Introduction
Toxoplasma gondii infections are prevalent in humans and animals worldwide. The ingestion of undercooked infected meat or the consumption of food and water contaminated with oocysts excreted in cat feces are the main sources of infection. Cats are everywhere and a single cat can excrete millions of oocysts that can remain viable in the environment for months under natural conditions. Estimation of oocyst contamination of the environment is difficult because of low numbers present in soil or water and because there are no molecular markers to distinguish live vs dead oocysts.
Domestic free-range (FR) chickens (Gallus domesticus) are excellent sentinels of environmental contamination with T. gondii oocysts because they feed on the ground, they are comparatively inexpensive, can be easily infected with T. gondii and seldom develop clinical toxoplasmosis (Ruiz and Frenkel, Reference Ruiz and Frenkel1980; Dubey, Reference Dubey2010a, Reference Dubey2010b).
Until 2000, T. gondii was generally considered to have low genetic diversity and strains were considered clonal. Interest in genetic diversity of T. gondii was spurred because some isolates were found to be more virulent (as assessed in mice) than others and certain genotypes were associated with clinical toxoplasmosis in humans (Dubey, Reference Dubey2010a).
Beginning in 2000, a collaborative research project was initiated at the United States Department of Agriculture (USDA) facility in Beltsville, Maryland, and the project terminated in 2019. The main objective was to study the genetic diversity of T. gondii using DNA derived from live parasites. Our initial focus was South America because, until then, little was known of the genetic diversity of T. gondii in this part of the world. The plan was to obtain tissues from chickens and bioassay them in outbred Swiss Webster mice and in cats at Beltsville. Thus, the biology of isolates could be compared using identical conditions. The ease of availability and the cost of purchasing chicken was also a factor in selecting this host species. Secondly, there was no restriction on importing chicken tissues into the USA at that time compared with no imports of tissues from other livestock (pigs, sheep, goats, cattle). A decade later, restrictions on the import of chickens were imposed because of H5N1 virus infection. The greatest success was obtained through collaboration with scientists in several institutions in Brazil. It was possible to isolate viable T. gondii from most regions of Brazil (discussed later). This was very labour-intensive and costly research. Initially, a door to door survey of houses with backyard chickens in Rio de Janeiro was conducted. The chicken sampled were from properties that were about 1 km apart and no more than 10 chickens were sampled from each property (da Silva et al., Reference da Silva, Bahia-Oliveira, Shen, Kwok, Lehman and Dubey2003; Dubey et al., Reference Dubey, Graham, da Silva, Lehmann and Bahia-Oliveira2003a). It meant purchasing chickens from individual houses, holding them live at a local facility, euthanizing them a day before departure from Brazil, and bringing them personally or by overnight courier service to Beltsville for bioassay. The project was extended to 19 other countries (see Dubey et al., Reference Dubey, Laurin and Kwowk2016). At Beltsville, tissues were bioassayed in outbred Swiss Webster mice (five for each tissue) so that mortality data could be compared. A system was proposed to designate the T. gondii isolates-Tg (for T. gondii) Ck (for chicken) and the country (e.g. Br for Brazil). Information on all viable T. gondii isolates is scattered in many publications. Genotyping was performed using PCR-RFLP, and the results were published piecemeal as more markers were developed in the last two decades (Su and Dubey, Reference Su and Dubey2020). We now summarize all data using 10 PCR-RFLP markers and correct errors in reporting.
Here, we review toxoplasmosis in chickens for the past decade, add new genotyping data, and correct mistakes in the literature. The review is divided into natural and experimental infections.
Natural infections
Prevalence
Serologic investigations
Worldwide serologic prevalences are summarized in Table 1 and Fig 1. Results varied with management, serological techniques and the cut-off values used. Virtually all chickens become infected after hatching because vertical transmission is extremely rare in chickens (Dubey, Reference Dubey2010b). Several studies documented an increasing prevalence with age (Table 1). Infections were higher in poorly managed farms. Infections were low (3.7% of 384) even in adult laying hens in large farms (>1000 per unit) compared with 11.7% of 470 backyard chickens in Germany; all chickens tested had outdoor access (Schares et al., Reference Schares, Bangoura, Randau, Goroll, Ludewig, Maksimov, Matzkeit, Sens, Bärwald, Conraths, Opsteegh and van der Giessen2017a). Infections in caged chickens were lower than in FR chickens (Yan et al., Reference Yan, Yue, Yuan, He, Yin, Lin, Dubey and Zhu2009; Cui et al., Reference Cui, Fang, Gu, Guo and Sun2010; Tian and Cui, Reference Tian and Cui2010; Xu et al., Reference Xu, Song, Wang, Wang, Cao and Liu2012; Mahmood et al., Reference Mahmood, Zahid, Sthanadar, Shah and Hussain2014; Matsuo et al., Reference Matsuo, Kamai, Uetsu, Goto, Takashima and Nagamune2014; Rodrigues et al., Reference Rodrigues, Moreira, Coutinho, Dubey, Cardoso and Lopes2019; Duong et al., Reference Duong, Appiah-Kwarteng, Takashima, Aye, Nagayasu and Yoshida2020).
Table 1. Seroprevalence of T. gondii in chickens (2009–2020).
