Introduction
Smallholder cocoa production systems in Bolivia under change
Cocoa cultivation combined with annual crops in shifting cultivation forms the main livelihood strategy of most farming families in the Alto Beni region at the eastern foothills of the Bolivian Andes. Such livelihoods based on tropical small-scale agriculture tend to be highly vulnerable to climate change impacts, since they offer limited coping optionsReference Borron 1 , Reference Verchot, Van Noordvik, Kandji, Tomich, Ong, Albrecht, Mackensen, Bantilan, Anupama and Palm 2 . Although Bolivia produced only 0.05% of the world's carbon emissions in 2009 3 , Reference McDowell and Hess 4 , Bolivian smallholder farmers are disproportionately affected by the negative impacts of global climate changeReference McDowell and Hess 4 . This is espec-ially true for small-scale cocoa farmers, as cocoa has been shown to be highly sensitive to changes in climateReference Anim-Kwapong and Frimpong 5 . In particular, cocoa is highly susceptible to droughts, which affect both growth and productionReference Anim-Kwapong and Frimpong 5 . Hence, cocoa production bears a high risk of being affected by climate change impacts. Strategies for making cocoa cultivation more resilient to climate change are urgently needed, since it is an important source of income not only in Alto Beni, but for 5–6 million tropical smallholder farmers worldwideReference Shapiro and Rosenquist 6 . Resilience means reducing the sensitivity of a system to stress factors and disturbances, while maintaining its capacity for self-organization, to learn and to adapt to changeReference Ifejika Speranza 7 . Our understanding of resilience includes self-organization, which implies that adaptation measures are organized by the actors, according to their own needs and visions. In our definition of adaptation, we follow Berkes and JollyReference Berkes and Jolly 8 , who understand adaptation as the result of short-term responses to changes in land-based activities, as well as of longer-term responses based on the actors’ cultural and ecological repertoires. By linking adaptation to self-organization, we approach adaptation as an active process. Resilient adaptation to climate change can therefore be regarded as a guiding principle in research for developmentReference Ifejika Speranza 7 .
Different agroforestry systems using timber trees, fruit trees and leguminous trees as shade canopies, windbreaks or buffer zones have been discussed as sustainable permanent land-use systemsReference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Veldkamp and Wanger 9 – Reference Nair, Kumar and Nair 11 . Although results from research and development projects agree that agroforestry is the most suitable way to produce cocoa in fragile ecosystems such as those in Alto Beni, most producers worldwide grow their cocoa in full sun monocultures; there is even a trend toward shifting from shaded to monoculture cocoa cultivation in order to increase short-term yieldsReference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Veldkamp and Wanger 9 . A minority of cocoa producers in Alto Beni began to form associations to organize more sustainable, certified organic cocoa cultivation in the 1980s. This resulted in a network of cocoa cooperatives grouped under the umbrella organization El Ceibo and other local organizations who promote cocoa cultivation without agrochemicals and organize organic and fair trade certification. Local organizations and development projects have been supporting the implementation of simple agroforestry as well as more complex successional agroforestry. However, to what degree these different systems are helping to build resilience has not yet been investigated systematically.
Resilience in agroecosystems
Resilience thinking provides an approach that integrates ecological and social aspectsReference Adger 12 , Reference Milestad and Darnhofer 13 . Increasing resilience means reducing the system's sensitivity, for example, to climate change, and finding ways to better deal with risks, shocks and uncertaintyReference Ifejika Speranza 7 . This is done mainly based on endowments (e.g., livelihood assets) and diversity (e.g., of crops and wild flora and fauna)Reference Ifejika Speranza 7 , Reference Milestad and Darnhofer 13 . Although resilience can be regarded as the opposite of vulnerability—because it increases the capacity to cope with stressReference Adger 12 —we argue that it is more than that. A system can become more resilient through one strategy to a range of risks that make it vulnerable; moreover, resilience focuses on inherent strengths and potentialsReference Ifejika Speranza 7 , Reference Obrist, Pfeiffer and Henley 14 . For example, increasing soil organic matter (SOM) maintains or enhances soil fertility and nutrient availability to plants and at the same time reduces the system's vulnerability to water stress, as SOM helps to retain humidity; the strategy of increasing SOM thus improves overall soil resilienceReference Ifejika Speranza 7 , Reference Nair 10 , Reference Altieri and Nicholls 15 , Reference Muller, Osman-Elasha, Andreasen, Halberg and Muller 16 . Resilience is composed of buffer capacity, self-organization and the ability to build capacity for learning and adaptationReference Milestad and Darnhofer 13 , Reference Folke 17 . Buffer capacity refers to ‘the amount of change a system can undergo while maintaining its functions and structures within the same stability domain’Reference Milestad and Darnhofer 13 , and refers mostly to ecosystem resilienceReference Lundberg and Moberg 18 —or, when dealing with farming systems, to agroecosystem resilience, which is the main focus of this paper. It seems obvious that meaningful strategies for resilience building cannot be devised by scientists alone. Local actors need to be involved in developing such strategies if they are to accept and implement them. Their perception of external influences is an important factor determining their decisions and strategy of actionReference Wiesmann 19 , Reference Gbetibouo 20 . However, although the effects of climate change on tropical smallholder agriculture have been discussed in the literature, very few studies have examined to what degree farmers actually perceive the effects of climate change described by experts and scientistsReference Gbetibouo 20 . There is hence a need for assessing, comparing and combining scientists’ and local actors’ knowledge about climate change impacts. This requires a transdisciplinary approach. Transdisciplinarity refers to cooperation between scientists and non-scientific actors based on the principle that knowledge generated by different groups of actors is taken into accountReference Wiesmann 19 , Reference Hurni, Wiesmann, Hurni, Wiesmann and Schertenleib 21 , Reference Rist, Chidambaranathan, Escobar, Wiesmann and Zimmermann 22 .
