by study staff who were Hispanic and fluent in both Spanish and English. Beverage-related behaviours were discussed to identify and address any specific barriers to adherence of consuming study beverages. These ‘check-ins’ lasted 5–10 min and followed a script to elicit conversation. Additionally, during this time, participants were asked to return any unconsumed beverages which, if presented, study staff then measured and recorded. Weekly beverage journals were collected, and new ones distributed for use the following week. To increase retention, common strategies were incorporated including: (i) collecting contact information of participants and at least two family members; (ii) programme reminders; (iii) incentives to complete assessments; and (iv) contacting participants at their preferred time by their preferred method (i.e. call or text) in their preferred language. Participants received a total of $US 75 for completing the study.
Participant-reported tolerance
Potential risks from consumption of green tea and Mediterranean lemonade may have included but were not limited to: nausea, vomiting, frequent bowel movements, flatulence (gas), acid reflux, excess burping, heartburn and bloating. If a participant experienced any of these signs/symptoms, s/he was given the option to withdraw from the study or change to the alternative beverage intervention (but not flavoured water) after a 1-week washout period. Participants were asked during weekly ‘check-ins’ if there were any issues related to their beverage consumption. If any issues were raised, they were recorded in participant folders.
Treatment satisfaction/acceptability
At the completion of the study, participants took part in an exit interview where they were asked to rate their overall satisfaction with the intervention, if they would consider a longer-term beverage intervention and, finally, if they would recommend the programme to others. Participants were asked questions regarding satisfaction with their overall progress and for changing dietary beverage habits. Each item was rated on a Likert scale with higher scores indicating greater programme favourability. Open-ended questions were used to seek participant input on modifications that could be made to improve acceptability and effectiveness of the intervention. The responses were used to identify which recruitment and intervention components were well received, which could be improved, and which were not acceptable.
Methods for assessing study outcomes
Height and body weight were measured using standard anthropometric procedures with the participant in light weight clothes and not wearing shoes. Fasting blood samples (venepuncture; 25 ml) were collected at baseline and 8 weeks to examine changes in lipids, HbA1c and fasting glucose.
Self-reported questionnaires
Validated self-reported questionnaires were used to measure acculturation, diet and physical activity. The Acculturation Rating Scale for Mexican-Americans–II (ARSMA-II)( Reference Cuellar, Arnold and Maldonado 36 ) was used to measure acculturation related to language, ethnic identity and ethnic interaction. The reliability and validity of the ARSMA-II are well established in English and Spanish( Reference Cuellar, Arnold and Maldonado 36 ). The Southwestern FFQ (SWFFQ)( Reference Taren, Tobar and Ritenbaugh 37 – Reference Garcia, Taren and Teufel 39 ), a bilingual FFQ adapted from the Arizona FFQ, includes 158 food items commonly consumed by Mexican-Americans (e.g. nopalitos, corn/flour tortillas, chorizo) and uses Mexican names for food items commonly given different names by other Spanish speakers (e.g. naranja, not china, for ‘orange’). Step-by-step instructions for completion were attached to each questionnaire in the participants’ preferred language. When completing the questionnaire, participants were asked to describe their average use (ranging from three or more times daily to rarely/never) and portion size for each food item listed. Participants were also asked eleven more-detailed multiple-choice questions regarding specific eating habits (e.g. ‘How often do you eat the skin on chicken?’, ‘What kind of fat do you usually use?’) and were able to write in additional food items not already included in the questionnaire. Data retrieved from the SWFFQ allowed us to calculate total daily energy intake (kilocalories). Internal validity of the SWFFQ compares favourably with 24 h recall (r = 0·82)( Reference Taren, Tobar and Ritenbaugh 37 ). Physical activity was assessed using the validated Global Physical Activity Questionnaire (GPAQ)( Reference Hoos, Espinoza and Marshall 40 , Reference Cleland, Hunter and Kee 41 ) which is available in both English and Spanish and provided minutes per week of physical activity of varying intensity and type. All completed questionnaires were reviewed in-person with each participant during the initial visit (i.e. baseline assessment) to ensure all questions were understood and completed properly.