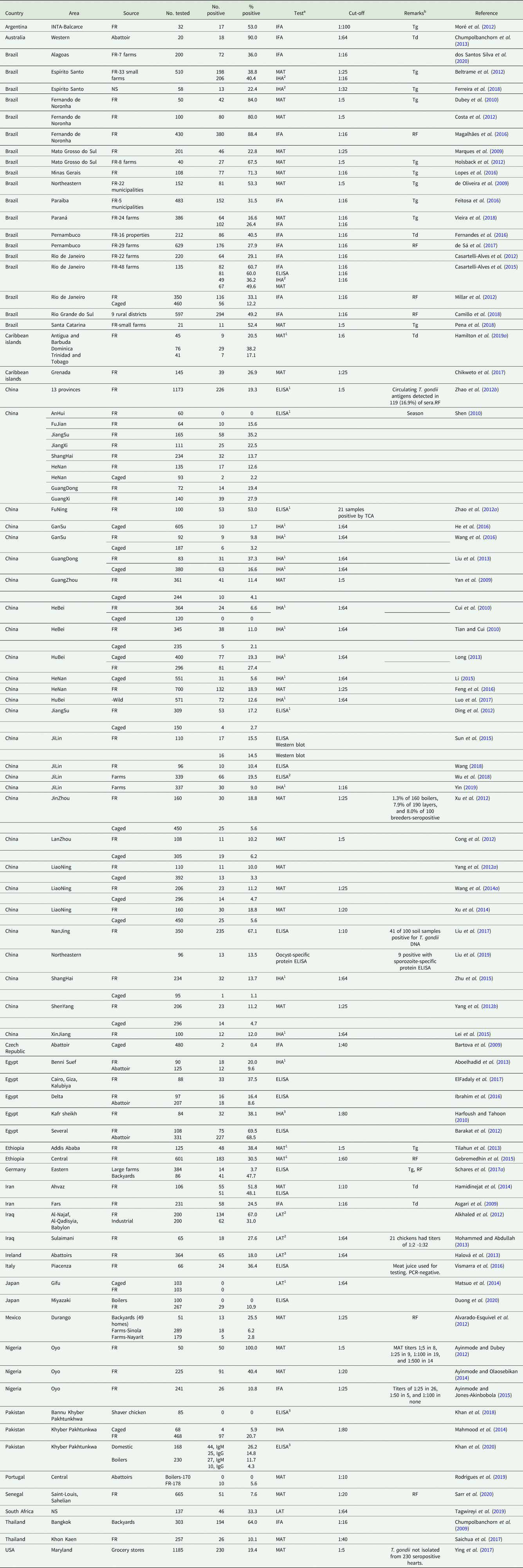
a ELISA = enzyme-linked immunosorbent assay. Unless stated otherwise, ELISA = ELISA in-house. 1ELISA (R&B Scientific, USA); 2ELISA (Military Veterinary Institute, Chinese Academy of Military Medical Sciences, Changchun, Jilin Province, China); 3(Bio-ELISA toxo-IgM and IgG kits (Biokit, S.S., Barcelona, Spain).
IFA = Indirect fluorescent antibody test.
IHA = Indirect hemagglutination antibody test. 1IHA (Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Science, Lanzhou, Gansu Province, China); 2Immuno-HAI Toxo, Wama Diagnostics, São Paulo, Brazil); 3IHA (SERFIB, France)
LAT = Latex agglutination test. 1Toxocheck-MT, Eiken Chemical, Tokyo, Japan; (1) Toxo-HAI Fumouze Diagnostics, Le Malesherbes, Levallois Perret, France; 2Toxoplasmosis Latex Test (Plasmatec, United Kingdom); 3Toxoreagent RST701, Mast Group, United Kingdom.
MAT = Modified agglutination test (Dubey and Desmonts, Reference Dubey and Desmonts1987). 1MAT (Toxo-Screen DA®, Biomerieux, Marcy l'Etiole, France). This is the same test as MAT.
b RF = risk factors, Td = T. gondii DNA detected. Tg = viable T. gondii isolated. NS = not stated.
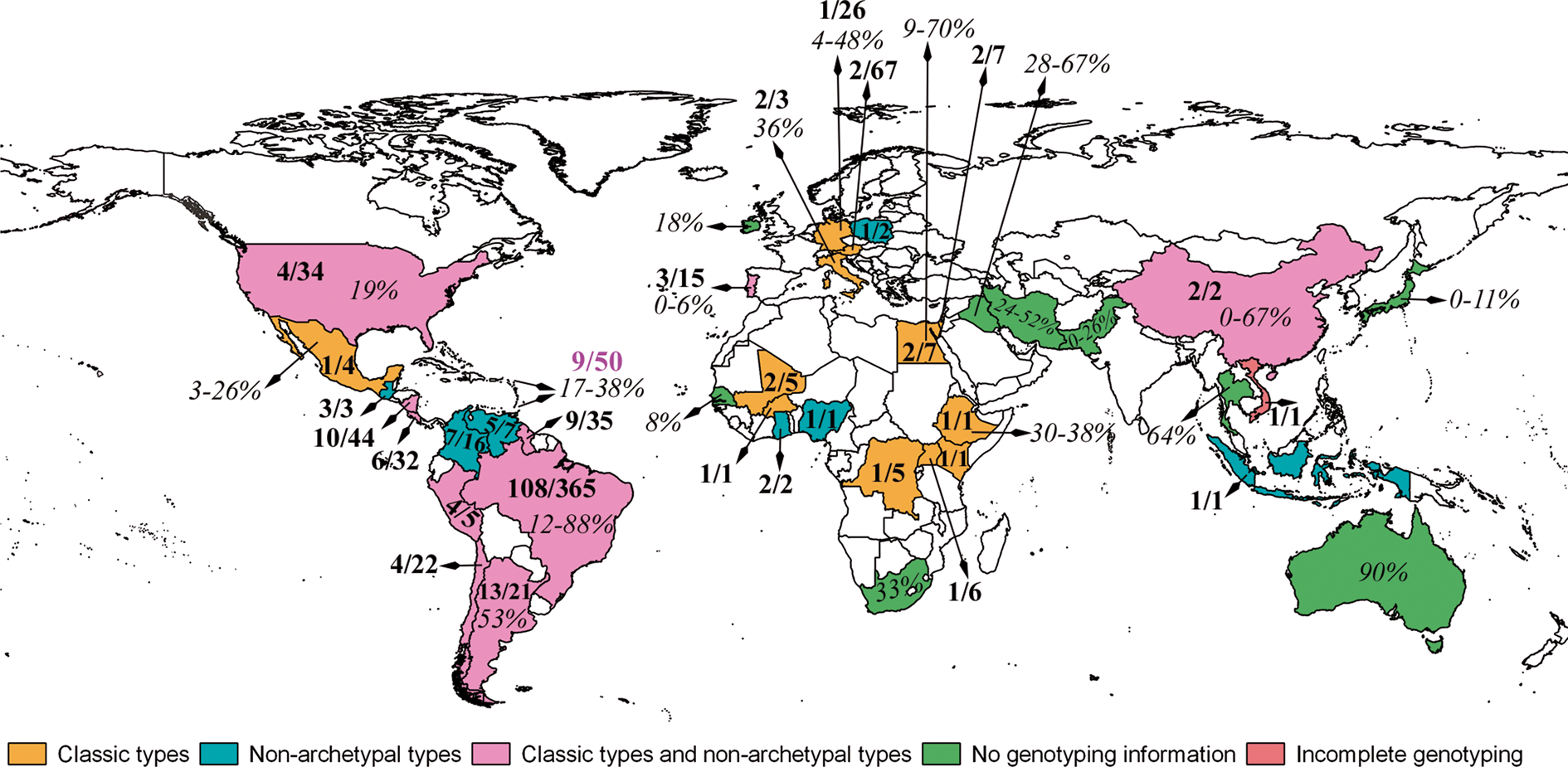
Fig. 1. Worldwide distribution of T. gondii infections in chickens. Numbers in bold are the number of T. gondii genotypes/number of viable isolates. Seroprevalences are given as %.
