Introduction
Although the DSM-5 no longer considers atypical language development a key characteristic in identifying children with autism spectrum disorder (ASD; American Psychiatric Association, 2013; Constantino & Charman, Reference Constantino and Charman2016), clinicians consider poor understanding of common words at 18–24 months of age as one of the strongest predictors of developmental level at the age of three (Wetherby, Watt, Morgan & Shumway, Reference Wetherby, Watt, Morgan and Shumway2007). Indeed, in children with ASD, impairments in both receptive and expressive language abilities are found to emerge at 12 months of age and are still present in later childhood (Landa & Garrett-Mayer, Reference Landa and Garrett-Mayer2006; Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith & Bryson, Reference Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith and Bryson2006; Yirmiya, Gamliel, Shaked & Sigman, Reference Yirmiya, Gamliel, Shaked and Sigman2007; Zwaigenbaum, Bryson, Rogers, Roberts, Brian & Szatmari, Reference Zwaigenbaum, Bryson, Rogers, Roberts, Brian and Szatmari2005). It is therefore vital to better understand why children with ASD display compromised vocabularies already in infancy as well as to investigate possibilities to improve their word learning abilities as early as possible. The current study therefore assesses whether infants at elevated risk for developing ASD can learn novel words from their parents.
It is, however, noteworthy that evidence for vocabulary impairments in children at elevated risk for ASD often comes from parental questionnaires, which can be prone to biases, as some parents require their child to react explicitly as proof for word understanding (Houston-Price, Mather & Sakkalou, Reference Houston-Price, Mather and Sakkalou2007). Nonetheless, children may not always respond to words according to their parents’ expectations. This might specifically be true for children with ASD, who have trouble with social interactions. Parental questionnaires might therefore underestimate a child's vocabulary size. Besides relying on parental questionnaires, we therefore also test word learning experimentally.
Surprisingly, there is little experimental evidence that infants at elevated risk for ASD are indeed limited in their vocabulary formation (but see Gliga, Elsabbagh, Hudry, Charman, Johnson & BASIS Team, Reference Gliga, Elsabbagh, Hudry, Charman and Johnson2012, with three-year-olds). There is one study that suggests that delays in vocabulary do not stem from problems with the word learning per se. Bedford and colleagues (Bedford, Gliga, Frame, Hudry, Chandler, Johnson, Charman & BASIS Team, Reference Bedford, Gliga, Frame, Hudry, Chandler, Johnson and Charman2013) show that two-year-olds at elevated versus lower risk for ASD were equally able to select a novel object among familiar objects when the experimenter used a novel word. This finding implies that both groups exhibit the word learning constraint also known as the mutual-exclusivity bias (Markman, Wasow & Hansen, Reference Markman, Wasow and Hansen2003), widely considered to be a driving force for vocabulary expansion. Another way to test novel word learning is by means of a preferential looking paradigm, which offers the advantages of testing novel word learning in the context of other novel words, and which does not require infants to make an explicit reaction. The current study specifically uses eye-tracking to assess children's on-line ability to learn two novel words.
As a first step to test on-line word learning abilities in this sample of children, we relied on parents to teach their infants novel words. Parents are the first in line when it comes to teaching young children new skills. Consequently, early interventions for ASD usually train parents to improve the quality and quantity of their parent-child interactions (McConachie & Diggle, Reference McConachie and Diggle2007). Such a training successfully improves infants’ language abilities (Rogers, Vismara, Wagner, McCormick, Young & Ozonoff, Reference Rogers, Vismara, Wagner, McCormick, Young and Ozonoff2014). In typically-developing children, parents can also boost early vocabulary growth, as compared to child care providers (Marulis & Neuman, Reference Marulis and Neuman2010). In addition, a recent study revealed that typically-developing two-year-olds show evidence of novel word learning when it is the mother who provided the accompanying speech, whereas teaching by an unfamiliar person did not result in significant word learning (van Rooijen, Bekkers & Junge, Reference van Rooijen, Bekkers and Junge2019).