Statistical methods
Sample size
As with most pilot studies, there was inadequate power to detect important differences in outcomes, and this was not the primary focus of the present study( Reference Thabane, Ma and Chu 42 – Reference Bell, Whitehead and Julious 44 ). We therefore based our sample size on the precision of our primary feasibility outcomes (recruitment, retention). We estimated that a total sample size of 50 would provide 95 % CI for recruitment and retention that were no wider than 0·28 (0·14). Estimates of the variance components from the current study will be used to power a future definitive trial( Reference Bell, Whitehead and Julious 44 ).
Feasibility outcomes
The primary outcomes were recruitment and retention. We aimed to recruit, on average, approximately two or three participants per week during the active recruitment phase. A recruitment rate of less than this would indicate a lack of feasibility. We recorded the number of Hispanic adults who contact the researchers and expressed interest in participation, the number screened for eligibility, and the number ineligible for study inclusion and the reason for their ineligibility. Retention was assessed by calculating the proportion of participants who completed the study out of the number enrolled, with a 95 % CI.
Preliminary efficacy outcomes
The statistical analysis plan was pre-specified. Descriptive statistics were calculated for the preliminary efficacy outcomes (cholesterol/lipid levels) and the secondary outcomes (fasting glucose and HbA1c). Linear mixed models were used to model all continuous outcomes, with fixed effects of intervention arm, time and their interaction to allow for different patterns of change between arms. A random participant effect was used to account for the longitudinal design. Using these models, we estimated changes from baseline and differences between arms, as well as comparing differences between groups. Mixed models provide unbiased estimation for missing completely at random and missing at random data, and allow data from all patients who were randomized to be included in the analysis( Reference Bell and Fairclough 45 ). In modelling energy intake, we excluded participant surveys reporting an intake of more than 20 290 kJ/d (5000 kcal/d).
Sensitivity analyses
We performed a sensitivity analysis to assess the influence of one individual with weight over 160 kg. We also carried out non-parametric tests (Wilcoxon signed rank and rank sum) on energy consumed, as these data were skewed, even with excluding implausible values as described above.
Results
Participant characteristics
Of the 102 eligible participants recruited, fifty were randomized in a 2:2:1 ratio to one of three beverage groups: GT, ML or FW. Demographic and clinical characteristics of participants are shown in Table 1. At baseline, the mean age of participants was 44·7 (sd 10·1) years and their mean BMI was 35·9 (sd 4·6) kg/m2. Participants were mostly female (78 %), employed (60 %) and earning an annual income of $US 30 000 or less (70 %). Seventy-two per cent held the equivalent of a high-school diploma or greater. Heritage identification was predominantly Mexican and Mexican-American (86 %), with 72 % reporting Spanish as the primary language used at home and 60 % being foreign born. On the ARSMA-II acculturation scale, 66 % fell within the ‘Very Mexican-oriented’ and ‘Mexican-oriented to approximately bicultural’ categories.
Table 1 Demographic and clinical characteristics of the fifty Hispanic participants according to intervention beverage group, Tucson, AZ, USA, August 2016–August 2017

FW, flavoured water control; ML, Mediterranean lemonade; GT, green tea; GED, General Educational Development; MOS, Mexican Orientation Subscale; HbA1c, glycated Hb.
Values displayed are means and standard deviations for continuous variables and counts (numbers) and percentages for categorical variables. Categorical percentages summing to less than 100 % are due to missing response. MOS and acculturation scores excluded five participants who did not identify as Mexican/Mexican-American because the acculturation instrument (Acculturation Rating Scale for Mexican-Americans–II) was developed for Mexican-Americans.