Results of different reports in Table 1 are difficult to compare because several serological tests with different cut-off values were used. Among those serological tests, the MAT was most commonly used (discussed later).
Several enzyme-linked immunosorbent assay (ELISA), indirect fluorescent antibody (IFA), and indirect haemagglutination (IHA) tests were used to detect T. gondii antibodies in chicken sera. Of these, the IHA is generally considered insensitive (Dubey, Reference Dubey2010a), but the results vary with the cut-off used, and the stability of reagents. In a study from Brazil, of 510 sera tested by MAT and IHA, the seropositivity was 40.4% by IHA (cut-off 1:16), compared with 38.8% by MAT (1:25) but different cut-off values were compared (Beltrame et al., Reference Beltrame, Pena, Ton, Lino, Gennari, Dubey and Pereira2012). In another study from Brazil, four serological tests were compared: of 135 sera from chickens tested, T. gondii antibodies were detected in 67 (49.6%) by MAT (1:16), in 82 (60.7%) by IFA (1:16), in 49 (36.2%) by IHA (1:16) and in 81 (60.0%) by ELISA (Casartelli-Alves et al., Reference Casartelli-Alves, Boechat, Macedo-Couto, Ferreira, Nicolau, Neves, Millar, Vicente, Oliveira, Muniz, Bonna, Amendoeira, Silva, Langoni, Schubach and Menezes2014) indicating variation among different tests.
The IHA test was used almost exclusively in surveys in China (Table 1); data on validation of this test in naturally infected chickens are not available. In the surveys that used IHA for T. gondii antibodies in chickens in China, the results of different studies are comparable because the same test kit and the same cut-off (1:64) were employed (Dong et al., Reference Dong, Su, Lu, Wang, Liu, Jian and Yang2018).
Different antigens, including recombinant antigens and total lysate antigens (TLAs), and total soluble antigens (TSAs), have been employed in ELISA (Sun et al., Reference Sun, Wang, Li, Wei and Liu2015). Similar results were obtained by using GRA1, GRA7, TSA and western blotting (Sun et al., Reference Sun, Wang, Li, Wei and Liu2015). Comparable results were obtained by MAT (51.8%, 1:16) and an ELISA (48.1%) on 106 chicken sera in Iran (Hamidinejat et al., Reference Hamidinejat, Nabavi, Mayahi, Ghourbanpoor, Pourmehdi Borojeni, Norollahi Fard and Shokrollahi2014), and between IFA (17.2%, 1:16) and ELISA (21.2%) in Brazil (Millar et al., Reference Millar, Alves, Teixeira, Vicente, Menezes, Sobreiro, de Almeida Pereira and Amendoeira2012). Several immunoreactive proteins were identified in sera of experimentally infected chickens; these may be useful for use in western blots for confirmation of results obtained with other tests (Wen et al., Reference Wen, Zou, Huang, Wen, Tselmeg and Liu2019).
Parasitologic investigation
Isolation of viable T. gondii: Results of isolation of viable T. gondii and genotyping of each isolate are summarized in Tables 2 and 3. Most isolations were made from FR chickens in Brazil.
Table 2. Isolation and genetic characterization of viable T. gondii from feral chickens by bioassay in mice.
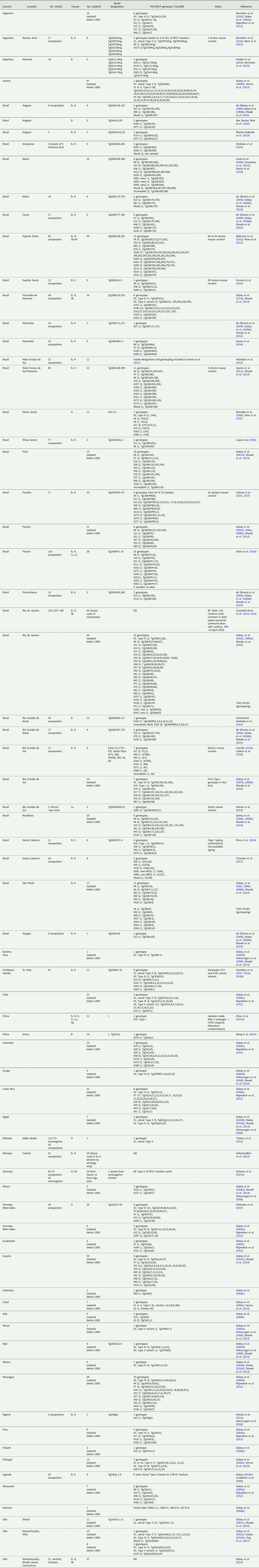
ND, no data; B, brain; H, heart; Li, liver; Lu, lung; Sk, skeletal muscle
Table 3. Distribution of PCR-RFLP (ToxoDB) T. gondii genotypes from chickens from different continents/countries; data were based on genotyping from viable T. gondii isolates.