To study word learning abilities in two-year-olds at elevated risk for ASD we adopt the same paradigm as used by van Rooijen and colleagues (Reference van Rooijen, Bekkers and Junge2019), originally based on a study of Ma and colleagues (Ma, Golinkoff, Houston & Hirsh-Pasek, Reference Ma, Golinkoff, Houston and Hirsh-Pasek2011). In this eye-tracking paradigm, two novel objects are displayed on a computer screen in several short animations. The corresponding auditory stimuli are presented as sentences at the bottom of the screen, such that the child's parent can read these sentences aloud during the experiment. During these animations the parent names both objects several times. At test the two novel objects appear side-by-side on the screen while the parent labels only one of them. Learning is inferred when infants fixate the labeled object longer than the unnamed object.
Since ASD cannot be diagnosed reliably before the age of three years, it is becoming increasingly common to turn to prospective risk studies for information about the early development of children with this disorder (Gliga, Jones, Bedford, Charman & Johnson, Reference Gliga, Jones, Bedford, Charman and Johnson2014; Loth et al., Reference Loth, Charman, Mason, Tillmann, Jones, Wooldridge and Banaschewski2017). Compared to the typical population, who have around a 1% chance of receiving an ASD diagnosis, prospective longitudinal studies often chart development in younger siblings of children with ASD, as they have elevated risk (i.e., 15–30% likelihood) of receiving an ASD diagnosis themselves (Ozonoff, Young, Carter, Messinger, Yirmiya, Zwaigenbaum, Bryson, Carver, Constantino, Dobkins, Hutman, Iverson, Landa, Rogers, Sigman & Stone, Reference Ozonoff, Young, Carter, Messinger, Yirmiya, Zwaigenbaum, Bryson, Carver, Constantino, Dobkins, Hutman, Iverson, Landa, Rogers, Sigman and Stone2011). Consistent with the retrospective findings in children with an ASD diagnosis, some prospective studies show that elevated risk siblings, even those who continue to develop typically, also have delays in receptive and expressive language abilities (Mitchell et al., Reference Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith and Bryson2006; Yirmiya et al., Reference Yirmiya, Gamliel, Shaked and Sigman2007; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Rogers, Roberts, Brian and Szatmari2005). In our study we therefore contrast performance in a sample of elevated risk with a sample of lower risk two-year-olds. All children are followed up on their autistic symptoms at three years of age (Lord, Rutter, DiLavore, Risi, Gotham & Bishop, Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012).
To summarize, we collected parental questionnaires on current vocabulary size as well as experimental evidence of two-year-olds’ word learning ability. We hypothesized that parents report their infants at elevated risk for ASD to have smaller vocabularies (e.g., Mitchell et al., Reference Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith and Bryson2006). For our word learning paradigm, we expected results to mirror findings from Bedford et al. (Reference Bedford, Gliga, Frame, Hudry, Chandler, Johnson and Charman2013): similar performances for children with elevated risk compared to children with a lower risk for ASD. Such a finding would suggest that smaller vocabularies do not solely originate from word learning difficulties, at least not when the parent provided the teaching. Another possibility is that the elevated risk children perform worse on our task than the lower risk children. This would mean that elevated risk children are generally impaired in word learning, in accordance with parental questionnaires, even in the optimal word learning situation in which parental speech is provided.
Method
Participants
Our dataset comprised 33 two-year-olds: 21 children in the elevated risk group (10 boys; M age = 2;0 years, range 1;11–2;2 years) and 12 children in the lower risk group (7 boys; M age = 2;1 years, range 1;11–2;4 years). Four additional children were excluded because no eye-tracking data was collected (n = 2) or due to experimental errors (n = 2). Groups did not differ significantly in age. All children had an older sibling, for whom parents completed the Social Communication Questionnaire (SCQ; Rutter, Bailey & Lord, Reference Rutter, Bailey and Lord2003; for Dutch: Warreyn, Raymakers & Roeyers, Reference Warreyn, Raymakers and Roeyers2004). All siblings of children in the elevated risk group had received a diagnosis of ASD from a clinician: diagnoses were confirmed with higher SCQ scores (M = 22.44, SD = 5.60, range 13–32). In contrast, the siblings from the lower risk group were not diagnosed with ASD and had lower SCQ scores (M = 8.36, SD = 6.44, range 0–24; t(27) = -6.21, p < 0.001, Cohen's d = 2.33). This study was embedded in a large multi-site prospective longitudinal cohort study looking at the development of ASD (EU-AIMS project; see Loth et al., Reference Loth, Charman, Mason, Tillmann, Jones, Wooldridge and Banaschewski2017). The project was approved by the Medical Ethical Committee of the Arnhem-Nijmegen Region (protocol NL42726.091.13), and conducted in accordance with the Helsinki Declaration. Parents signed informed consent prior to participation and received monetary compensation for their time, travel costs when applicable, and a small present for the child in appreciation of their participation.
