There is an increasing interest in using in vitro digestion models, both to understand how food is being digested and to simulate human digestion; thus, many approaches have been suggested and designed for different purposes. A recent review by Hur et al. (Reference Hur, Lim and Decker1) has shown large variations in existing models, concerning variables such as pH, use of one, two or more gastrointestinal phases, enzyme sources and chemicals used. In vitro–in vivo correlations in digestion models are important. Despite this, most models still need validation with human data, and there is an urgent need for developing more physiologically relevant models(Reference Fatouros and Mullertz2). To better simulate human physiological conditions, one approach is to aspirate gastrointestinal juices from human volunteers to be used in in vitro models. Previously, human aspirates have been used in several in vitro studies to investigate protein degradation and to detect the formation of bioactive peptides(Reference Almaas, Holm and Langsrud3–Reference Faye, Tamburello and Vegarud6). However, few studies have been conducted on lipid digestion(Reference Miled, Canaan and Dupuis7), and to our knowledge, no comparisons of commercial v. human gastrointestinal enzymes for this purpose have been performed.
The mechanisms of lipid breakdown during digestion, including the release of specific fatty acids (FA) and their bioaccessibility in the gut, are important to understand due to the various health implications of lipids(Reference Wilde and Chu8). The health benefits of the n-3 long-chain PUFA (n-3 LC-PUFA) EPA (20 : 5) and DHA (22 : 6) have been well documented with regard to reducing the risk of CVD and having a positive effect on autoimmunity and mental disorders(Reference Uauy and Valenzuela9–Reference Appleton, Rogers and Ness14). Salmon is an important source of these FA in the human diet(Reference Ackman15), and salmon oil (SO) was therefore chosen as a substrate in the present study. As reviewed by McClements & Li(Reference McClements and Li16), lipid digestion has been reported to be more complex than protein digestion, both with regard to enzymes and to physiological conditions in the gut(Reference Carey, Small and Bliss17–Reference Armand, Borel and Pasquier20). Several factors could affect the hydrolysis of dietary lipids, such as food matrix and buffering capacity, type of emulsion (oil in water or water in oil) and individual secretion of both digestive enzymes and bile salts (BS)(Reference Pafumi, Lairon and de la Porte21–Reference Gallier and Singh26). In humans, three lipolytic enzyme systems are important for lipid digestion: lingual, gastric and pancreatic lipase systems(Reference Armand27). These are responsible for hydrolysing FA from specific positions on TAG (sn-1, -2 and -3).
Lingual and gastric lipases are secreted in the mouth and in the fundic region of the gastric ventricle, respectively(Reference Abrams, Hamosh and Lee28, Reference Moreau, Laugier and Gargouri29). They are both active in the stomach and might be responsible for 10–30 % of dietary lipid hydrolysis(Reference Armand, Borel and Pasquier20, Reference Pafumi, Lairon and de la Porte21) and the limited extent of gastric lipolysis has been suggested to be due to feedback inhibition by lipid products(Reference Carey, Small and Bliss17, Reference Hamosh, Ganot and Hamosh30, Reference Patton and Carey31). Lingual and gastric lipases hydrolyse only one position on each TAG, and has a preference for the sn-1 and sn-3 positions, but also a limited sn-2 activity(Reference Tiruppathi and Balasubramanian32). This lipolytic activity, yielding one NEFA and one diacylglycerol(Reference Carey, Small and Bliss17, Reference Patton, Rigler and Liao33–Reference Hayes, Pence and Scheinbach35), gives a more efficient fat emulsification with BS(Reference Armand, Borel and Dubois19, Reference Armand, Borel and Pasquier20), helping large lipid droplets to disperse into smaller micelles(Reference Reis, Holmberg and Watzke36). The formation of micelles facilitates subsequent duodenal TAG hydrolysis by pancreatic lipases(Reference Gargouri, Pieroni and Riviere37), and is a prerequisite for further lipolysis in the duodenum. Pancreatic lipase, which is the main enzyme system responsible for lipolysis in the human gastrointestinal tract, hydrolyses about 95–98 % of the remaining dietary TAG(Reference Porsgaard, Xu and Gottsche38). This enzyme system has a preference for FA located in the sn-1 and sn-3 positions of TAG(Reference Mattson, Benedict and Martin39, Reference Rogalska, Ransac and Verger40), yielding two NEFA plus one sn-2 monoacylglycerol(Reference Mattson, Benedict and Martin39, Reference Mattson and Volpenhein41). Pancreatic lipase is inhibited by surface-active agents from the bile(Reference Reis, Holmberg and Watzke36, Reference Borgstrom and Erlanson42–Reference Reis, Watzke and Leser44), mainly the bile acids (cholic acid, deoxycholic acid and chenodeoxycholic acid)(Reference Wilde and Chu8), which are the protonated form of the BS. To overcome the inhibition facilitated by BS, colipase is necessary as a mediator(Reference Borgstrom and Erlanson42, Reference Morgan and Hoffman45). Colipase anchors pancreatic lipase to its substrate(Reference Freie, Ferrato and Carriere46) by binding both pancreatic lipase (Reference Donner, Spink and Borgstom47, Reference Patton, Albertsson and Erlanson48) and BS(Reference Borgstrom, Erlanson-Albertsson and Wieloch49), helping the lipolytic activity to proceed.