Detection of T. gondii DNA: Results are summarized in Table 4. Results varied among different studies depending on the source of chickens, PCR method used, type and the amount of tissue tested. A study from Iran reported T. gondii DNA in tissues of 27 of 29 seropositive chickens (Asgari et al., Reference Asgari, Motazedian, Esmaeelzadeh, Kalantari and Hatam2009). Usually, it is difficult to get good quality DNA from naturally infected tissues for genotyping; however, Zou et al. (Reference Zou, Nie, Zhang, Zou, Zhu and Cong2017) successfully genotyped four isolates as ToxoDB genotype #9. Given that PCR is known to be sensitive to contamination, it is often difficult to assess the PCR results without obtaining live parasites. A study from Argentina (Pardini et al., Reference Pardini, Moré, Rudzinski, Gos, Campero, Meyer, Bernstein, Unzaga and Venturini2016; Bernstein et al., Reference Bernstein, Pardini, Moré, Unzaga, Su and Venturini2018) also had success in isolating good quality DNA from the brains of two naturally infected chickens from Argentina; one sample was ToxoDB genotype #19, the other was #286 based on 10 PCR-RFLP markers (Table 4).
Table 4. Toxoplasma gondii DNA in tissues of chickens.

B, brain; H, heart; Li, liver; Lu, lung; Sk, skeletal muscle, NS = Not stated
Histopathology and immunohistochemistry: The T. gondii burden in tissues of asymptomatic chickens is low (Dubey, Reference Dubey2010a). Thus, the chances of detection of the parasite by histopathology and immunohistochemistry are low (Casartelli-Alves et al., Reference Casartelli-Alves, Boechat, Macedo-Couto, Ferreira, Nicolau, Neves, Millar, Vicente, Oliveira, Muniz, Bonna, Amendoeira, Silva, Langoni, Schubach and Menezes2014; Ibrahim et al., Reference Ibrahim, Abdel-Ghaffar, Osman, El-Shourbagy, Nishikawa and Khattab2016). In a study from Brazil, serologic results, bioassay and histopathology results were compared. Histological sections of the brain, heart and thigh muscle were examined microscopically and after immunohistochemical staining (IHC) reactivity with T. gondii antibodies. Toxoplasma gondii was detected in tissues of eight (5.9%) of naturally infected chickens, in hearts of five and in brains of three. Only tissue cysts were found, and they were not associated with lesions (Casartelli-Alves et al., Reference Casartelli-Alves, Boechat, Macedo-Couto, Ferreira, Nicolau, Neves, Millar, Vicente, Oliveira, Muniz, Bonna, Amendoeira, Silva, Langoni, Schubach and Menezes2014). In a study from Egypt, blood and brains of 304 chickens were tested for T. gondii infection; 34 (11.8%) of blood sera were seropositive by ELISA and T. gondii was detected histologically in sections of formalin preserved brains of 21 (6.9%) (Ibrahim et al., Reference Ibrahim, Abdel-Ghaffar, Osman, El-Shourbagy, Nishikawa and Khattab2016). The finding of T. gondii in Giemsa stained smears of livers (38.4%), kidney (20.5%) and spleen (12.8%) of 39 seropositive but asymptomatic chickens in another study from Egypt is an overestimation of infection (Mohammed and Abudullah, Reference Mohammed and Abdullah2013); the illustrations provided indicate that artefacts were diagnosed as T. gondii (J.P.D. own opinion).
Validation of MAT serologic results with the isolation of viable T. gondii
Among the serological tests, the MAT was most commonly used in the studies in the last two decades. As stated earlier, a unique opportunity became available to evaluate the efficiency of detection of T. gondii in naturally exposed chickens (Dubey et al., Reference Dubey, Laurin and Kwowk2016). In that study, 2066 FR chickens from Argentina (Dubey et al., Reference Dubey, Venturini, Venturini, Piscopo, Graham, Dahl, Sreekumar, Vianna and Lehmann2003e; Dubey et al., Reference Dubey, Marcet and Lehmann2005g), Austria (Dubey et al., Reference Dubey, Edelhofer, Marcet, Vianna, Kwok and Lehmann2005b), Brazil (Dubey et al., Reference Dubey, Graham, Blackston, Lehmann, Gennari, Ragozo, Nishi, Shen, Kwok, Hill and Thulliez2002, Reference Dubey, Graham, da Silva, Lehmann and Bahia-Oliveira2003a; da Silva et al., Reference da Silva, Bahia-Oliveira, Shen, Kwok, Lehman and Dubey2003; Dubey et al., Reference Dubey, Navarro, Graham, Dahl, Freire, Prudencio, Sreekumar, Vianna and Lehmann2003d; Dubey et al., Reference Dubey, Gennari, Labruna, Camargo, Vianna, Marcet and Lehmann2006a; Dubey et al., Reference Dubey, Rajendran, Costa, Ferreira, Kwok, Qu, Su, Marvulo, Alves, Mota and Silva2010), Chile (Dubey et al., Reference Dubey, Patitucci, Su, Sundar, Kwok and Shen2006b), Colombia (Dubey et al., Reference Dubey, Gomez-Marin, Bedoya, Lora, Vianna, Hill, Kwok, Shen, Marcet and Lehmann2005c), Congo (Dubey et al., Reference Dubey, Karhemere, Dahl, Sreekumar, Diabaté, Dabiré, Vianna, Kwok and Lehmann2005d), Costa Rica (Dubey et al., Reference Dubey, Su, Oliveira, Morales, Bolaños, Sundar, Kwok and Shen2006c), Egypt (Dubey et al., Reference Dubey, Graham, Dahl, Hilali, El-Ghaysh, Sreekumar, Kwok, Shen and Lehmann2003b), Grenada (Dubey et al., Reference Dubey, Bhaiyat, de Allie, Macpherson, Sharma, Sreekumar, Vianna, Shen, Kwok, Miska, Hill and Lehmann2005a), Israel (Dubey et al., Reference Dubey, Salant, Sreekumar, Dahl, Vianna, Shen, Kwok, Spira, Hamburger and Lehmann2004c), Italy (Dubey et al., Reference Dubey, Huong, Lawson, Subekti, Tassi, Cabaj, Sundar, Velmurugan, Kwok