Apparatus
The study was performed at two sites in the Netherlands: Utrecht (ER n = 10; LR n = 5) and Nijmegen (ER n = 11; LR n = 7). In Utrecht we conducted home visits. We assembled a testing booth to minimize distraction, in which infants sat in a high-chair at approximately 65cm distance from a 24” screen with a Tobii TX300 eye-tracker underneath (sampling rate 300 Hz, 5-point calibration). The task was programmed in Matlab version R2014b (MathWorks Inc., USA) and Psychtoolbox (version 3.0.12, Brainard & Vision, Reference Brainard and Vision1997).
In Nijmegen, all assessments took place at the Baby Research Center of the Radboud University. Infants were seated in a high-chair at a distance of 60–65 cm from a 17” screen, with a Tobii T120 eye-tracker underneath (sampling rate 60 Hz, 5-point calibration). The task was run in Matlab version R2013a with Psychtoolbox version 3.0.11.
Stimuli
We used the same stimuli (visual stimuli, novel words and sentences) as in van Rooijen et al. (Reference van Rooijen, Bekkers and Junge2019); see also Figure 1. The visual stimuli comprised images of two familiar (an apple and a book) and two novel objects. These novel objects appeared first as animations in the training phase, and then static for the test and reminder trials. The two Dutch pseudowords were ‘gemer’ /ˈxemər/ and ‘miekel’ /ˈmikəl/. Sentences were presented on the screen below the visual stimuli, such that the child's caregiver could read them aloud during the experiment.
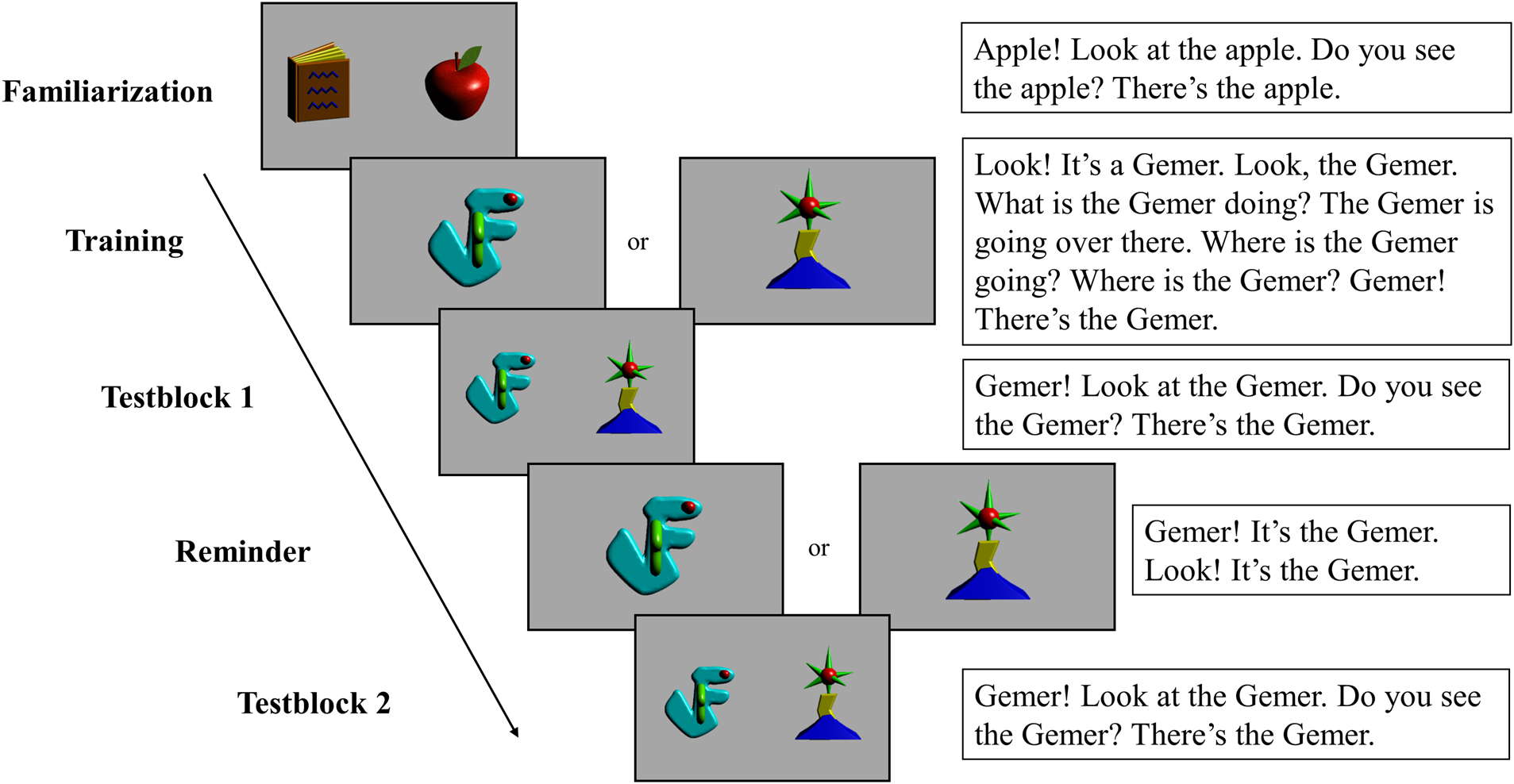
Figure 1. The screen displays per experimental phase. On the right, the text accompanying every trial, translated in English. These sentences contain the target word ‘gemer’ for half of the trials; in the other trials it is replaced by the target word ‘miekel’.
Procedure
At both test sites, the caregiver was positioned behind the child to discourage direct eye contact. The caregiver was instructed to interact with their child as little as possible and, if needed, redirect them to the screen without pointing or using the pseudowords.