The present study aimed to compare two different enzyme sources, human duodenal juice (HDJ) and porcine pancreatin plus bile mix (PB), on the in vitro duodenal digestion of SO, to reveal differences in mechanisms by the two different lipolytic enzyme systems. SO has a naturally high content of the two n-3 LC-PUFA, EPA and DHA, which are known to be of nutritional importance. Consequently, it was of importance to study the specificity of the enzyme systems used, the kinetics of the reaction and the liberation of these specific FA as well.
Experimental methods
Chemicals and materials
SO from Atlantic salmon (Salmo salar L.) was obtained from Denomega (Denomega Nutritional Oils), and the lipid composition is described in Table 1. Pancreatin (porcine, EC no.: 232–468-9) and bile extract (ovine and bovine, EC no.: 232–369-0) were obtained from Sigma Chemical Company. Human duodenal enzymes were obtained by collecting HDJ from six persons according to Ulleberg et al. (Reference Ulleberg, Comi and Holm50) and Holm et al. (Reference Holm, Krogdahl and Hanssen51). In brief, a three-lumen tube (Maxter Catheters) enabled both simultaneous instillation of a stimulation solution in the duodenum and aspiration of gastric and duodenal juices. The stimulation solution (sucrose 70 g/l, NaCl 1·8 g/l, l-phenylalanine 3·2 g/l and l-valine in water 2·3 g/l) was instilled close to the papilla of Vateri (100 ml/h) to stimulate the production of pancreatic enzymes. HDJ was aspirated approximately 10 cm distally. Aspirates were collected on ice, centrifuged (4500 g, 10 min) to remove mucus and cell debris before being frozen in batches and stored at − 20°C before use. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by Norwefian Regional Ethics Committee, REK. Approved by project no. 2012/2230 and Biobank no. 2012/2230. All subjects signed up as volunteers in the project.
Table 1 Lipid composition of oil from Atlantic salmon (SO, Salmo salar L.)

FA, fatty acids.
Total protein content
Total protein concentrations were measured in triplicate using the [Q]QubitTM Fluorometer (Invitrogen) with the Quant-ITTM protein assay kit (Invitrogen) according to the manufacturer's instructions.
In vitro digestion
The in vitro digestion model employed was based on duodenal digestion described by Aura et al. (Reference Aura, Harkonen and Fabrititus52). A procedure was developed for using HDJ (1·37 mg protein/ml, diluted with 0·15 m-NaHCO3) or a commercial porcine enzyme mix, PB (1·2 mg pancreatin/ml and 11·8 mm-BS in 0·9 % NaCl and 0·15 m-NaHCO3). For each time point, one tube was prepared, each tube containing, in total, 8·1 ml of the respective enzyme solutions. pH was adjusted to 7·0 before the tubes were placed in a rotary incubator (37°C, 215 rpm) for 15 min, followed by the addition of 0·125 g SO. After incubation for 0, 20, 40, 60, 80 and 110 min, the tubes were placed on ice and CHCl3–methanol (2:1) was added in order to stop lipid hydrolysis. The digestion experiments were carried out in duplicate or more, except for the incubations at 110 min, which were examined only once due to the lack of material.
In order to optimise the procedure, the experiments were performed by varying the substrate concentration (SO) from 4·5 to 38·0 mg/ml for the commercial PB system, the commercial porcine pancreatin concentration from 1·4 to 13·6 mg/ml and the BS concentration from 0 to 118 mm for both enzyme systems (pancreatin and HDJ). The experiments were carried out in duplicate or more.