Taking stock of how the different cocoa growing systems (monoculture and different forms of agroforestry) coexist in Alto Beni, this study aimed to: (a) assess how cocoa farmers perceive climate change, and build a set of indicators of agroecosystem resilience based on a transdisciplinary approach; (b) determine resilience—mainly of the agroecosystem (aspects of buffer capacity)—under the different cocoa cultivation systems; and (c) explore to what degree self-organization and learning capacities enhance agroecosystem resilience in cocoa cultivation or, more specifically, what role organic cooperatives and organic certification play in building resilience to climate change.
Materials and Methods
The study area
The region of Alto Beni is located in the eastern foothills of the Andes, at an altitude of 350–800 m above sea levelReference Ortiz and Somarriba 23 . Annual precipitation is about 1500 mm, with a dry period in winter, between May and August. The mean temperature is about 25 °C. According to the Köppen climate classification, the climate is rainy tropical and winter dryReference Elbers 24 . The local Yungas mountain range is known for its widespread traditional cultivation of coca (Erythroxylum coca)—which is prohibited, but was nevertheless existent in Alto Beni at the time of our research (2010–2012). Other perennial crops such as cocoa, coffee and citrus trees are regarded as viable substitutes for coca by the authorities and have been officially promoted and supported in the Yungas. As a result, cocoa cultivation is now abundant in Alto Beni.
The largest share of inhabitants in the study area is settlers from the Bolivian highlands (Altiplano) who moved to the rainforests over the past 60 yearsReference Elbers 24 . Motivated by government support in the form of land, seedlings and assistance, they began to cultivate rice and corn in Alto Beni. Average farms are 12 ha in size; landholdings have remained indivisible by law until today. Cocoa plantations were promoted by the government's settlement program. The first cooperatives were founded in the 1960s to improve farmers’ participation along the value chain and to avoid intermediariesReference Bebbington, Quisbert and Trujillo 25 . International organizations began to support these cooperatives by providing technical and institutional assistance and connecting them to international specialty markets. In 1987 the umbrella organization of cocoa cooperatives El Ceibo achieved organic certification and became the world's first exporter of certified organic cocoaReference Elbers 24 . El Ceibo is the country's most important cocoa producer, has been certified for organic production (European Union, Naturland, US Department of Agriculture) and fair trade (Fairtrade International), and exports about half of the volume of dry cocoa beans it produces to Europe and the USA. An additional small volume is exported as processed organic chocolate bars. The rest is processed and distributed on the national market. Farmers’ groups can apply for certification through El Ceibo if they organize themselves in cooperatives of at least ten members. Then they enter a 3-year phase of transition to organic agriculture, during which cocoa plots undergo annual examinations as part of El Ceibo's system of external and internal certification.
Agroecosystems in the study area
The most widespread land use in the area of Alto Beni is shifting cultivation of annual crops (such as rice and corn) and perennial crops (such as cocoa, plantain, banana, papaya and citrus varieties)Reference Elbers 24 . Most of these crops are planted in full sun monocultures, which is also common for cocoaReference Ortiz and Somarriba 23 . This practice causes soil degradation and loss of arable land, primary forests and biodiversity, among other problemsReference Elbers 24 . Moreover, monocultures are susceptible to droughts and extreme weather eventsReference Anim-Kwapong and Frimpong 5 , Reference Altieri and Nicholls 15 , Reference Altieri, Funes-Monzote and Petersen 26 . In order to make cocoa plantations more sustainable, development projects and extension services promoted the shift from monoculture to agroforestry systems with diversified plantations combining cocoa and multifunctional shade trees (referred to as ‘simple agroforestry’ in this study). A special form of agroforestry practiced in the study area is successional agroforestry. This cultivation system aims to mimic natural succession with agricultural species. Crops are grouped according to the different phases in the successional system: for example, pioneers include rice and corn; secondary or transition species include papaya and leguminous trees of the Inga genus; and primary species include mahogany and cocoa. This leads to a cultivation system with structures and functions similar to those of the natural ecosystemReference Altieri and Nicholls 15 . In contrast to other farming systems, in successional agroforestry all crops and biomass accumulating species are sown and planted at the same time or shortly after each other, with the aim of achieving the highest possible plant density and diversity and occupying as many ecological niches as possible. Pioneers develop first, with the species of the next phase growing more slowly in their shade. Constant systematic pruning and selective weeding help to accumulate organic material in the soil and to ensure that sufficient light reaches the smaller plants. Schulz et al.Reference Schulz, Becker and Götsch 27 mention that plants have an allelopathic effect at the end of their life cycle, reducing the growth of neighboring plants, whereas young plants stimulate growth and vegetative development in adjacent plants. Accordingly, in successional agroforestry, ageing trees and crops are cut down after harvesting and chopped to serve as organic litter. Successional agroforestry has been reported to have a high resistance to external perturbationsReference Schulz, Becker and Götsch 27 , Reference Schulz, Montagnini, Francesconi and Rossi 28 , that is why it was considered in the present study.
Perceptions and experiences of climate change impacts: defining critical factors
Defining measurable indicators of agroecosystem resilience in a regional climate change context poses two major problems: first, the main climate change impacts have been assessed scientifically at various regional levels, including for Bolivia (compiled, e.g., by the World Bank 29 ), where they comprise increasing heat, droughts, floods and more frequent weather extremes. From an actor-oriented perspectiveReference Wiesmann 19 , however, it is important to examine which of these externally identified impacts farmers actually perceive, as only the perception of an impact will lead farmers to take adaptive measures. Accordingly, the first step must be to evaluate how farmers perceive changing climate patterns and how they translate them into new management practicesReference Gbetibouo 20 . Second, owing to the rather abstract definition of resilience, there are innumerable ways of defining resilience indicatorsReference Cabell and Oelofse 30 , Reference Darnhofer, Fairweather and Moller 31 . If this is done by scientists alone, then the risk is high that the resulting indicators will not be sufficiently relevant to local actors.
One way of addressing the above problem is to take a transdisciplinary approachReference Wiesmann 19 , Reference Rist 32 . This means considering simultaneously how both farmers and scientists perceive climate change impacts, and what main strategies both groups derive for adaptation to climate change. A transdisciplinary approach makes it possible to define resilience indicators that integrate scientists’ and farmers’ views in a way that prevents the cognitive gaps between the two groups from hampering communication and collaboration.