and Su2008a), Mexico (Dubey et al., Reference Dubey, Morales and Lehmann2004b), Nicaragua (Dubey et al., Reference Dubey, Sundar, Pineda, Kyvsgaard, Luna, Rimbaud, Oliveira, Kwok, Qi and Su2006d), Peru (Dubey et al., Reference Dubey, Levy, Sreekumar, Kwok, Shen, Dahl, Thulliez and Lehmann2004a), Poland (Dubey et al., Reference Dubey, Huong, Lawson, Subekti, Tassi, Cabaj, Sundar, Velmurugan, Kwok and Su2008a), Portugal (Dubey et al., Reference Dubey, Vianna, Sousa, Canada, Meireles, da Costa, Marcet, Lehmann, Dardé and Thulliez2006e), Sri Lanka (Dubey et al., Reference Dubey, Rajapakse, Ekanayake, Sreekumar and Lehmann2005h), USA (Dubey et al., Reference Dubey, Graham, Dahl, Sreekumar, Lehmann, Davis and Morishita2003c; Dubey et al., Reference Dubey, Webb, Sundar, Velmurugan, Bandini, Kwok and Su2007c), Venezuela (Dubey et al., Reference Dubey, Lenhart, Castillo, Alvarez, Marcet, Sreekumar and Lehmann2005e) were serologically tested by MAT and chicken hearts were bioassayed for the isolation of viable T. gondii (Dubey et al., Reference Dubey, Laurin and Kwowk2016). These chickens would have been exposed to many pathogens, including protozoans Eimeria species, Cryptosporidium species, Sarcocystis species, Neospora caninum, various helminthic and bacterial infections that may react with T. gondii. Thus, there was a chance to study cross-reactivity against other pathogens. Needless to say that these studies were very expensive to conduct with respect to money, time and resources. All chickens were bioassayed, irrespective of serological status. In many instances, seronegative chicken hearts were pooled and fed to cats and the feces of cats were tested for excretion of T. gondii oocysts; cats excrete oocysts even after ingesting few T. gondii (Dubey, Reference Dubey2010a). All serological results were done by one operator, minimizing procedure variability.
Viable T. gondii was isolated from 528 of 2066 chickens by bioassay in mice (Dubey et al., Reference Dubey, Laurin and Kwowk2016). The isolation rate of T. gondii generally increased with the MAT titer. It is noteworthy that viable T. gondii was isolated from six of 1025 chickens with MAT titer of <1:5 (considered seronegative). Likely, these chickens had not yet seroconverted or there was a prozone (the lower dilutions are negative, but higher dilutions are positive). The isolation rates with different titers in increasing order were 15.2% of 105 at a titer of 1:5, 11.4% of 79 at a titer of 1:10, 42.9% of 98 at a titer of 1:20 and 59.9% of 759 chickens at titers of 1:40 or higher (Dubey et al., Reference Dubey, Laurin and Kwowk2016). This result suggests that the higher the titer, the higher the parasite tissue load in chickens.
Additionally, hearts pooled from 1028 chickens were bioassayed in 29 cats. It was noteworthy that the 23 cats fed hearts pooled from 802 seronegative (MAT < 1:5) chickens did not excrete T. gondii oocysts, thus supporting the specificity of the test (Dubey et al., Reference Dubey, Laurin and Kwowk2016). Cats are highly sensitive to T. gondii infection after ingestion of T. gondii stages. Experimentally, cats orally inoculated with single bradyzoites (freed from tissue cysts) excreted millions of oocysts (Dubey, Reference Dubey2001). Cats can consume more than 200–500 g of tissues in a matter of 3–4 days and excrete oocysts in feces that can be easily detected by microscopic examination of feces. Thus, it was assumed that a cat would have excreted oocysts if any of the 802 hearts fed to cats were infected with T. gondii (Dubey et al., Reference Dubey, Laurin and Kwowk2016).
Comparison of serology, PCR techniques, and bioassay for the detection of T. gondii
An extensive study was conducted to determine the efficacy of 3 serological tests (MAT, IFAT, ELISA), magnetic-capture (MC) real-time PCR (RT PCR), and T. gondii burden in brain, heart, drumstick in chickens (Schares et al. Reference Schares, Koethe, Bangoura, Geuthner, Randau, Ludewig, Maksimov, Sens, Bärwald, Conraths, Villena, Aubert, Opsteegh and van der Giessen2018). Two PCR methods (conventional RT PCR, and on acidic pepsin digests [PD-RT PCR]) were used to detect DNA. Antibodies to T. gondii were determined using blood serum and meat juice. The following conclusions were drawn: (i) substantial agreement was found between the mouse bioassay and MC-RT PCR or the mouse bioassay and conventional PD-RT PCR. (ii) The PD-RT PCR was more sensitive than MC-RT PCR. (iii) The organ tested affected the diagnostic sensitivity of MC-RT PCR;100 times higher parasite burdens were found in brain and heart tissues than pectoral muscles, thigh or drumstick muscles. (iv) using sera of naturally exposed chickens, diagnostic sensitivities of ELISA, IFAT and MAT were: 87.5%, 87.5% and 65.2%, respectively, and diagnostic specificities of 86.2%, 82.8% and 100%, respectively. Testing of meat juice by three serological tests revealed that the MAT with meat juice from pectoral muscles was less consistent than those of ELISA and IFAT and the MAT performed similar to ELISA and IFAT when applied to test meat juice samples collected from heart, thigh or drumstick musculature (Schares et al., Reference Schares, Koethe, Bangoura, Geuthner, Randau, Ludewig, Maksimov, Sens, Bärwald, Conraths, Villena, Aubert, Opsteegh and van der Giessen2018).