Our paradigm was identical to van Rooijen and colleagues (Reference van Rooijen, Bekkers and Junge2019). Figure 1 gives a schematic overview of the task, which comprised five phases: familiarization, training, test block 1, reminder, and test block 2. The familiarization phase served to ensure that infants become familiar with the procedure: they saw two familiar objects accompanied with speech directing them to look at one of the objects (e.g., apple). The training phase introduced the two novel objects in short animations. Each animation showed one object, labeled nine times throughout the animation. The animation of each object was presented twice, in alternating order. Training was followed by a test phase, interleaved with a reminder phase. Each test block comprised two (in Utrecht) or four (in Nijmegen) test trials. Each test trial showed images of the two novel objects side-by-side, and parents asked their children to look at one of them (and the other served as non-target object). During a test trial, the target object was named four times. In the reminder phase (two trials, one for each novel object) infants saw again each object in isolation, while the parent labelled it correctly three times. Then, the test block was repeated. As a result, the target object was explicitly paired with the referent 18 times during the familiarization phase, and another three times in the reminder phase intermittent the two test blocks. Across participants, we counterbalanced the labeling of the two objects and the order of presentation in the training phase as well as the test phase, independently from one another. Moreover, the side of presentation of the objects during test trails was counterbalanced, independently from the order of labeling during training and test phases.
The experimenter manually started a trial the moment the infant was fixating a fixation star in the middle of the screen, and ended the trial once the speaker had finished the sentence. The familiarization, test and reminder trials all had a minimum duration of 10 s, and training trials lasted at least 20 s. However, as trial length was dependent on the speech rate of the caregiver, trial duration was variable. The task had a total duration of approximately five minutes.