Anim-Kwapong and FrimpongReference Anim-Kwapong and Frimpong 5 proposed a set of methods for such a transdisciplinary assessment of climate change impacts on cocoa cultivation in Ghana. These methods include focus group discussions and key informant interviews with different stakeholders. In the present study, we adopted this methodology to capture farmers’ perceptions of climate change impacts. In a first phase, we defined critical external influences on cocoa production based on five expert interviews and three focus group discussionsReference Anim-Kwapong and Frimpong 5 , 33 with cocoa producers. Climate change impacts and adaptation strategies from the cocoa producers’ perspective were further assessed in a final workshop with 30 cocoa producers from Alto Beni. The workshop followed an interactive methodology for the participatory evaluation of risks and adaptation possibilities suggested by the Livelihood and Forestry Programme Nepal 34 . The resulting factors influencing cocoa production are listed in Fig. 4 in the Results section. El Ceibo provided climate data for the village of Sapecho collected by their experimental station.
Plot sampling and interviews with cocoa producers
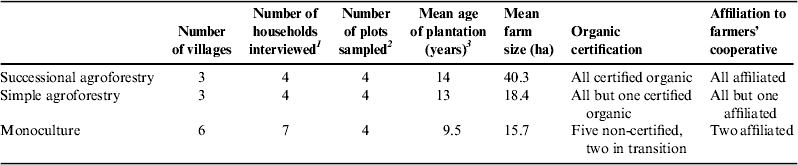
The transdisciplinary process including focus group discussions, the participatory workshop and the combination of scientific predictions on most probable climate change effects and local experiences of climate change impacts led to the definition of eight key variables to be used as agroecosystem resilience indicators (see Fig. 4). Major problems in cocoa cultivation and related adaptation measures mentioned by the participants were translated into indicators by the research team. The team cross checked the resulting indicators against the available literature before adopting them for use in the subsequent steps of this study. Accordingly, agroecosystem resilience was operationalized by the following variables: Ah horizon (depth of topsoil), SOM, soil bulk density, tree species diversity, diversity of crop varieties, ant species diversity, cocoa yield and infestation of cocoa trees with Moniliophthora perniciosa. These variables were sampled during a second phase of fieldwork. Research was carried out together with ecologists from the University of La Paz (UMSA) in 2010 and 2011 and with the assistance of the person in charge of a forest seed bank maintained by El Ceibo. Farms were selected according to their cocoa cultivation system (monoculture, simple agroforestry or successional agroforestry), assuring that locations, ecological preconditions and plot age were comparable. In terms of socio-economic characteristics, farms were selected so that (1) they represented the two cocoa farming systems most typically practiced by the two major local population groups: farmers having migrated to the region from the highlands, and farmers belonging to the locally rooted indigenous group; and (2) although the total farm size could vary considerably from one group to the other, the surfaces used for growing cocoa (1–5 ha) and other agricultural crops were rather similar. Sites were selected in six villages along the Beni River (Fig. 1). On each of 12 farms—four practicing monoculture, four simple agroforestry and four successional agroforestry (Fig. 1, Table 1)—a sampling plot of 48×48 m was installed in the main cocoa plantation, resulting in four sampling plots for each of the three cultivation systems (stratified sampling 35 ). These sampling plots were each divided into four sub-plots of 24×24 m. Soil and biodiversity data were sampled in each sub-plot; from these sub-plot data, means for the entire sampling plot were calculated for further analysis. In order to determine yields and management practices, semi-structured interviews were conducted on the 12 sampled farms as well as three additional farms practicing monoculture (total n=15). We surveyed farm crop diversity and cocoa yield over the past year, previous use of the cocoa plot where we sampled data and management practices such as pest and disease control. According to the standards of qualitative researchReference Martin 36 , we kept questions open and avoided phrasing them in a suggestive way. Abstract concepts such as resilience were not discussed during the interviews or focus groups. In order to gather information on socio-economic aspects of resilience (self-organization and adaptive capacity), we asked farmers about their affiliation and interaction with cocoa cooperatives, participation in courses on cocoa cultivation and motivations behind their affiliation to a cocoa cooperative. These data were complemented with expert interviews with five agricultural consultants engaged in the promotion of agroforestry and/or cocoa cultivation in the region of Alto Beni.

Figure 1. The study area of Alto Beni. SAFS=successional agroforestry; SAF=simple agroforestry; Mono=monoculture.
Table 1. Characteristics of 12 sampled/interviewed and three interviewed farms practicing different cocoa cultivations systems.
1 Crop varieties, cocoa yield, infestation with Moniliophthora perniciosa.
2 Soil analysis, tree species diversity, ant species diversity.
3 Not significantly different (Kruskal–Wallis rank sum test).
Assessment of agroecosystem resilience
Soil data
In terms of soil indicators, we assessed SOM, depth of Ah horizon and bulk density. SOM is a crucial variable in the context of adaptation to climate change and agroecosystem resilienceReference Cabell and Oelofse 30 because it helps to retain water in the soil and to make nutrients available to plants. Bulk density indicates soil compaction, which is a measure of susceptibility to soil erosion and water retention capacity. Depth of Ah horizon gives an impression of the vertical distribution of SOM and, in combination with SOM concentration and bulk density, shows whether the organic material is concentrated or well distributed. Ah horizon was measured by means of a soil profile, measuring the part containing visibly organic material. SOM and clay content were sampled based on mixed samples taken every 5 m in a grid with a soil auger at 0–25 cm depth at the sub-plot level, resulting in four mixed samples per plot, which were then converted to a mean value for 0–25 cm for each plotReference Schroth and Sinclair 37 . Bulk density was sampled using metal cylinders in the middle of the 0–25 cm core, at 12.5 cm depth. The samples were analyzed by the Laboratorio de Calidad Ambiental, University of La Paz (UMSA), following the ISRIC guidelinesReference van Reeuwijk 38 .