Baseline physical activity data were collected on forty-eight individuals. Mean leisure-time physical activity was 1·3 (sd 2·9) h/week. Thirty-nine participants completed the baseline and 8-week SWFFQ, although seven questionnaires that reported daily energy consumed greater than 20 290 kJ (5000 kcal) were excluded. Among the thirty-two remaining, mean energy intake was 9460 (sd 4322) kJ/d (2261 (sd 1033) kcal/d). Mean blood cholesterol values were 192·6 (sd 38·2) mg/dl for total, 48·1 (sd 9·4) mg/dl for HDL and 116·9 (sd 30·6) mg/dl for LDL. Mean blood glucose and HbA1c were 92·6 (sd 11·7) mg/dl and 5·5 (sd 0·3) %, respectively. Age, weight, total cholesterol and LDL-cholesterol were qualitatively imbalanced across groups, with maximal differences in means of 9·1 years (age), 9·2 kg (weight), 17·4 mg/dl (total cholesterol) and 14·9 mg/dl (LDL-cholesterol).
Recruitment, retention, tolerance
Figure 1 shows results of the recruitment process. Participants were recruited over 24 weeks via fliers, online, friends/family, swap meet and by other means. A total of 236 Hispanic adults were screened for eligibility. Of 102 eligible participants, fifty were randomized (0·49; 95 % CI 0·39, 0·59). This is an average recruitment rate of approximately two per week. Additional measures of study feasibility are reported in Table 2. Most participants (88 %; 95 % CI 76, 95 %) completed the 8-week assessment, which is well above our feasibility criterion of 70 %. Among those who did not complete, four were lost to follow-up and two were excluded prior to treatment due to health concerns. Two participants in the ML group switched to GT at three weeks due to gastrointestinal distress. Self-reported compliance was high among completers, with 93 % of the assigned ounces of beverage consumed.
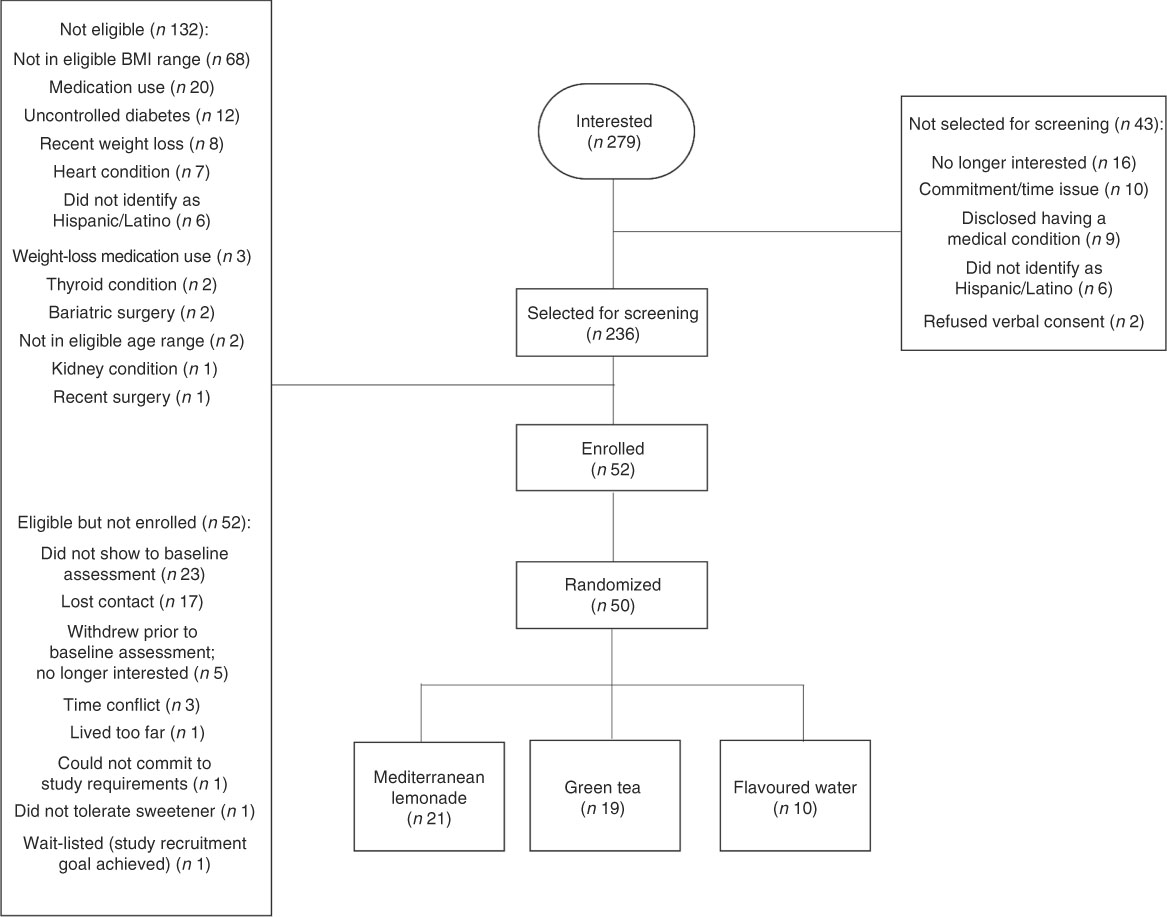
Fig. 1 Recruitment and screening process
Table 2 Retention and tolerance to beverages of the fifty Hispanic participants according to intervention beverage group, Tucson, AZ, USA, August 2016–August 2017

FW, flavoured water control; ML, Mediterranean lemonade; GT, green tea.