Genetic diversity of viable T. gondii isolates
Genotypes of T. gondii from chickens in each publication are summarized in Table 2, and by continent in Table 3 and Fig 1. Viable T. gondii parasites were isolated from most geographical regions, including Africa, Europe, Caribbean, Central America and South America. Data from Asia are very limited (Tables 2 and 3). Overall, genotype distribution follows the global patterns recognized previously (Shwab et al., Reference Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014), with ToxoDB genotypes #1 and #3 (collectively known as Type II), and genotype #2 (known as Type III) being dominant in Africa and Europe (Table 3). In the Caribbean region, genotypes #2 and #13 were frequently identified, and diverse genotypes were also present. In Central America, genotype #7 is common in chickens, as well as the presence of many unique genotypes. Toxoplasma gondii isolates are highly diverse and there is no clear dominance of any genotypes in South America. Of the 471, T. gondii samples analysed in South America, 365 were from Brazil, from which 108 genotypes were identified (Supplementary Tables S1, S2; Supplementary Fig.1).
Clinical infections
Chickens are considered resistant to T. gondii and hence there are only rare reports of clinical toxoplasmosis in chickens (Dubey, Reference Dubey2010a). An outbreak of clinical toxoplasmosis was reported on an avian farm from Brazil that had 47 FR chickens (Vielmo et al., Reference Vielmo, Pena, Panziera, Bianchi, De Lorenzo, Oliveira, Alves, Gennari, Pavarini, de Barros and Driemeier2019). Of these, 13 adult chickens were sick and nine died. The birds had apathy and diarrhea. Four of these nine chickens were examined at necropsy. The affected chickens were in poor body condition. Microscopically, necrosis and inflammation were noted in several tissues, including air sacs, myocardium, brain, kidney, lungs, liver, small intestine and spleen, tissue cysts or tachyzoites were identified in lesions. Viable T. gondii was isolated from tissues of two chickens by bioassay in mice. PCR-RFLP genotyping revealed a unique ToxoDB genotype, designated #280 and the results were confirmed by microsatellite typing. Antibodies to T. gondii were detected in the serum of one dead chicken and sera of four other chickens; the MAT titers were 10, 320 and 2560 (three chickens).
Epidemiology and use of sentinel chickens
In a study in China, T. gondii DNA was found in 41 of 100 soil samples on chicken farms, indicating the presence of oocysts (Liu et al., Reference Liu, He, Han, Zhang, Li, Wang, Xu, Yan and Li2017). Serological results using oocyst-based protein ELISA indicated that chickens acquired infection by ingesting oocysts (Liu et al., Reference Liu, Wang, El-Ashram and Liu2019). Follow up of T. gondii infection in sentinel chickens can provide valuable information concerning the epidemiology of toxoplasmosis on farms. The results of two studies in Argentina and the USA are summarized here.
Moré et al. (Reference Moré, Maksimov, Pardini, Herrmann, Bacigalupe, Maksimov, Basso, Conraths, Schares and Venturini2012) studied T. gondii infection in 202, one-week-old sentinel chickens placed on 10 chicken farms in Argentina. The chickens were bled 68 or 74 days later; 13 chickens developed T. gondii antibodies in the IFA test (1:100 in eight and 1:200 in five); however, attempts to isolate viable T. gondii were not successful by bioassay in mice inoculated with tissues of any of the 13 seropositive chickens.
An experiment in the USA was initiated to study the epidemiology of T. gondii transmission on three pig farms in three New England states that had a high prevalence of T. gondii infection (Dubey, Reference Dubey2010a). Toxoplasma gondii seronegative, sentinel chickens were placed on three (30 each) swine farms in November 2003. Chickens were bled monthly and their sera were tested for T. gondii antibodies by MAT (cut-off 1:25). Chickens that seroconverted were euthanized on the farm and their tissues were bioassayed in mice, cats or both. Over the course of the experiment (7 months), 31 of 71 chickens seroconverted (MAT 1:100 or higher); three chickens seroconverted after 1 month, eight chickens after 2 months, five chickens after 3 months, two chickens after 4 months, one chicken after 5 months, and seven chickens after 6 months. Tissues of 26 seropositive chickens were bioassayed in both cats and mice; viable T. gondii was isolated, by bioassay in mice, from hearts (whole) of all 26 chickens, brains (whole) of three chickens and leg muscles (25 g) of 11 chickens; 21 of 26 cats fed 250 g of leg muscle from seropositive chickens excreted T. gondii oocysts. Results confirmed earlier findings that indicated low T. gondii burden in poultry skeletal muscle and heart being the tissue of choice for isolation of viable parasites (Dubey, Reference Dubey2010b).
The number of mice that became infected with T. gondii was higher when inoculated with heart tissue vs the brain and leg muscles; of 130 mice used for bioassay, 5 (3.8%), 28 (21.5%) and 115 (88.4%) mice became infected with T. gondii after inoculation with brain, leg muscle and heart, respectively. Of the 27 cats fed leg muscles from 27 seropositive chickens, 23 excreted T. gondii oocysts. The two cats fed tissues of 40 seronegative chickens did not excrete oocysts. As stated earlier, in another investigation, viable T. gondii was isolated from 26 chickens, hearts of all 26 and legs of only three (Schares et al., Reference Schares, Bangoura, Randau, Goroll, Ludewig, Maksimov, Matzkeit, Sens, Bärwald, Conraths, Opsteegh and van der Giessen2017a). Thus, the heart is confirmed once more as the organ of choice for isolating viable T. gondii in chickens.
Little is known of the dynamics of T. gondii in chickens under natural conditions. While feeding from the ground provides exposure to T. gondii oocysts, the USA study was performed during the winter months. It is not clear how chickens became infected with T. gondii during the winter months. Winters in New England states are harsh, and the ground is frozen; thus, chickens are unlikely to ingest oocysts on the ground from the previous year. It is more likely that the grain fed to these chickens was contaminated with oocysts excreted by cats on the farm. It is interesting to note, that among the three farms studied, no chickens were infected on one farm, a few were infected on the second farm, and all chickens were infected on the third farm, indicating that risk factors differed among these three farms (Dubey, Reference Dubey2010a).