Additional measures
N-CDI
Parents filled out the MacArthur Communicative Development Inventory – Words and Sentences (CDI; Fenson et al., Reference Fenson, Dale, Reznick, Thal, Bates, Hartung, Pethick and Reilly1993; Dutch version: N-CDI; Zink & Lejaegere, Reference Zink and Lejaegere2002), which measures infants’ receptive and expressive vocabulary size over various semantic categories (720 items).
ADOS-2
We measured autistic traits at 36 months, with the Autism Diagnostic Observation Schedule 2 (ADOS-2; Lord et al., Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012). The ADOS-2 is a 30–45 minute, semi-structured play assessment of communication, social interaction, play skills, and restricted interests/repetitive behavior (Lord et al., Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012). It was developed to diagnose ASD across a wide range of chronological and mental ages and is normed on individuals ranging from 12 months of age through 40 years. The ADOS-2 was administered by a trained psychologist who met requirements for research reliability and who was blind to the outcome of the study. Based on the level of expressive language of the child she administered module 1 (1 child) or module 2 (25 children). To compensate for a difference in test modules, we used the comparison score for each infant. Three of the participants (one elevated risk and two lower risk children) dropped out before the 36-month-old test session, so for these children we have no ADOS-2 scores.
Data analysis
We first separated fixations from saccades, using the Identification by 2-Means Clustering algorithm, specifically designed for noisy infant eye-tracking data (Hessels, Niehorster, Kemner & Hooge, Reference Hessels, Niehorster, Kemner and Hooge2017). We interpolated data loss up to 100 ms in the raw data when at least 2 samples of valid data were available at each end. A moving window of 200 ms width was used for fixation classification. Fixations were merged when they were not more than 30 pixels apart and within a time range of 30 ms. Fixations of 40 ms or shorter were removed.
For the training phase, we reported total proportion looking time to the screen. For the test trials, we determined total fixation time on both the target and the non-target object in the time window of 200–2200 ms from first word onset: we added the durations of all fixations on each image (areas of interest: left and right side of the screen, separated by a 40-pixel wide gap in the middle of the screen). We then calculated the proportion of target looking by dividing the total fixation time on target by the total fixation time on target and non-target combined. Test trials were excluded when the child had no valid fixations on the objects on the screen over this two-second time period. Children with less than two valid test trials were excluded from analyses (elevated risk: n = 3; lower risk: n = 1). Therefore, the final sample comprised 18 elevated risk and 11 lower risk children.
For statistical analyses independent samples t-tests were used to test differences in target looking, N-CDI and ADOS-2 scores between elevated and lower risk children. We also explored whether reclassification after the ADOS-2 yielded similar results.
Results
Word learning paradigm
Results from the training phase revealed no difference in the proportion time both groups spent on fixating the screen (elevated risk: M = 0.73, SD = 0.13; lower risk: M = 0.70, SD = 0.19; t(27) = −0.52, p = .61, Cohen's d = 0.18). Statistical tests indicated no relevant main effects of test site (F(1,28) = 1.01, p = .32) or test block (F(1,26) = 1.56, p = .22) on target fixation times in the test trials, nor any interaction effects between these variables, risk group and fixation times. We therefore collapsed data across sites and test blocks for all analyses. An independent samples t-test revealed that groups did not differ in their fixation times on the target (elevated risk: M = 0.56, SD = 0.10; lower risk: M = 0.53, SD = 0.14; t(27) = -.54, p = .60, Cohen's d = 0.25). A subsequent one-sample t-test showed that overall children looked at the target object significantly above chance (chance level = 0.50; t(28) = 2.22, p = 0.035, Cohen's d = 0.41)Footnote 1. Figure 2 displays the proportion of looking time to the target and non-target for each risk group separately. These results imply that, irrespective of their risk status, these infants were able to form new word-object mappings.

Figure 2. Proportion of looking time to target and non-target, per risk group (ER = elevated risk; LR = lower risk). Dark grey bars represent the proportion of time spent looking at the target and light grey bars represent the proportion of time spent looking at the non-target. Error bars represent one standard deviation from the mean.