Lipid extraction and analysis
Lipids were extracted from the digesta according to Bligh & Dyer(Reference Bligh and Dyer53) and separated into lipid classes, i.e. NEFA, neutral lipids (monoacylglycerol, diacylglycerol and TAG) and polar lipids, using an automated solid-phase extraction (Gerstel MPS Autosampler; Gerstel GmbH) based on a modified and in-house validated method(Reference Ruiz, Antequera and Andres54). Internal standards, C23 (NEFA) and C21 (TAG), were used for the quantification of FA in the NEFA and neutral lipid fractions, respectively. Neutral lipids were eluted with CHCl3–methanol, and NEFA with diethyl ether–acetic acid. The solvent was removed by evaporation under N2, and the contents of FA in the fractions were measured as FA methyl esters using GC with flame ionisation detection. Briefly, the lipids were derivatised and analysed as methyl esters using an Agilent 6890 capillary gas chromatograph (GC) equipped with a BPX-70 column (60 m × 0·25 mm inner diameter, 0·25 μm film; SGE Analytical Science Private Limited). The temperature program started at 70°C for 1 min, increased by 30°C/min to 170°C, 1·5°C/min to 200°C and 3°C/min to 220°C with a final hold time of 5 min. Peaks were integrated with Agilent GC ChemStation software (version A.05.02; Agilent Technologies), and identified using external standards. Coefficients of variation were < 5 %. Hydrolysis was measured as mg NEFA/g SO added.
Bile salt concentration
BS concentrations were analysed in duplicate after dilution with distilled water (1:50) at the Central Laboratory of the Norwegian School of Veterinary Science (Oslo, Norway) using Advia® 1650 (Bayer HealthCare), an automated analysis system for clinical chemistry. Principles of analyses were based on enzymatic amplification determining total 3α-hydroxy bile acids using a kit (Bio-Stat Diagnostic systems). In the presence of Thio-NAD, the enzyme 3α-hydroxysteroid dehydrogenase converts bile acids to 3-keto steroids and Thio-NADH. The rate of formation of Thio-NADH was measured spectrophotometrically at 410 nm. BS concentration was determined using a standard curve of known concentrations of taurocholic acid.
Statistical analyses
Data are presented as means and standard deviations. Student's t test (two-sample, assuming unequal variance) was used to estimate significant differences (GraphPad Prism × 6). Differences were considered as significant when P< 0·05.
Results
The lipid composition of SO is described in Table 1, containing approximately 6 % EPA (20: 5) and 9 % DHA (22 : 6). The other main FA of SO were 16 : 0, 18 : 1n-9 and 18 : 2, constituting of 13, 28 and 9 %, respectively.
The amount of the released FA from TAG, using the commercial porcine PB, was substrate-dependent, as shown in Fig. 1. Incubation of 15·5 mg SO/ml PB released 417 (sd 29) mg NEFA/g SO, which was significantly higher than 4·4 mg SO/ml PB (P= 0·0010) and 7·0 mg SO/ml PB (P= 0·0144), resulting in a release of 235 (sd 22) and 246 (sd 6) mg NEFA/g SO, respectively (Fig. 1). A further increase in SO to 38·0 mg SO/ml PB did not give a significant increase in the release of FA compared with 15·5 mg SO/ml PB (result not shown).
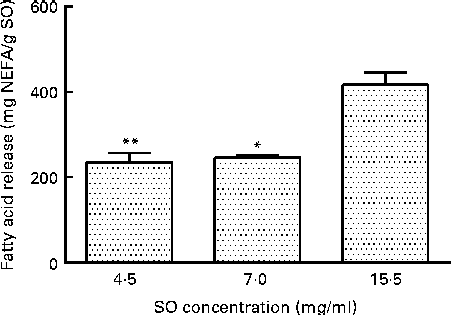
Fig. 1 Effect of the substrate (salmon oil; SO) concentration on release of fatty acids (FA; mg NEFA/g SO) measured after incubation at 37°C for 80 min, using a commercial pancreatin plus bile. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that following incubation of SO at 15·5 mg/ml: *P= 0·0144, **P= 0·0010.