Experimental infections
Clinical and diagnosis
Chickens inoculated intravenously with T. gondii tachyzoites generally remained asymptomatic, irrespective of the dose (Chumpolbanchorn et al., Reference Chumpolbanchorn, Anankeatikul, Ratanasak, Wiengcharoen, Thompson and Sukthana2009; Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Daugschies and Bangoura2014; Schares et al., Reference Schares, Bangoura, Randau, Goroll, Ludewig, Maksimov, Matzkeit, Sens, Bärwald, Conraths, Opsteegh and van der Giessen2017a,Reference Schares, Herrmann, Maksimov, Matzkeit, Conraths, Moré, Preisinger and Weigendb). Chickens orally inoculated with oocysts can develop diarrhoea, and the effect may be neurogenic rather than the destruction of enterocytes (Bonapaz et al., Reference Bonapaz, Hermes-Uliana, Santos, da Silva, Araújo and Sant'Ana2010; Braga et al., Reference Braga, Silva, Sant'Ana and Araújo2011).
Chickens inoculated with T. gondii seroconverted as early as 4 days post-inoculation (p.i.), but more commonly between 10 and 21 days p.i. (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Daugschies and Bangoura2014; Wang et al., Reference Wang, Zhao, Wang, Xie, Zhang, Yuan, Hassan, Liu, Xu, Yan, Song and Li2014b; Hiob et al., Reference Hiob, Koethe, Schares, Goroll, Daugschies and Bangoura2017; Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018). Antibody titers (IFA) persisted until euthanasia at 10 weeks p.i. (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Daugschies and Bangoura2014). In some chickens, antibodies declined to undetectable levels by 4 weeks p.i. (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Daugschies and Bangoura2014). Based on DNA detection, T. gondii burden was sparse and detectable in heart, retina, pancreas and drumstick of four of 12 chickens euthanized at 10 weeks p.i. (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Daugschies and Bangoura2014); clinical acute toxoplasmosis developed in 7–10 days old chickens inoculated intraperitoneally with large numbers (1–50 million) of five strains of T. gondii in China (Wang et al., Reference Wang, Zhao, Wang, Xie, Zhang, Yuan, Hassan, Liu, Xu, Yan, Song and Li2014b; Wang et al., Reference Wang, Zhao, Wang, Zhang, Shen, Hassan, Xie, Yan, Song, Xu and Li2015). Age was a factor in the pathogenesis of acute toxoplasmosis. Of the chickens infected at 7, 14, 21 and 28 days of age, only the chickens inoculated at 7-day old, died of acute toxoplasmosis, chickens inoculated at 14 days had mild signs, but no mortality and those inoculated on 21 and 28 days old chickens remained asymptomatic (Wang et al., Reference Wang, Han, Mu, Yuan, Zhang, He, Yang and Li2014a).
In an experiment from China, 30 chickens (35-day old) were inoculated intravenously with 4.3–10 million tachyzoites (Yan et al., Reference Yan, Yue, Yuan, Lin, He, Yin, Xu, Song and Zhu2010). The chickens were euthanized on 7, 14, 21, 28 and 35 days p.i. and their tissues were tested for parasite DNA and sera were evaluated by the MAT and IHA. This study provided valuable information concerning a commercial IHA kit marketed by Lanzhou Veterinary Research Institute, China; this IHA kit has been used extensively for T. gondii serological surveys in animals in China, including chickens (Table 1). The inoculated chickens remained asymptomatic. By MAT, antibodies (titer 1:160 or 1:640) peaked around 21 days p.i. and were present in low titers (1:10, 1:40, 1:40, 1:160) in four chickens killed on day 35 p.i. By IHA, peak titers (1:10, 1:10, 1:160, 1:160) were detected in four chickens euthanized on day 21 p.i. and the titers had dropped to 1:5 or <1:5 on days 28 or 35 p.i. Thus, the results obtained by IHA were inconsistent and mostly below the cut-off of 1:64 used in various surveys (Table 1). Toxoplasma gondii DNA was extracted from several tissues of these chickens; the heart and lungs were more consistently infected (Yan et al., Reference Yan, Yue, Yuan, Lin, He, Yin, Xu, Song and Zhu2010).
Valuable serological diagnostic information was obtained from chickens orally inoculated with different strains of T. gondii oocysts (Hotop et al., Reference Hotop, Buschtöns, Bangoura, Zöller, Koethe, Spekker-Bosker, Hotop, Tenter, Däubener, Straubinger and Groß2014; Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Maksimov, Daugschies and Bangoura2019). Unusual and inconsistent results were obtained by ELISA using recombinant proteins: by rGRA1- and rGRA9-based ELISA, high levels of antibodies were detected only between days 7 and 10 p.i., dropped to undetectable levels and mildly increased between 42 and 63 days p.i. By the rGRA6-ELISA, the initial peak was between days 14 and 21 p.i. and antibodies persisted until day 63 p.i. By rSAG1-ELISA, antibodies peaked between days 14 and 21 and then were not detectable (Hotop et al., Reference Hotop, Buschtöns, Bangoura, Zöller, Koethe, Spekker-Bosker, Hotop, Tenter, Däubener, Straubinger and Groß2014). By contrast, chickens developed MAT antibodies between 4 and 7 days p.i., and antibodies persisted until the termination of the experiment on day 84 p.i. (Hotop et al., Reference Hotop, Buschtöns, Bangoura, Zöller, Koethe, Spekker-Bosker, Hotop, Tenter, Däubener, Straubinger and Groß2014). Seroconversion and the rate of parasitization varied among chickens inoculated with different strains (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Maksimov, Daugschies and Bangoura2019). Antibody titers as high as 1: 512 000 were detected in chickens by IFA. Parasite DNA was detectable in many tissues, but the heart was the most persistently infected tissue (Geuthner et al., Reference Geuthner, Koethe, Ludewig, Pott, Schares, Maksimov, Daugschies and Bangoura2019).