Biodiversity data
Biodiversity is an integral part of resilience, as it buffers against perturbations and provides seeds for reorganizationReference Lundberg and Moberg 18 , Reference Cabell and Oelofse 30 , Reference Kremen and Miles 39 . It is measured by determining species diversity, in our case of trees, crops and ants. Tree species diversity is obviously higher in agroforestry than in monoculture; however, we use it in combination with the diversity of crops and ants as an indicator of the agroecosystem's ecological buffer capacity, which is a distinctive feature of resilience. Tree species diversity was considered as an important attribute of a resilient cocoa cultivation system by our focus groups; this was in line with scientific findings stressing the generally high conservation value of cocoa agroforestry, which is especially important in biodiversity hot spots such as Alto BeniReference Verchot, Van Noordvik, Kandji, Tomich, Ong, Albrecht, Mackensen, Bantilan, Anupama and Palm 2 , Reference Bhagwat, Willis, Birks and Whittaker 40 , Reference Sperber, Nakayama, Valverde and de Siqueira Neves 41 . Tree species in the cocoa sampling plots were determined with the help of the staff of El Ceibo's forest seed bank. All trees other than cocoa with a diameter at breast height of >5 cm were categorized. We surveyed crop diversity by listing all crops of the farm during a transect walk, and later complemented these data with information from the interviews. This was useful because the interviews revealed that some crops that the families used for various purposes, such as dyeing or medicinal treatments, have not been recognized as such during fieldwork or have not been present in the plot at the time of sampling due to a short life cycle. Richness and abundance of ant species has been recommended in the literature as an indicator that describes the proximity of an agricultural system to a natural systemReference Bisseleua, Missoup and Vidal 42 , Reference Philpott, Bichier, Rice and Greenberg 43 . We agree that this proximity may not automatically lead to resilient agroecosystemsReference Denison, Kiers Stuart and West 44 . In a rainy tropical context like our study area, however, an agroecosystem with a structure and function similar to those of natural rainforest is, according to various studies, better adapted to climate change impacts, erosion processes and insect calamities than a monocultureReference Altieri, Funes-Monzote and Petersen 26 , Reference Bisseleua, Missoup and Vidal 42 , Reference Borkhataria, Collazo and Groom 45 . Diversity of wild species is also important as a proxy for antagonists of pests, such as insectivorous birdsReference Borkhataria, Collazo and Groom 45 . Here, ant individuals were sampled at the plot level in six foliage samples per sampling plot and identified by an entomologist at the laboratories of the Instituto de Ecología, University of La Paz (UMSA).
Cocoa yield
To determine this variable, we counted cocoa pods >5 cm on ten trees in the center of each plot during the main harvesting season. However, the amount of cocoa pods varied because of the different harvesting cycles. For this reason, we also recorded cocoa yields as reported by the farmersReference Philpott, Bichier, Rice and Greenberg 43 during the main harvesting period. They were asked how much they harvested in the previous year and how much they expected to harvest in the current year. Based on the data from these two sources—pod counts in plots and yields reported by farmers—we calculated a mean value for each farm, which we then used as this farm's annual cocoa yield.
Infestation with M. perniciosa
This fungal disease, also known as witches’ broom, can cause severe yield losses and is one of the most damaging cocoa diseases in the study areaReference Milz 46 . Since its symptoms appear on different parts of the tree, we were able to include all plots in the comparison, even those where there were no pods. The intensity of infestation was sampled by counting the number of infested parts of ten trees at the center of each plot and rating them with an index of 0–3 (0=no infestation visible, 1=one infestation, 2=one to ten infestations, 3=more than ten infestationsReference Gerold and Faust 47 ).
The response variables (one mean per farm calculated from the four sub-plots)—depth of Ah horizon, SOM, soil bulk density, tree species diversity, diversity of crop varieties, ant species diversity, cocoa yield and infestation of cocoa trees with M. perniciosa—were tested for significant differences between the explanatory variables simple agroforestry, successional agroforestry and monoculture. Owing to the fairly large plot size and the four replications, we had only four means per group. This implied that model assumptions for ANOVAs could not be fully verified. We therefore tested the data non-parametrically by means of Kruskal–Wallis rank sum tests and post hoc by means of Wilcoxon rank sum tests, using the open-source software R, version 2.14.1 48 . Correlations were tested using Pearson's product moment correlation.
Results
‘As if the sun was getting closer to the Earth’: main changes in the local climate
The results of focus group discussions and the final workshop with 30 cocoa producers show that farmers effectively perceive gradually evolving climate changes along a timeline (Fig. 2). Periods of extreme heat and an extended dry season were experienced as the main climate problems, as expressed in the vivid statement by one of the cocoa producers quoted in the section heading. However, the arrival of fungal cocoa diseases—witches’ broom (M. perniciosa) in the 1980s and frosty pod rot (Moniliophthora roreri) in 2010/2011—was mentioned as a severe impact, as well. Moreover, periods of excessive rainfall were associated with increased incidence of black pod disease (Phytophthora palmivora).

Figure 2. Timeline of climate and environmental events as remembered by cocoa producers from Alto Beni, 2010.
Climate data from the village of Sapecho show increasing maximum temperatures (Fig. 3), indicating that farmers’ perceptions of rising temperatures are in line with scientific evidence (see alsoReference Gbetibouo 20 ).

Figure 3. Monthly temperature maxima in the village of Sapecho, 1964–2010. Data: El Ceibo Research Station, 2011.
According to the World Bank's country note on climate change aspects in agriculture for Bolivia 29 , the main risks resulting from climate change are floods and droughts due to increasing rainfall in humid months and decreasing rainfall in dry months, as well as rising temperatures. Cocoa producers regarded heat, droughts and floods as the greatest threats to their livelihoods and attributed all of them to global climate change. As the best adaptation strategies for their cocoa plantations, farmers mentioned increasing SOM, planting more trees, enhancing biodiversity, diversifying production and controlling pests and diseases by means of improved management practices. The combination of cocoa farmers’, experts’ and scientists’ knowledge about climate change that informed the definition of agroecosystem resilience indicators is presented in Fig. 4.

Figure 4. Transdisciplinary process for defining agroecosystem resilience indicators.