N-CDI scores
An independent samples t-test indicated that compared to the lower risk children, the elevated risk children understood fewer words (elevated risk: M = 343.29, SD = 162.70; lower risk: M = 512.50, SD = 145.62; t(25) = 2.71, p = .012, Cohen's d = 1.10) and produced fewer words (elevated risk: M = 148.47, SD = 105.59; lower risk: M = 346.60, SD = 178.09; t(12.80) = 3.20, p = .007, Cohen's d = 1.35; equal variances not assumed). Figure 3 shows the means of both measures per risk group. Thus, according to parents, the groups differed on vocabulary size at the moment of testing.
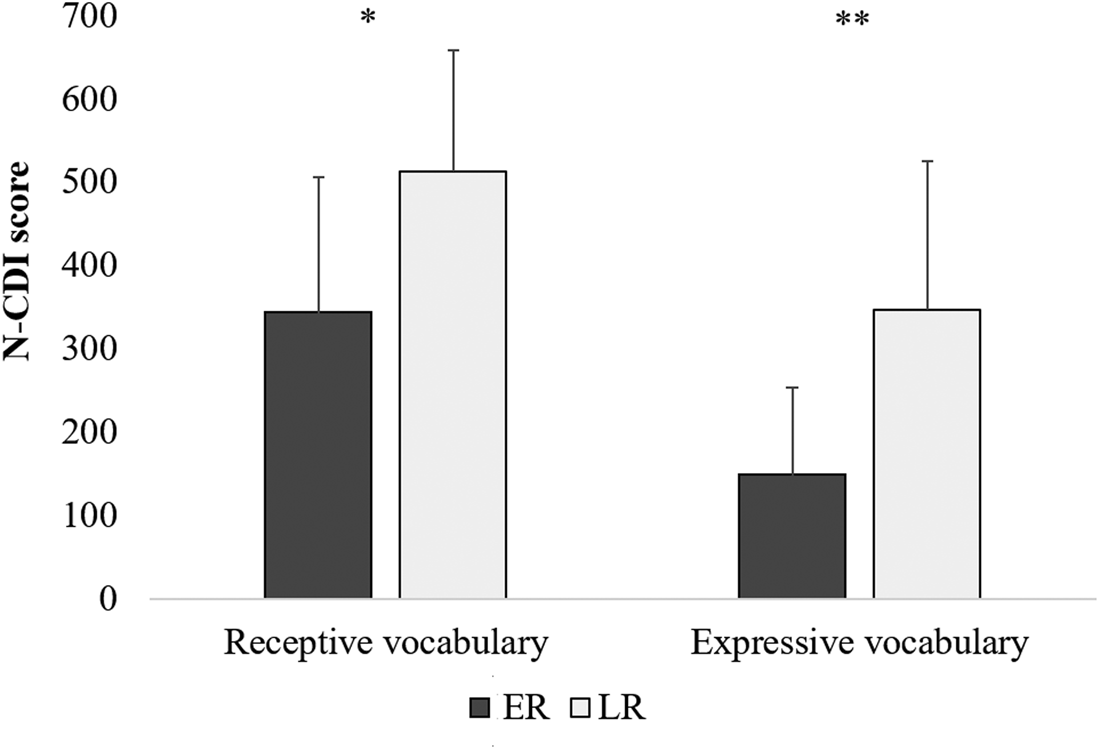
Figure 3. Mean receptive and expressive vocabulary scores per risk group. Dark grey bars represent elevated risk children; light grey bars represent lower risk children. Error bars represent one standard deviation from the mean. * p < 0.05; ** p < 0.01.
ADOS-2 scores
Test site had no effect on ADOS-2 scores (p = .46), so we collapsed all data. An independent samples t-test indicated that elevated and lower risk children did not differ in their ADOS-2 comparison scores at 36 months (elevated risk: M = 3.12, SD = 2.21; lower risk: M = 2.11, SD = 0.93; t(23.29) = −1.63, p = .12, Cohen's d = 0.60; equal variances not assumed).