An extensive investigation was undertaken by a Japanese study concerning the use of recombinant and nascent proteins for the serodiagnosis of toxoplasmosis in chickens (Appiah-Kwarteng et al., Reference Appiah-Kwarteng, Saito, Toda, Kitoh, Nishikawa, Adenyo, Kayang, Owusu, Ohya, Inoue-Murayama, Kawahara, Nagamune and Takashima2019). Chickens (n = 21) were inoculated with 10 or 100 million tachyzoites intravenously (three strains, RH, CTG, PLK) or intravenously and intraperitoneally (ME49) and were tested for antibodies and parasites. The chickens remained asymptomatic and were bled on days 7, 14, 21 and 28 p.i. Antibodies were assessed by the commercial latex agglutination test (LAT, Eiken Kagaku, Japan), western blot and ELISA using nascent and recombinant proteins (SAG1, GRA7). By LAT, antibodies were detected only on day 7 p.i., but not afterwards; this is a noteworthy observation because LAT has been used to detect T. gondii antibodies in many species of animals, including chickens. By ELISA, antibodies peaked day 7 or 14 p.i. and were detectable until the termination of the experiment on day 28 p.i.; the antibody response was stronger and more consistent by using nascent proteins then recombinant E. coli-derived recombinant proteins (Appiah-Kwarteng et al., Reference Appiah-Kwarteng, Saito, Toda, Kitoh, Nishikawa, Adenyo, Kayang, Owusu, Ohya, Inoue-Murayama, Kawahara, Nagamune and Takashima2019). The results were confirmed by western blotting using crude T. gondii lysate. To locate the T. gondii in tissues, 7-day old chickens were inoculated with a fluorescent-tagged protein T. gondii strain, TgCatJpGi1/TaJ/GRA Red. Fluorescent-tagged parasite images were visible in the hearts, lungs, livers and brains of the three of seven chickens that died 7 days p.i., but not in tissues of chickens that survived the acute phase; results were confirmed by bioassay in mice. These observations are in marked contrast to the findings that viable parasites are easily isolated from the hearts of chronically infected mice (see isolation Table 2). A luciferase-linked GRA8-ELISA was developed in Japan for the detection of T. gondii antibodies in sera of experimentally infected chickens (Duong et al., Reference Duong, Appiah-Kwarteng, Takashima, Aye, Nagayasu and Yoshida2020).
Serotyping
Toxoplasma gondii strains are genetically diverse but strains from Europe, North America and Africa fall into two main lineages (Types II, III). The information is based on the characterization of parasite DNA extracted from live T. gondii isolated from infected hosts. Only limited information is available based on the serotyping of samples from humans (Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018). Information on a large panel of 101 synthetic peptides was obtained on sera from chickens intravenously inoculated with tachyzoites of three strains of T. gondii (RH-Type I, Me49-Type II and NED-Type III). The authors concluded that by using selected peptides, it was possible to serotype strains up to 9 weeks p.i. (Maksimov et al., Reference Maksimov, Basso, Zerweck, Schutkowski, Reimer, Maksimov, Conraths and Schares2018).
Effect of breed/strain of chickens, T. gondii genotype on toxoplasmosis in chickens
Breed or strain of chicken can influence the course of T. gondii infection (Schares et al., Reference Schares, Herrmann, Maksimov, Matzkeit, Conraths, Moré, Preisinger and Weigend2017b). One-day-old chickens of two lines (white layer, line A, brown layer, line B) inoculated intravenously with tachyzoites of a cross line of Type II/Type III T. gondii strain; higher mortality was observed in line A chickens (Schares et al., Reference Schares, Herrmann, Maksimov, Matzkeit, Conraths, Moré, Preisinger and Weigend2017b). Serum antibody levels assessed by SAG1-ELISA at 31 days p.i. were higher in chickens of line B. By using RT-PCR and 25 mg aliquots of brain and lungs, T. gondii burden was higher in the brain than in lungs.
Concurrent infections
Coccidial infections are common in chickens and Eimeria tenella is the most pathogenic among the seven or more species of Eimeria that infect chickens. Chickens are also commonly infected with T. gondii. Therefore, the effect of concurrent infections of these two coccidians was investigated. Results indicated that E. tenella and T. gondii could interact in vivo and in vitro (Zou et al., Reference Zou, Huang, Yin, Ding, Liu, Wang, Chen and Suo2011; Tang et al., Reference Tang, Yin, Qin, Tao, Suo, Liu and Suo2016; Hiob et al., Reference Hiob, Koethe, Schares, Goroll, Daugschies and Bangoura2017; Zhang et al., Reference Zhang, Thabet, Hiob, Zheng, Daugschies and Bangoura2018). By using moderate doses of E. tenella and T. gondii, an adverse or synergistic effect was not demonstrated in dually infected chickens (Hiob et al., Reference Hiob, Koethe, Schares, Goroll, Daugschies and Bangoura2017).
Conclusions
Here, we summarized seroprevalence, clinical disease, epidemiology and genetic diversity of T. gondii strains isolated from chickens worldwide for the past decade. It is obvious that T. gondii infection in FR chickens is common and chickens are excellent sentinels to monitor T. gondii contamination in the environment. Chickens, in general, are resistant to T. gondii infection. Genetic studies revealed low genetic diversity in Europe, Asia, Africa and the USA, intermediate diversity in Caribbean Islands, but higher diversity of T. gondii from FR chickens in South America. Controlled experiments using chickens on farms in Argentina and the USA revealed the dynamic of infection and distribution of the parasites in these animals. It will be good to have similar studies from other parts of the world and to conduct genetic analyses of T. gondii isolates from sentinel chickens over a period time, which can shed light on the dynamics of T. gondii infections on farms, to reveal single or multiple exposures T. gondii strains.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001134.
Acknowledgements
This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE was managed by ORAU under DOE contract number DE-SC 0014664. All opinions expressed in this paper were the authors’ and did not necessarily reflect the policies and views of USDA, ARS, DOE or ORAU/ORISE. S. M. Gennari received a fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq).
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.