The characteristics of the selected cocoa farms are presented in Table 1. Seven of the cocoa farmers interviewed were certified for organic production and eight were not. The pattern of affiliation to a farmers’ cooperative was closely related to that of certification: all agroforestry farmers but one were members of a cooperative, whereas only two monoculture farmers—those who were in the process of shifting to organic agriculture—were affiliated to a cooperative. Results from the plot sampling and interviews regarding the agroecosystem resilience indicators are presented in Table 2.
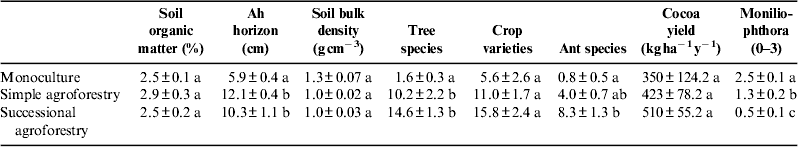
Table 2. Overview of results for agroecosystem resilience indicators, with standard error of means. N=12 for Ah horizon, soil organic matter, soil bulk density, tree species and ant species. N=15 for crop varieties, cocoa yield and infestation with Moniliophthora perniciosa. a, b, c: No significant differences for value sharing the same letter.
Agroecosystem resilience indicators
SOM concentration ranged from 2.08 to 3.45%, which is rather low for cocoa plantationsReference Juhrbandt, Duwe, Barkmann, Gerold, Marggraf, Tscharntke, Leuschner, Veldkamp, Faust and Guhardja 49 . SOM showed no significant differences between the different cultivation systems (Table 2) and correlated significantly with clay content (r 2=0.44, P<0.05, Pearson's product moment correlation). The region of Alto Beni is locally known for its fertile soils, but experts stated in the interviews that the land is prone to rapid soil degradation, which poses a constant threat in plantations without soil cover and trees. Previous land use could not be identified for all farms. Two monocultures had been established on plots that had previously been part of primary ecosystems (riverine vegetation), the others on a former plantain and a former papaya plot. Three agroforestry plantations have been established on plots that had previously been fallow, at least two had replaced the former cocoa monocultures, and at least one had been established on grazing land. None of the interviewees had installed their cocoa plantation on terrain covered by primary forest. Ah horizon was significantly deeper in plots under successional agroforestry and simple agroforestry than in monocultures (P<0.05, Wilcoxon rank sum test, Table 2). Soil bulk density was higher in monocultures, but the difference was not significant (Kruskal–Wallis rank sum test). Ah horizon was negatively correlated with bulk density (r 2=−0.64, P<0.05, Pearson's product moment correlation, Table 2).
Tree species diversity was higher in shaded cocoa plots, with the highest total tree species diversity found in successional agroforestry systems (total of 74 species found on all successional agroforestry plots), followed by simple agroforestry systems (54 species in total), and monocultures (four species in total). The maximum of tree species found in one plot was 38, in a successional agroforestry plot. As we had expected, the difference between agroforestry systems and monocultures was significant (P<0.05, Wilcoxon rank sum test) (Table 2). In total, 89 species of woody perennials from 34 botanical families were found. The most abundant families were Fabaceae, Rutaceae and Bombacaceae. The most frequent shade tree species were Bactris gasipaes, Amburana cearensis, Attalea phalerata, Inga spp., Swietenia macrophylla and Centrolobium ochroxylum. Bactris and Attalea are multipurpose palm trees. Inga was planted to improve the soil and for its fruit. The others are high-value timber trees.
Crop varieties diversity: in total, interviewees mentioned 37 different crop varieties, including cocoa, papaya and varieties of Citrus and Musaceae as the most common crops. The highest number of crops was found on a farm practicing successional agroforestry (21 different crops, without counting fruit, timber and medicinal trees found in the sampling plot). The overall average number of crop varieties per farm was 10.3. Successional agroforestry farms averaged 15.8 different crops, simple agroforestry farms 11, and monoculture farms 5.6 (difference not significant, Kruskal–Wallis rank sum test, Table 2). All families reported that they consume part of all crops cultivated. Rice and Musaceae were mentioned as the most important subsistence crops, whereas cocoa was consumed in rather small quantities. On-farm diversity of crops other than cocoa played an important role for all families participating in the present case study. Shade and multiple forest products such as timber, fruit and natural medicines were mentioned as important to cocoa producers and their families by all farmers interviewed, including those growing their cocoa in monoculture.
Ant species diversity: we found a total of 5227 individuals belonging to 22 genera. Of these, 1341 individuals (18 genera) were found in successional agroforestry plots, 293 individuals (11 genera) in simple agroforestry plots and 326 individuals (five genera) in monoculture plots. The maximum found in one individual plot was 15 genera, found in a successional agroforestry plot. The difference between successional agroforestry and monocultures was significant (P<0.05, Wilcoxon rank sum test). Ant species diversity was significantly correlated with tree species diversity (r 2=0.74, P<0.05, Pearson's product moment correlation, Table 2).
Cocoa yields varied considerably from year to year due to climate conditions, pests and diseases, management and other factors. It was reported that plots are abandoned when cocoa prices are low; when they improve, the plot is pruned and revitalized through labor input. Overall cocoa yields (427.8 kg ha−1 yr−1) were close to the world average (474.3 ha−1 yr−1, data from FAOSTAT for 2010) but varied from 138 to 680 kg ha−1 yr−1. The difference in yields was not significant (Kruskal–Wallis rank sum test), but nonetheless, sample yields were higher in agroforestry systems (Table 2).
Infestation with M. perniciosa: the difference in infestation intensity was significant between all systems (P<0.05, Wilcoxon rank sum test). The highest infestation was observed in monocultures and the lowest in successional agroforestry systems. These findings were backed by statements made by all interviewees with agroforestry systems, who reported less witches’ broom infestation in shaded cocoa plantations (Table 2). All agroforestry farmers reported that they control the disease by regularly removing infested plant material, and that this is monitored by agricultural consultants from cooperatives and other local organizations. Two of the monoculture farmers stated that they do not control witches’ broom due to a lack of knowledge, and two others reported that they remove infested parts very rarely due to a lack of time. It was stated in the literature that fungal cocoa diseases may profit from the higher humidity in cocoa plantations diversified with shade trees (Schroth et al. cited inReference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Veldkamp and Wanger 9 ). This may hold true for black pod or frosty pod rot, but seems not to apply for witches’ broom (see alsoReference Borkhataria, Collazo and Groom 45 , Reference Milz 46 , Reference Rice and Greenberg 50 ). Three agroforestry farmers from this survey stated that black pod and frosty pod rot only appear in shaded cocoa if plants are not pruned and diseases are not controlled manually.