All displayed values except adherence are counts (numbers) and percentages. Adherence to treatment is defined as the average percentage of ounces consumed of the total required per week and values displayed are mean and minimum–maximum percentages.
* Includes participants who changed treatment.
Participant satisfaction
Among forty-one participants who completed the satisfaction survey, overall satisfaction was high with a mean of 3·3 (sd 0·9) on a 4-point scale with 1 = ‘low satisfaction’ and 4 = ‘high satisfaction’. Patients were highly satisfied with instructions and their progress and were highly likely to recommend the programme (Table 3).
Table 3 Patient satisfaction survey results among forty-one of the forty-four Hispanic participants who completed the study according to intervention beverage group, Tucson, AZ, USA, August 2016–August 2017

FW, flavoured water control; ML, Mediterranean lemonade; GT, green tea.
Displayed values are means and standard deviations of responses on a 1 (‘low’) to 4 (‘high’) scale.
Preliminary efficacy outcomes
The preliminary efficacy outcomes, shown in Table 4, were analysed in the intention-to-treat population, where all participants were analysed in the group that they were randomized. There were no statistically significant changes from baseline to week 8 for the primary efficacy outcomes (total, HDL or LDL cholesterol). In addition, there was no statistically significant change in HbA1c, a secondary efficacy outcome. However, fasting glucose increased significantly by 5·2 mg/dl (P = 0·0003) and 3·3 mg/dl (P = 0·02) in the ML and GT groups, respectively. Weight decreased significantly by 1·8 kg (P = 0·005) in the FW group. Reported energy intake decreased significantly in the FW (–3146 kJ (−752 kcal), P = 0·04) and GT (–3113 kJ (−744 kcal), P = 0·01) groups among thirty-seven participants with at least one plausible value for total daily energy intake (<20 290 kJ/d (<5000 kcal/d)). There was no statistically significant change from baseline to week 8 for leisure-time physical activity.
Table 4 Efficacy outcomes and weight among the fifty Hispanic participants according to beverage intervention group, Tucson, AZ, USA, August 2016–August 2017

FW, flavoured water (control); ML, Mediterranean lemonade; GT, green tea; HbA1c, glycated Hb; LTPA, leisure-time physical activity.
Values displayed are estimates and their 95 % confidence intervals for change from baseline (week 8 – week 0) within arms and comparisons of change from baseline between arms.
* P<0·05
** P<0·01
*** P<0·001.
A significant increase in fasting blood glucose from baseline to 8 weeks was shown in the GT and ML groups. Further, compared with FW control, fasting glucose change from baseline in the GT and ML arms was significantly greater (6·6 mg/dl, P = 0·004; 4·7 mg/dl, P = 0·04). Weight loss was significantly greater in the FW group than in the ML group (P = 0·03). No other statistically significant comparisons between the arms were observed.
We performed a sensitivity analysis to assess the influence of one individual randomized to FW with weight over 160 kg. For glucose, change from baseline comparison between GT and FW control was no longer significant. For weight, change from baseline comparison between ML and FW was not significant. All other comparisons were similar and led to the same conclusions as in the primary analysis. Non-parametric tests on energy consumed were also consistent with the primary analysis.