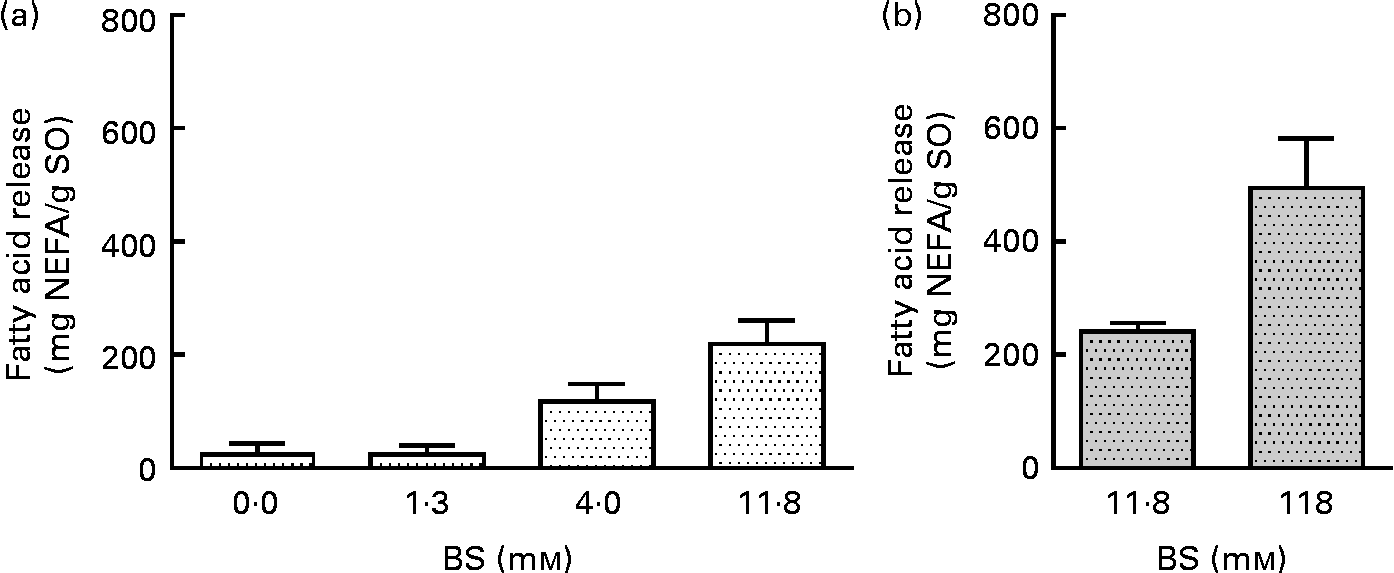
BS were added to pancreatin (1·4 mg/ml) in concentrations ranging from 0 to 11·8 mm, resulting in a 4-fold increase in the liberation of total FA (Fig. 2(a)), from 24·6 to 220 mg NEFA/g SO. No significant difference was observed at a BS concentration of 11·8 mm while increasing pancreatin from 1·4 to 13·6 mg/ml (Fig. 2(a) and (b)). A ten times increase in PB concentration (13·6 mg pancreatin/ml and 118·0 mm-BS) only increased NEFA liberation by a factor of 2, from 241 (sd 16) to 496 (sd 87) mg NEFA/g SO (Fig. 2(b)). This experiment demonstrates a highly BS-dependent lipolysis by the porcine enzyme system (pancreatin), contrary to the human lipolytic enzyme system (HDJ) (Fig. 3).

Fig. 2 Effect of bile salt (BS) concentration on the release of fatty acids (FA; mg NEFA/g salmon oil (SO)) measured after incubation at 37°C for 80 min. Commercial pancreatin are used as the lipolytic enzyme source in two different concentrations: (a) 1·36 mg/ml and (b) 13·6 mg/ml. Values are means, with standard deviations represented by vertical bars.
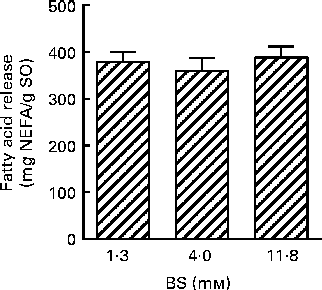
Fig. 3 Effect of bile salt (BS) concentration on the release of fatty acids (FA; mg NEFA/g salmon oil (SO)) after incubation at 37°C for 80 min, using human duodenal juice as a lipolytic enzyme source. Values are means, with standard deviations represented by vertical bars.