Figure 5 visualizes the differences in agroecosystem resilience indicators between the different cultivation systems by means of a multi-dimensional spider diagram using a percentage scale. The diagram plots the three cultivation systems’ resilience indicators against each other. For each indicator, the 100% value is the maximum found in any single one of the 12 plots. Successional agroforestry systems were the agroecosystems with the highest resilience: these systems had the highest diversity and cocoa yields, as well as the lowest infestation with M. perniciosa. Simple agroforestry systems had the deepest Ah horizon; most other values ranged in between successional agroforestry and monoculture. Accordingly, we consider the resilience of simple agroforestry to be intermediate. Monocultures had rather good soils, with the same value of SOM as successional agroforestry systems, but concentrated in the upper centimeters. However, due to this system's lack of diversity and the fact that it had the lowest yields and by far the highest infestation with M. perniciosa, we consider it to have the lowest agroecological resilience among the three systems. Qualitative results show that the farmers who implemented a successional agroforestry system had organic certification and were well connected to local farmers’ organizations, indicating resilience also with regard to socio-economic factors.
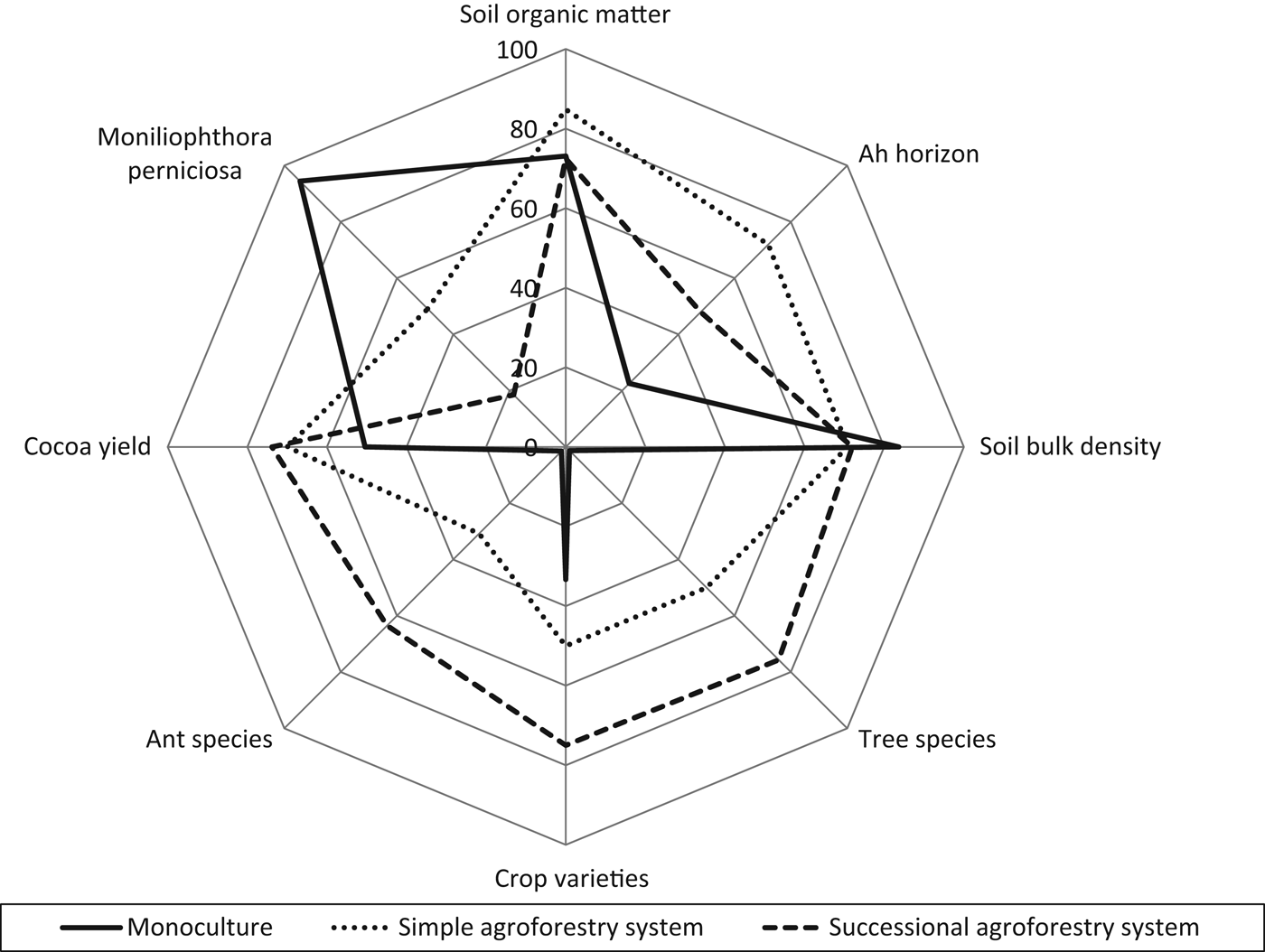
Figure 5. Comparison of agroecosystem resilience indicators under monoculture, simple agroforestry and successional agroforestry. All variables refer to the cocoa sampling plot, except for crop varieties, which refers to the whole farm. N=12 for Ah horizon, soil organic matter, soil bulk density, tree species and ant species. N=15 for crop varieties, cocoa yield and infestation with Moniliophthora perniciosa.
Self-organization and adaptive capacity
In order to relate the results for agroecosystem resilience to the socio-economic variables that are comprised in the concept of socio-ecological resilience, we also evaluated aspects related to self-organization and adaptive capacity.