Discussion
In this pilot randomized controlled trial, we successfully recruited our goal of fifty Hispanic adults in 6 months, despite the reported challenges in engaging this population in clinical research( Reference George, Duran and Norris 46 ). Our recruitment efforts highlight the importance of face-to-face interactions when engaging this high-risk, underserved population. Our most effective recruitment strategies took place at a local swap meet and patient referrals from a local community health clinic. These findings are consistent with previously reported effective recruitment strategies for the Hispanic population( Reference Reidy, Orpinas and Davis 47 ). Notably, our study staff also were bilingual and bicultural. This allowed for study recruitment efforts to be tailored for language preference and literacy level( Reference Reidy, Orpinas and Davis 47 – Reference Roger, Elias and Carla 49 ). These factors have been demonstrated to be important in recruiting the Hispanic population( Reference Reidy, Orpinas and Davis 47 , Reference Occa, Morgan and Potter 48 ). Our findings reiterate the importance of cultural competency and face-to-face interactions (where preliminary details of the study may be discussed) to establish trust and rapport with individuals early on in the research process.
Forty-four of our fifty participants (88 %) completed the pilot study. Retention rates compare favourably with other beverage interventions in adults. For example, in one of these limited studies, Zoellner et al.( Reference Zoellner, Hedrick and You 17 ) investigated the effectiveness of a 6-month behavioural and health literacy intervention for adults targeting SSB consumption (SIPsmartER) compared with a physical activity intervention (MoveMore). There was 74 % retention in both groups; however, only 1 % of the population was Hispanic. In a study specific to Hispanics, Rodriguez-Ramirez et al.( Reference Rodriguez-Ramirez, Gonzalez de Cosio and Mendez 18 ) reported a 76·0 % retention rate for Mexican women receiving the water plus education intervention compared with 64·9 % for women receiving the education alone; however, no specific retention strategies were discussed. One potential explanation for our retention success was the culturally responsive retention strategies implemented to increase participant engagement. First, all research activities took place in a community-based building located in an area where most of the study participants resided. Second, we provided a flexible schedule to participants so that they could come in for clinical assessments and beverage pick-ups on the days and times that best suited their schedules. Participant family members were allowed to attend beverage pick-ups, if necessary. Third, reminders for appointments and beverage pick-ups were made by the same bilingual research staff member according to each participant’s preferred method of contact (e.g. call or text) and preferred time. This was done to establish rapport with participants. Lastly, monetary incentives were provided for participants throughout the study to complete clinical assessments.
The importance of these retention strategies to the Latino community has been investigated by Reidy et al.( Reference Reidy, Orpinas and Davis 47 ) where participants of Mexican origin in Familias Fuertes were asked to rate the importance of each individual retention strategy. Participants indicated that bilingual and bicultural programme facilitators, convenient time and location, and monetary incentives were important in their decision to continue participating in the study. While our retention rates and treatment satisfaction were high, it is important to acknowledge that two participants who were originally randomized to ML experienced minor gastrointestinal discomfort and opted to continue participating in the study in the GT intervention. For those who completed the current pilot study, adherence to consuming the beverages based on self-reported data was 93 %. Because beverage logs were primarily used to measure adherence to intervention, we cannot say with certainty that participants consumed the ounces reported; however, participants were asked to return any unconsumed beverages to study staff as a secondary means of measuring adherence. On average, 81·4 fl.oz (2407 ml) of unconsumed beverages was returned to study staff for each participant. This accounted for 6·1 % of beverages unconsumed during the study, which suggests there was agreement with self-reported adherence data.