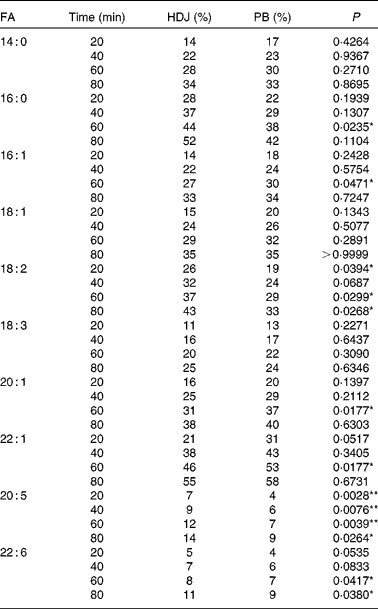
The in vitro digestion was carried out for 110 min, indicating that the release of FA reached a plateau at 80 min using both enzyme systems. Due to the lack of parallels, data obtained at 110 min are not shown, but the results confirmed the plateau. After 80 min of incubation, both PB (11·8 mm-BS) and HDJ (1·4 mm-BS) liberated approximately the same total amount of FA (352 (sd 23) and 379 (sd 18) mg NEFA/g SO, respectively), indicating about 67 % hydrolysis (from sn-1 and sn-3) of TAG in the substrate added (Fig. 4). There was no significant difference in the kinetics of liberation between the two lipolytic enzyme sources, PB and HDJ, releasing 189 (sd 24) and 173 (sd 24) mg NEFA/g SO at 20 min, 253 (sd 15) and 257 (sd 35) mg NEFA/g SO at 40 min and 318 (sd 16) and 312 (sd 11) mg NEFA/g SO at 60 min, respectively. However, the release of specific FA was found to be different for some of the FA analysed (Table 2). Fig. 5 shows the significant differences found at 60 min when the level of overall hydrolysis caused by HDJ was similar to PB (Fig. 4) and the data therefore primarily reflecting selective hydrolysis. Compared with PB, HDJ caused a significantly higher release of EPA at all times (P< 0·05), and the release of DHA was found to be significantly higher at 60 (P= 0·0417) and 80 min (P= 0·0380) (Table 2). HDJ also gave a higher release of 18 : 2 at 20 min (P= 0·0394), 60 min (P= 0·0299) and 80 min (P= 0·0268), and of 16 : 0 at 60 min (P= 0·0235). On the other hand, PB showed a significantly higher ability to release 16 : 1 (P= 0·0471), 20 : 1 (P= 0·0177) and 22 : 1 (P= 0·0177) at 60 min (Table 2). Furthermore, the results showed that both enzyme preparations released approximately the same amounts of other FA (constituting < 3·5 %). A significantly higher ability for HDJ to release EPA compared with DHA was observed throughout the in vitro digestion, at 20 (P= 0·0054), 40 (P= 0·0058), 60 (P= 0·0003) and 80 (P= 0·0409) min.
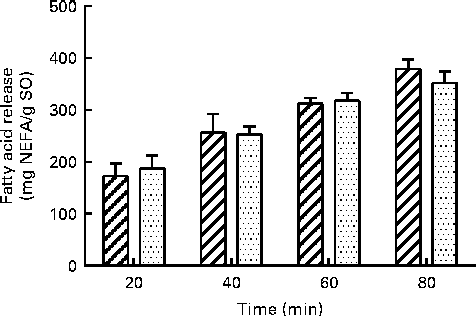
Fig. 4 Release of total fatty acids (FA; mg NEFA/g salmon oil (SO)) measured after incubation at 37°C for 80 min using porcine pancreatin and bile (![]() ) and human duodenal juice (
) and human duodenal juice (![]() ) as lipolytic enzyme sources. Values are means, with standard deviations represented by vertical bars.
) as lipolytic enzyme sources. Values are means, with standard deviations represented by vertical bars.
Table 2 Release of specific fatty acids (FA) (% of total FA in salmon oil) during digestion with commercial pancreatine and bile (PB) and human duodenal juice (HDJ)

*P< 0·05, **P< 0·01.