El Ceibo, the federation of local cooperatives, was the largest regional farmers’ organization at the time of this research. The members of local cooperatives obtain certification for organic cocoa production through El Ceibo. Certification is accompanied by the supply of shade tree seedlings and technical assistance for their planting and management in the cocoa plots. This encourages farmers to shift from monoculture to agroforestry systems of cocoa cultivation (expert interviews). In addition, El Ceibo regularly organizes knowledge exchange platforms for local farmers in cooperation with other farmers’ organizations. Accordingly, all agroforestry systems (with one exception) were certified organic (Table 1). El Ceibo recommended a simple shade canopy of 30%, whereas other organizations propagated more diverse successional agroforestry systems (expert interviews). Table 1 indicates that agroforestry was strongly linked to organic certification, and organic certification was organized through cooperatives. Among the 15 families interviewed, nine were affiliated to a cooperative (Table 1), including two monoculture farmers, who had joined a cooperative and were entering the 3-year phase of transition toward organic production.
A better price for certified cocoa was the most frequently mentioned reason for joining a cooperative. All agroforestry farmers stated that trees are keys to adapting their cocoa plots to climate change, mainly because they help to counteract the negative effects of droughts, but also because it is easier to work in the shade given the increased heat. With regard to adaptive capacity, four out of the six monoculture farmers had never attended a course on cocoa cultivation and reported that they do not have access to extension services regarding cocoa. All farmers with agroforestry systems and organic certification had participated in at least one course. All interviewees were interested in capacity building for sustainable cocoa cultivation. Learning needs mainly concerned the question of how to address the constraints related to the practical implementation of successional agroforestry systems, gaps in general knowledge about how to further increase resilience, deficiencies of existing extension services, and lack of labor, time and equipment for adequately pruning the trees.
Discussion
Agroecosystem resilience in cocoa cultivation in Alto Beni and the key role of diversity
Indicators of agroecosystem resilience tended to be more favorable under agroforestry than under monoculture (Fig. 5). Successional agroforestry had the highest tree species diversity, crop diversity and ant species diversity, as well as the highest yields and the lowest infestation with M. perniciosa. We expected SOM concentration to be higher under agroforestry—especially successional agroforestry—due to high biomass inputs from roots and from pruningReference Nair, Kumar and Nair 11 , Reference Schulz, Becker and Götsch 27 . This was not confirmed. However, results may have been influenced by the previous uses of the plots: two of the monoculture plantations had been established on terrain previously covered with primary vegetation, whereas this was not the case for either of the agroforestry systems. On the contrary, at least one successional agroforestry system has been established on grazing land with the aim of soil recuperation, a potential ascribed to successional agroforestry by other authorsReference Schulz, Montagnini, Francesconi and Rossi 28 , Reference Vieira, Holl and Peneireiro 51 . Results for SOM are not entirely clear due to the influence of various other factors such as plot age (it may take more time for substantial differences to develop in response to the different cultivation systems), location, soil structure and the lack of baseline soil data. However, as expected, the Ah horizon was deeper and soil bulk density was lower under agroforestry than under monoculture, and the two indicators were significantly negatively correlated, indicating a positive effect of agroforestry on soil structure and the distribution of SOM. The presence of trees might help to distribute organic material into deeper soil layers and inhibit soil compaction. Sperber et al.Reference Sperber, Nakayama, Valverde and de Siqueira Neves 41 found that tree diversity in Brazil was correlated with faunal composition and showed that tree species richness was positively correlated with parasitoid abundance and hence with natural enemies of cocoa pests. Accordingly, they found a lower level of pest outbreaks in cocoa under shade, which was also confirmed by agroforestry farmers in our study. Findings of other authors also indicate a higher agroecosystem resilience to pest calamities in cocoa plots diversified with shade treesReference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Veldkamp and Wanger 9 . Clough et al.Reference Clough, Barkmann, Juhrbandt, Kessler, Wanger, Anshary, Buchori, Cicuzza, Darras, Putra, Erasmi, Pitopang, Schmidt, Schulze, Seidel, Steffan-Dewenter, Stenchly, Vidal, Weist, Wielgoss and Tscharntke 52 showed that ant species diversity (and other wild species’ richness) and tree species diversity do not necessarily decrease with increasing cocoa yield in a study in Indonesia, indicating that cocoa plantations can be farmed intensively (e.g., by means of labor or organic fertilizer inputs) while maintaining a high biodiversityReference Clough, Barkmann, Juhrbandt, Kessler, Wanger, Anshary, Buchori, Cicuzza, Darras, Putra, Erasmi, Pitopang, Schmidt, Schulze, Seidel, Steffan-Dewenter, Stenchly, Vidal, Weist, Wielgoss and Tscharntke 52 . Similar to our findings, wild species richness was correlated with shade tree density and diversity in cocoa or coffee plantations in other studies as wellReference Bisseleua, Missoup and Vidal 42 , Reference Philpott, Bichier, Rice and Greenberg 43 , Reference Borkhataria, Collazo and Groom 45 . Biodiversity in the agroforestry cocoa plots in our study was strongly associated with organic certification and membership in cooperatives (Table 1). It is thus an effect of local farmers’ capacity of self-organization that helped them to increase their learning capacities and establish a self-organized extension service. This extension service recommends growing cocoa in agroforestry systems and, in doing so, goes beyond the minimal organic certification requirements of the European Union and the US Department of Agriculture. This additional commitment was achieved by facilitating cooperative members’ capacity building, as well as providing tree seedlings and technical assistance in transforming monoculture plantations to agroforestry systems. Interviews indicated that farmers’ motivation for participating in the certification process was strongly enhanced by their interest in capacity building and in achieving technical knowledge. Accordingly, certification can be seen as a social process during which management practices are shared and transmitted between cocoa producers, thus allowing them to further increase their self-organization and learning capacities which, in turn, are the drivers behind increasing agroecosystem resilience.