Based on the ADOS-2 classification we regrouped all children as high-likelihood of developing ASD (‘ASD-group’, with ADOS-2 module 1 total score > 7; ADOS-2 module 2 total score > 6; n = 7), or as probably typically-developing (‘non-ASD group’, n = 19), and rerun analyses. For the eye-tracking task, there was still no difference in fixation on the target object between the ASD and the non-ASD group (ASD group: M = 0.57, SD = 0.08; non-ASD group: M = 0.56, SD = 0.12; t(24) = −.24, p = .81, Cohen's d = 0.10). The risk groups also no longer differed in their receptive N-CDI scores (ASD group: M = 418.29, SD = 168.37; non-ASD group: M = 385.95, SD = 171.34; t(24) = −0.43, p = .67, Cohen's d = 0.19) nor in their productive N-CDI scores (ASD group: M = 243.71, SD = 167.75; non-ASD group: M = 192.74, SD = 141.93; t(24) = −0.72, p = .49, Cohen's d = 0.33; equal variances not assumed).
Discussion
The current study tested the hypothesis that noticeable delays in vocabulary size in two-year-olds with elevated risk of ASD could stem from difficulties with word formation. Our results showed that elevated risk infants are equally able as typically-developing children to learn novel words, even though their parents reported them to know fewer words than their peers. Below we discuss both findings in more detail.
Our first finding suggests that children at elevated risk for ASD can efficiently process their parents’ voices to learn new word-object pairings as well as typically-developing children do. Even though sample sizes were very small, making it difficult to interpret null-findings, confidence intervals (Levine & Ensom, Reference Levine and Ensom2001) for both groups further suggest that there is considerable overlap between groups in proportion target looking (ER group: 95% C.I. |0.514–0.606|; LR group: 95% C.I. |0.447–0.613|). Moreover, our results are in accordance with previous research which shows no deficiency in novel word formation in elevated risk 24-month-olds when interacting with an experimenter (Bedford et al., Reference Bedford, Gliga, Frame, Hudry, Chandler, Johnson and Charman2013): both elevated and lower risk groups showed similar performances in a fast mapping task. However, whereas the Bedford et al. study further demonstrated that groups subsequently diverged in the long-term storage of the novel words after feedback had been given, the present study lacks data on any form of long-term retainment of the novel words. Future research could confirm whether it is the long-term storage of words, instead of the word-object pairings, that is impaired in children with ASD.
Moreover, it could still be that novel word formation is subtly impaired, which only becomes apparent when testing under more stringent conditions. For instance, we presented the target words multiple times, which could potentially be more advantageous for slow learners. Although we did not observe an interaction between Group and Testing Block (which serves as a proxy for the repetition factor) it could be that with different paradigms, different findings emerge. Furthermore, we opted for the caregiver to provide the verbal input, as intervention studies highlight the important role of the caregiver in diminishing ASD symptoms (McConachie & Diggle, Reference McConachie and Diggle2007). It could be that with unfamiliar speakers, evidence of word learning becomes smaller (van Rooijen et al., Reference van Rooijen, Bekkers and Junge2019). Nevertheless, our results further underscore the suggestion that infants at elevated risk of ASD can form novel words at least in some situations.
An alternative explanation for the absence of a group difference is that the elevated risk group does not contain many children who turned out to develop ASD (6 out of 18 ER children and one LR child reached ADOS-2 classification at 36 months). We know from previous research that even elevated risk children who develop typically initially fall behind in their vocabulary size, yet catch up over the second year of life (Hudry, Chandler, Bedford, Pasco, Gliga, Elsabbagh, Johnson & Charman, Reference Hudry, Chandler, Bedford, Pasco, Gliga, Elsabbagh, Johnson and Charman2014). As we tested two-year-olds, it is possible that the typically-developing children in our elevated risk group had already caught up, and therefore compensate for the weaker performances by the children who eventually are diagnosed with ASD. However, the N-CDI scores show that the elevated risk children as a group are still behind in their vocabulary size, which makes this alternative explanation unlikely. Moreover, our findings remained even when we recalibrated children's risk status based on the ADOS-2 scores collected at the age of three years.