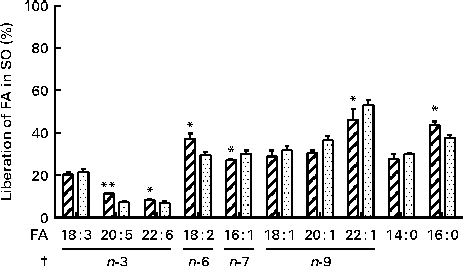
Fig. 5 Release of specific fatty acids (FA; %) per g FA in salmon oil (SO) added after incubation at 37°C for 60 min, using commercial pancreatin and bile (PB, ![]() ) and human duodenal juice (HDJ,
) and human duodenal juice (HDJ, ![]() ) as the lipolytic enzyme source. Values are means, with standard deviations represented by vertical bars. */** Mean value was significantly different (P< 0·05/P< 0·01) from that following incubation with PB (20 : 5, P= 0·0039; 22 : 6, P= 0·0417; 18 : 2, P= 0·0299; 16 : 1, P= 0·0471; 22 : 1, P= 0·0177; 16 : 0, P= 0·0235). † Where the first double bond in the FA is found (counted from the –COOH).
) as the lipolytic enzyme source. Values are means, with standard deviations represented by vertical bars. */** Mean value was significantly different (P< 0·05/P< 0·01) from that following incubation with PB (20 : 5, P= 0·0039; 22 : 6, P= 0·0417; 18 : 2, P= 0·0299; 16 : 1, P= 0·0471; 22 : 1, P= 0·0177; 16 : 0, P= 0·0235). † Where the first double bond in the FA is found (counted from the –COOH).
Discussion
The present study show a similar total lipolytic activity during the in vitro digestion of SO using two different enzyme sources: HDJ and a commercial porcine PB. The total protein content (1·37 mg protein/ml) was the same in both enzyme preparations, ensuring similar enzyme concentrations. The release of FA, measured as NEFA, reached a plateau at 80 min, indicating that 67 % of the accessible FA were hydrolysed from TAG in SO (Fig. 4.), which is higher than that reported in other in vitro studies often showing less than 44 % release of FA(Reference Martin, Nieto-Fuentes and Señoráns55, Reference Larsson, Cavonius and Alminger56). However, it is still lower than what is assumed to be digested and absorbed in vivo (98 %)(Reference Armand27). The lower outcome measured in the release of FA from TAG observed in static in vitro digestion compared with in vivo may be explained by saturated micellar solubilisation of lipolytic products, obstructing the interaction between pancreatic lipase and the substrate(Reference Martin, Nieto-Fuentes and Señoráns55). During in vitro lipolysis, there will be an accumulation of NEFA, which may lead to equilibrium between the substrate and the product(Reference Reis, Raab and Chuat57), giving a so-called product inhibition. To avoid this, certain dynamic in vitro digestion models have been developed using dialysis to extract the digested components, which closely approximates absorption through the lining of the gut in vivo (Reference Minekus, Marteau and Habenaar58).
To ensure maximum hydrolytic activity in the present in vitro model, optimal physiological concentrations of the enzymes and BS were determined. Droplet size of the emulsion is important when studying lipid digestion due to the surface activity of pancreatic lipase(Reference Wilde and Chu8). In the present study, a rotary incubator was used to simulate the movement of the gastrointestinal tract and to ensure similar conditions when comparing the two lipolytic enzyme systems. Standard variations within each enzyme system were low ( < 10 %), showing that the heterogeneous nature of the mixture gave reproducible results. However, there were other physiologically relevant parameters that were not included in the model. It has been reported that lingual and gastric lipase systems may be responsible for approximately 10–30 % of lipid hydrolysis(Reference Armand, Borel and Pasquier20, Reference Pafumi, Lairon and de la Porte21). Their activity occur mainly in the gastric ventricle, and facilitates the following TAG hydrolysis by HPL better by improving emulsification with BS(Reference Armand, Borel and Dubois19, Reference Armand, Borel and Pasquier20, Reference Gargouri, Pieroni and Riviere37). The static in vitro duodenal digestion model used in the present experiment was made to mimic lipid digestion in the duodenum, and the gastric step was not included. Pancreatic lipase was therefore the sole enzyme responsible for lipolytic activity. However, for the aspirated HDJ, there is a possibility for the leakage of gastric lipase from the gastric juice because of the procedure used(Reference Holm, Krogdahl and Hanssen51). Gastric lipase has a lower pH optimum than pancreatic lipase (4(Reference Carriere, Gargouri, Moreau and Woolley59) and 6·5–7·5(Reference Moreau, Gargouri and Lecat60), respectively), which makes it unlikely that gastric lipase has contributed in the liberation of FA in these experiments which is conducted at pH 7·0.