As a pilot trial, we must be careful when interpreting efficacy data. Overall, no positive effects were observed in our preliminary efficacy outcomes as a result of the beverage intervention. Individuals randomized to the FW group did demonstrate non-significant improvements in total, LDL and HDL cholesterol. These cardiometabolic changes in the FW group may be partially explained by the significant weight loss observed in this group, which was not apparent in the other beverage groups. Interestingly, fasting glucose increased significantly in the ML and GT groups by the end of the 8-week intervention period. This may have occurred for a few reasons. Because of our small sample size, we did not control for covariates, leading to the potential for factors such as diet and physical activity to have influenced our results. It is also possible that participants in these groups may not have complied with study protocol by consuming food within 12 h of their blood draw or adding caloric sweeteners to their beverage to increase palatability. Lastly, it is possible no significant changes were observed in efficacy outcomes given the short duration of the study. Our results suggest that the FW and GT groups significantly decreased their energy intake from baseline. This may partially explain the significant weight loss observed in the FW group. However, given this considerable decrease in energy, it is unclear why the GT group also did not experience significant weight loss. One explanation for this may include issues related to self-reported dietary assessments which are known to have high measurement error( Reference Prentice 50 ).
An important strength of our study was our ability to successfully recruit and retain this understudied population in a beverage intervention. We also measured acculturation, which is important when considering how to refine the intervention approach to be culturally responsive for future trials. However, the present pilot study has limitations, some of which are inherent to pilot studies, that should be addressed and limit interpretation of our findings. Given the study was 8 weeks long, modulated only one aspect of the diet and did not modify physical activity, it is possible that this was not an adequate amount of time to observe changes in our primary and secondary efficacy outcomes. In addition, the small sample size may have reduced effect size, thus hindering our capacity to detect changes in our efficacy outcomes. Due to our small sample size, we were unable to control for any covariates or effect modifiers such as BMI and energy intake that may have influenced the relationship between beverage intervention assignment and the outcomes explored. Further, the use of subjective dietary data collection (e.g. self-reported questionnaires) allows for the possibility of participant-reported bias. Importantly, our study was comprised predominantly of Mexican-origin participants, which limits the generalizability of our findings to other Hispanic subgroups. These limitations may be overcome in future definitive trials with the following adjustments: (i) increasing sample size; (ii) longer duration of dietary exposure; (iii) a cross-over design wherein individuals serve as their own control across groups over time; and (iv) use of 24 h diet recalls or clinically relevant biomarkers to better characterize dietary change with a beverage intervention including a more robust assessment of changes in simple and complex carbohydrate intakes, as well as substitutive energy selections.
Conclusion
In conclusion, the present pilot study seeks to expand our knowledge on beverage interventions as a means to improve cardiometabolic health in the large and growing Hispanic population. While we did not obtain statistical significance for efficacy outcomes, this intervention approach appears to be feasible and well accepted. Given the likelihood of beverages to contribute to excess intake and resultant obesity, and the fact that we have demonstrated that beverages are a modifiable dietary behaviour, future studies should robustly evaluate beverage interventions for effectiveness and eventual dissemination. Additionally, future beverage interventions in Mexican-origin adults may consider altering common sugary beverages (e.g. agua frescas or horchata) that are homemade or labelled ‘all natural’. This focus may improve upon the impact of beverage interventions for this population. Importantly, this pilot study provides the early efficacy data necessary to design a larger, adequately powered randomized controlled trial among Hispanic adults with obesity.
Acknowledgements
Acknowledgements: The authors would like to acknowledge the Diabetes Prevention & Education Center at the Banner – University Medical Center South Campus, the Tucson Tanque Verde Swap Meet, the University of Arizona Collaboratory for Metabolic Disease Prevention and Treatment, Nosotros staff, and all study participants. Financial support: Funding for this study was provided by the University of Arizona Mel and Enid Zuckerman College of Public Health through the Diabetes Development Fund. The funder had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflicts of interest. Authors’ contributions: D.O.G., C.A.T., M.L.B., J.A.M. and I.A.H. designed the research; D.O.G., K.E.M., B.A. and L.A.V. conducted the research; B.A.R. and M.L.B. analysed data; and D.O.G., K.E.M., B.A.R., M.L.B. and C.A.T. wrote the initial manuscript. D.O.G. had primary responsibility for the final content. All authors read, contributed to and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Arizona Institutional Review Board (#1606621176). Written informed consent was obtained from all participants. Clinical trial registry: This trial was registered at clinicaltrials.gov as NCT02911753.