Other studies of organic farming found that certified organic farmers in remote areas tend to have better management practices and therefore to be more successful with their crops than farmers with common practice agroecosystems that are often not optimally managedReference Altieri, Funes-Monzote and Petersen 26 ; see also 53 – Reference Scialabba and Hattam 55 . Results from our study that support these statements are the poor or entirely absent control of witches’ broom disease on uncertified cocoa farms despite significantly higher infestation, or the comparably low yields on monoculture farms (Table 2). Literature reviews comparing yields from organic and conventional cultivation systems found that yields frequently increase when farmers shift from a low-input system (which includes many traditional systems) or degraded soil to organic management 53 , Reference Badgley, Moghtader, Quintero, Zakem, Chappell, Aviles-Vazquez, Samulon and Perfecto 56 – Reference Panneerselvam, Halberg, Halberg and Muller 58 . In perennial plantations, yields from organic crops were found to be lower than from comparable conventional crops, but this was compensated by other products from organic agroforestry systemsReference Scialabba and Mueller-Lindenlauf 59 . Yields in our monoculture plots compared to cocoa monocultures in other parts of the world were low because they were managed without agrochemicals or any external nutrient inputs. Full-sun monocultures can significantly increase cocoa yields, but this requires high inputs and can be maintained only for a short time compared to agroforestry systemsReference Tscharntke, Clough, Bhagwat, Buchori, Faust, Hertel, Hölscher, Juhrbandt, Kessler, Perfecto, Scherber, Schroth, Veldkamp and Wanger 9 . Agricultural consultants from El Ceibo also stated that cocoa yields are highest in young, input intensive full-sun monocultures, but that soils under monoculture cocoa degrade rapidly. Other authors found that cocoa grown in successional agroforestry systems without external inputs can produce yields at a level that the surrounding cocoa plantations were only able to achieve through considerable input of mineral fertilizer and pesticidesReference Schulz, Becker and Götsch 27 .
Shapiro and RosenquistReference Shapiro and Rosenquist 6 state that one-third of the world's cocoa yield is lost to pests and diseases every year, and that many cocoa producers lack the training and resources to control them. The reasons for the poor performance of the non-certified monoculture farms in our study are thus to be found in the socio-economic context. To obtain certification without being part of a local organization in Alto Beni is difficult. Certification offers better cocoa prices and membership in a social movement, which comes with knowledge management and technical support for cocoa cultivation. Thanks to the growing worldwide demand for cocoa—and especially for organic cocoa—Alto Beni has experienced rising cocoa prices in the last few years. Certifying one's cocoa plantation with the aim of obtaining a better cocoa price was mentioned as the main reason for founding or joining a cocoa cooperative; organic certification can thus be regarded as an incentive for farmers’ self-organization, which, in turn, enhances their learning capacities.
Organic cocoa farming as an adaptation to climate change?
The three different forms of cocoa cultivation considered in this study can be understood as a trajectory from low agroecosystem resilience monoculture cocoa growing to higher agroecosystem resilience complex agroforestry, enhanced by organic certification. Accordingly, the monocultures in transition had already been framed with fruit trees, and the respective families reported that they planned to further diversify the system.
If organic certification enhances diversity and the plantation of shade trees—as suggested by the results of our case study—it bears a higher potential for adaptation to climate change impacts than local common practice agricultureReference Ifejika Speranza 7 , Reference Milestad and Darnhofer 13 . A special potential for increased agroecosystem resilience on cocoa farms was found in successional agroforestry systems, as they had the most favorable indicator values. These knowledge-intensive agroforestry systems are still underrepresented in the study area, and organic certification alone may not be sufficient for their enhancement. Extension services thus need to improve the outreach of their activities, which could further benefit from participatory research that aims to enhance exchange and learning platforms and facilitates, validates and enhances access to knowledge on the evolution of species diversity and related management strategies.
Studies from other regions indicate that agriculture using shade trees has been one of the most successful strategies for diversification and adaptation to climate change, with considerably increased yields for annual as well as for perennial cropsReference Altieri, Funes-Monzote and Petersen 26 . However, we suggest that further studies on the trade-offs of agroecological diversification should not focus only on quantitative variables such as yieldsReference Darnhofer, Fairweather and Moller 31 , since diversified agroforestry systems are characterized by complex interactions between ecology and local sociocultural norms and traditionsReference Nair 10 , and many benefits of agroforestry are not easily quantified. We consider an integrative research approach that takes into account how local actors perceive, value and interpret risks and conditions as appropriate for case studies like ours, as these factors frame local actors’ strategies of actionReference Wiesmann 19 , which, in turn, influence the way in which natural resources are managed.
Conclusion
This study focused on the resilience of smallholder cocoa farms, comparing three different cocoa cultivation systems with regard to farm-level agroecosystem resilience and farmers’ adaptive capacity. Agroecosystem resilience tended to be higher under successional agroforestry systems compared to simple agroforestry and monoculture systems. Accordingly, successional agroforestry can be regarded as a more promising adaptation strategy than mere certified organic production, as organic certification alone does not necessarily result in a high diversity of shade trees. The combination of successional agroforestry with organic certification can improve important agroecosystem resilience variables while providing high cocoa yields. We conclude that the establishment of successional agroforestry is a key strategy for adapting to risks such as climate change impacts as mentioned by the cocoa farmers from Alto Beni. This study also shows that local organizations facilitating organic certification support agroecological diversification and, by doing so, help to increase agroecosystem resilience to climate change impacts. They further enhance farmers’ learning and adaptive capacity by organizing courses and knowledge-exchange platforms. Thus, organic certification can support good farming practices and diversification through a support infrastructure for farmers. Organic producers frequently have more and better access to extension services and thus an increased agro-ecological adaptation potential. With a view to practice, these findings indicate that it might be highly beneficial to support local farmers’ organizations that focus on farm diversification, organic management practices and improved accessibility and management of knowledge.
Acknowledgements
We acknowledge the following institutions for their support: AVINA Foundation for initial funding, the Swiss National Centre of Competence in Research (NCCR) North-South (Research Project 13) and the Research Institute of Organic Agriculture (FiBL) for supervision of the study, the Commission for Research Partnerships with Developing Countries (KFPE) for financing an Échange Universitaire project which made it possible to collect data together with four students from Bolivia. We are also grateful for the valuable support of the participating students and their supervisors from the Facultad de Agronomía and Instituto de Ecología (University of La Paz), the field assistants and agricultural consultants from El Ceibo, and the cocoa farmers participating in the study.