Changes in pH will affect enzyme activity and alter its efficiency. In the small intestine, pH gradually increases from 6 to 7·4(Reference Fallingborg61) due to the breakdown and degradation of different food constituents, e.g. hydrolysed FA from TAG(Reference Rudman and Shank62). In the present study, pH was set to 7·0 and controlled both at 0 and 80 min. There were no changes in pH through digestion (results not shown), probably due to high buffer capacity in the in vitro system. The change in pH due to lipid hydrolysis was therefore not probably affecting the rate of lipolysis in the present experiment.
The positive impact of BS on lipase activity is well known(Reference Reis, Holmberg and Watzke36). Concentrations of total BS are found to be approximately 2·6 mm in the fasted state(Reference Kalantzi, Goumas and Kalioras63) and increase to approximately 11–14·5 mm in response to a meal(Reference Armand, Borel and Pasquier20, Reference Kalantzi, Goumas and Kalioras63, Reference Fausa64). This observation is compatible with BS concentration found in HDJ used in these studies, which has an average of 1·3 mm(Reference Ulleberg, Comi and Holm50) and is aspirated from semi-fasted subjects. The standard PB mix used contains 11·8 mm-BS, which is in the normal range in the ‘fed state’. On the basis of this finding, there is reason to believe that lipolytic activity in PB would be higher compared with the lipolytic activity of HDJ, which reflects a ‘fasted state’. However, the influence of BS concentration was different in the two enzyme preparations (Figs. 2 and (b) and 3). While porcine pancreatin showed increased activity with increasing BS concentration, the lipase activity of HDJ was constant. These results indicate that both HDJ and PB had optimal conditions for lipolytic activity, and that the experiments determining total lipid breakdown are comparable despite the different BS concentrations. The limited effect of BS on the lipolytic activity of HDJ might be due to several factors. The release of FA with 1·3 mm-BS may already have reached the equilibrium in the in vitro digestion system, due to higher enzyme activity. Furthermore, HDJ consists of pancreatic juice and bile, in addition to secretions from the gastrointestinal tract, in contrast to porcine pancreatin which is made from grinded pancreas. This might lead to HDJ having a higher share of other components, e.g. phospholipids and cholesterol, which could act as emulsifiers. The requisite of BS to optimise the lipolytic enzyme system in HDJ might therefore be lower. Both are plausible explanations and need to be further examined.
Previous studies have reported the specific positions of FA on TAG in marine oils(Reference Brockerhoff, Hoyle and Hwang65). The results from these studies showed that 16 : 1, 18 : 1, 20 : 1 and 22 : 1 are mainly found in the sn-1 and sn-3 positions (>70 %), whereas 14 : 0 (approximately 50 %), 16 : 0 (approximately 45 %) EPA (20 : 5, approximately 47 %), DHA (22 : 6, approximately 76 %) are mainly found in the sn-2 position. Even though 14 : 0, 16 : 0 and EPA are mostly found in the sn-2 position, these FA are, together with 18 : 2, more randomly distributed in all the sn positions than other FA on TAG. The positions of FA in marine oils and the known HPL position preference(Reference Carriere, Barrowman and Verger34, Reference Hayes, Pence and Scheinbach35) are in accordance with the results from the present study, demonstrating that the FA found in the sn-1 and sn-3 positions are released to a higher degree than the FA mainly found in the sn-2 position (Fig. 5). Furthermore, previous studies have shown that PUFA containing double bonds close to the carboxyl group are more resistant to attack by pancreatic lipases(Reference Bottino, Vandenburg and Reiser66). In EPA and DHA, the first double bond is at carbon number three (n-3), which might also explain why the release of these FA is relatively low at 80 min (10–14 %) when compared with the other FA analysed (33–58 %). These observations could also explain the differences between the observed effect of the positioning of FA on TAG, explaining why 14 : 0 and 16 : 0, found in the sn-2 position, seem to be released to the same degree as the FA found in the sn-1 and sn-3 positions. On the basis of these observations, it is likely that both the position of the FA on TAG and the appearance of double bonds in the FA affect both lipolytic enzyme systems.