Our second finding is that parents report children at elevated risk for ASD to understand and produce fewer words than their peers, which aligns with other research (Mitchell et al., Reference Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith and Bryson2006; Yirmiya et al., Reference Yirmiya, Gamliel, Shaked and Sigman2007; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Rogers, Roberts, Brian and Szatmari2005). Assuming that this discrepancy between groups is real, this begs the question what other explanations besides mapping ability alone might there be? It could be that these delays are caused by deficits in higher-level social demands of interactive communication, such as the ability to follow the speakers’ intentions (Baron-Cohen, Baldwin & Crowson, Reference Baron-Cohen, Baldwin and Crowson1997). Furthermore, there are reciprocal effects between how children and their parents behave that reduces both the quantity and quality of their social interactions: elevated risk children often display fewer bids (Saint-Georges, Mahdhaoui, Chetouani, Cassel, Laznik, Apicella, Muratori, Maestro, Muratori & Cohen, Reference Saint-Georges, Mahdhaoui, Chetouani, Cassel, Laznik, Apicella, Muratori, Maestro, Muratori and Cohen2011; cf. Wan, Green, Elsabbagh, Johnson, Charman, Plummer & BASIS Team, Reference Wan, Green, Elsabbagh, Johnson, Charman and Plummer2013), produce fewer communicative gestures (Özçalışkan, Adamson & Dimitrova, Reference Özçalışkan, Adamson and Dimitrova2016), or show reduced interest to sustain social interactions (Hudry, Aldred, Wigham, Green, Leadbitter, Temple, Barlow, McConachie & PACT Consortium, Reference Hudry, Aldred, Wigham, Green, Leadbitter, Temple, Barlow and McConachie2013; Wan et al., Reference Wan, Green, Elsabbagh, Johnson, Charman and Plummer2012). At the same time their parents appear less responsive and show more directive interaction behaviors (Hudry et al., Reference Hudry, Aldred, Wigham, Green, Leadbitter, Temple, Barlow and McConachie2013; Steiner, Gengoux, Smith & Chawarska, Reference Steiner, Gengoux, Smith and Chawarska2018), which may result in parents producing fewer unique words or shorter utterances (Fusaroli, Weed, Fein & Naigles, Reference Fusaroli, Weed, Fein and Naigles2019). These impoverished social encounters might limit the window of word learning. Thus it appears that while in infants with elevated risk of ASD their compromised vocabularies cannot be solely linked to difficulties in word formation, there remains a myriad of other reasons that could contribute to this.
Alternatively, it could be that this discrepancy in early vocabularies reflects a parent report bias. Perhaps parents of children with ASD set higher thresholds to denote word acquisition, and hence require more positive evidence, as these children do not always give adequate responses. (Wan et al., Reference Wan, Green, Elsabbagh, Johnson, Charman and Plummer2013). However, whether or not elevated risk children's vocabularies are compromised remains to be seen, as this group difference disappeared when we recalculated risk status based on the ADOS scores. This suggests that vocabulary delays in children with ASD might be smaller than is often assumed. Nevertheless, evidence from this study is not very strong as sample sizes are small and it seems premature to rely on this outcome to guide the interpretation of past work or the decision-making regarding the implementation of novel therapies.
To conclude, our results show that children at elevated risk for ASD are able to learn novel words in an on-line word learning paradigm, at least when it is a parent who provides the speech. Although elevated risk children initially show a lag in vocabulary size according to parental questionnaires, experimentally testing their on-line word learning abilities does not reveal such a deficiency. Therefore, the communication difficulties observed in children with ASD probably do not stem from the process of word-object pairings, but are more likely caused by higher level social demands of interactive communication. This is vital information for understanding the source of communication difficulties in ASD, and may inform the design of new interventions with a primary role for the children's caregivers.
Acknowledgements
We would like to thank all parents and children for their participation in this study; Roberta Golinkhoff and Derek Houston for kindly providing us with the stimuli as used by Ma and colleagues (Reference Ma, Golinkoff, Houston and Hirsh-Pasek2011); and Carlijn van den Boomen, Karlijn Blommers, Loes Vinkenvleugel, Manon de Korte and Yvette de Bruijn and all other testing assistants involved in the EU-AIMS project for their help. The project is funded through the Innovative Medicines Initiative Joint Undertaking (IMI grant number 115300) and a VENI grant (016.154.051) from the Dutch Organization for Scientific Research (NWO) awarded to author CJ.