Although no differences in total lipolytic activity were observed between HDJ and PB, the present results show significant differences in the hydrolysis of specific FA, suggesting that lipases of different origins may have different affinities to specific TAG or FA. Variations in the levels of overall hydrolysis might lead to the masking of selective hydrolysis. However, observed variations were small, and at 60 min when HDJ and PB caused almost identical levels of hydrolysis, the difference in the release of various FA was most pronounced. When taking all time points into account, HDJ gave, in general, a higher release of the PUFA 18 : 2, EPA and DHA. The digestion of long-chain FA, including the transport and distribution of its derived products in the body, is complex. Some studies have concluded that they are poorly broken down in the small intestine, demonstrating slow adsorption and recovery in the circulation postprandially(Reference Bottino, Vandenburg and Reiser66–Reference Brockerhoff68), whereas others have shown an efficient recovery of FA from marine oils in the adipose tissue as well as the mobilisation of these FA from the adipose tissue(Reference Garton, Hilditch and Meara69, Reference Storlien, Kraegen and Chisholm70). EPA and DHA are two of the PUFA which have been extensively studied, due to their potential health benefits. However, to our knowledge, no studies on the digestion and release of these specific n-3 LC-PUFA in the small intestine have been reported. In salmon, EPA is randomly distributed on the glycerol backbone, whereas DHA is detected mostly in the sn-2 position(Reference Amate, Ramirez and Gil71–Reference Mbatia, Adlercreutz and Adlercreutz74). In the present experiment using HDJ, a higher release was observed for EPA compared with DHA, 14 % against 10 % at 80 min, respectively. This difference may partly be explained by pancreatic lipase position specificity, which is the sn-1 and sn-3 positions of TAG(Reference Mattson, Benedict and Martin39, Reference Rogalska, Ransac and Verger40). The significantly higher portion of EPA released from TAG, observed in the present study, may partly explain the higher increase in circulating EPA compared with DHA, despite similar intakes, observed in human intervention studies(Reference Visioli, Rise and Barassi75–Reference Kirkhus, Lamglait and Eilertsen78). This has previously been explained with a more strict biological regulation of DHA levels(Reference Kirkhus, Lamglait and Eilertsen78). FA in the sn-2 position on TAG will be metabolised as monoacylglycerol and therefore are probably subjected to different metabolic pathways. The results from the present study showed that the differences between EPA and DHA might not just be due to different metabolism in the body after absorption, but also due to a specific preference of human pancreatic lipase in the release of the two different FA in the intestinal lumen. The present results also indicated that HDJ and PB had different impacts on the release of EPA and DHA. PB released 9 % of both FA at 80 min, whereas HDJ released 14 % EPA and 10 % DHA. Whether this is due to porcine enzymes in PB releasing FA also from the sn-2 position needs to be further investigated. HDJ also gave a significantly higher release of the PUFA 18 : 2 compared with PB. 18 : 2 is a precursor for the formation of the n-6 LC-PUFA arachidonic acid (20 : 4), known to be important for various physiological reactions in the body(Reference Mills, Windsor and Knight79, Reference Calder80). The effect and reason for the specific release of 18 : 2 needs to be further examined.
Conclusion and summary
In the present study, we examined the possibility to mimic an in vitro digestion model with human gastrointestinal juices (HDJ), using a commercial enzyme preparation consisting of porcine pancreatine and bile (PB). This was successful, and we were able to adjust the model by giving the same total liberation and the kinetics using both enzyme sources. A 67 % release of the available FA in SO was obtained, showing that the substrate and enzyme ratios, as well as the BS concentration used, were suitable for performing in vitro lipid digestion studies. Despite similar total lipolytic activity, HDJ and PB gave significant different liberation of specific FA. A higher release of EPA and DHA was obtained using HDJ, showing that the human lipolytic enzyme system might be better suited to release n-3 LC-PUFA known to have several positive implications on health. The results also showed that HDJ gave a significant higher release of EPA compared with DHA, which could be explained by enzyme specificity both for the specific FA per se and to the position on TAG.
Acknowledgements
The present study was supported by the Research Council of Norway, and carried out at the University of Life Sciences and Nofima, both located at Aas, Norway. We also wish to thank the Oestfold Hospital Trust for supplying the possibility for aspirating human gastric and duodenal juices from human volunteers. The authors are participants in the FA1005 COST Action INFOGEST on food digestion. K. E. A. was involved in the planning, conducting of the experiments and writing of the paper. B. K. participated in the planning, discussion and revision of the paper. H. H. participated in the discussion and revision of the paper. G. V. contributed to the planning a part of the experiments and the discussion of the results. M. J. was responsible for the aspiration of the gastrointestinal juices. G. E. V. contributed to the planning, discussion and revision of the paper. The authors report no conflict of interest and are alone responsible for the content and writing of the manuscript.