Randomized controlled trials have established the efficacy of periconceptional folic acid use to prevent neural tube defects (NTD), serious birth defects of the brain and spine( Reference Czeizel and Dudas 1 , 2 ). In 1992, the US Public Health Service (USPHS) recommended that all women capable of becoming pregnant consume at least 400 μg of folic acid daily to reduce the risk of having a pregnancy affected by an NTD( 3 ). In 1993, the US Food and Drug Administration (FDA) reviewed the evidence available regarding folic acid and the prevention of NTD, and determined that fortifying the food supply was the most effective way to provide folic acid to all women of childbearing age( 4 ). FDA mandated that by 1998 all enriched cereal grain products (ECGP) be fortified with 140 μg of folic acid per 100 g of flour( 5 ). In 1998, the Institute of Medicine (IOM) recommended that all women of childbearing potential consume at least 400 μg of folic acid from fortified foods and supplements daily, in addition to a diet high in folate-rich foods. The IOM set the tolerable upper intake level (UL) of usual folic acid intake from fortified foods and supplements at 1000 μg/d to ensure that usual folic acid intake would not mask signs of anaemia and thereby delay diagnosis of vitamin B12 deficiency, which could lead to irreversible neurological damage( 6 ). Since fortification, it is estimated that the prevalence of NTD in the USA has dropped by 27 %( 7 ).
The USA was not alone in implementing a folic acid fortification policy. Canada mandated in 1998 that all ECGP be fortified with 150 μg of folic acid per 100 g of flour( 8 ). Costa Rica implemented wheat and corn flour fortification at 180 μg/100 g as well as fortification of milk and rice in 1998( Reference Chen and Rivera 9 ). In 2000, Chile decided to fortify bread flour at 220 μg/100 g( Reference Hertrampf and Cortes 10 ). South Africa implemented mandatory fortification at 150 μg/100 g for wheat flour and 180 μg/100 g for maize flour in 2003( Reference Sayed, Bourne and Pattinson 11 ). In 2009, Australia began mandatory fortification at 200 μg/100 g of the final flour product( 12 ). Voluntarily fortified foods are available in many other countries, including the UK, Ireland and New Zealand.
The methods available for modelling the impact of folic acid fortification have improved since the US fortification policy was established. With the availability of improved data on the folic acid content of foods( Reference Raper, Perloff and Ingwersen 13 ), the use of measurement error models( Reference Carriquiry 14 ) and better reporting of supplement intake, more accurate estimates of usual folic acid intake are now possible. The modelling exercise reported in the present paper provides a framework to evaluate different options of folic acid fortification and supplementation levels for countries worldwide.
Methods
National Health and Nutrition Examination Survey
The National Health and Nutrition Examination Survey (NHANES) is a complex, multistage survey designed to be representative of the US civilian, non-institutionalized population. It includes extensive, in-person interviews and a physical examination. NHANES is conducted in two-year cycles by the National Center for Health Statistics (NCHS) at the US Centers for Disease Control and Prevention (CDC). We used data from the 2003–2004, 2005–2006 and 2007–2008 cycles. All study participants provided informed consent, and the study protocol received approval from the NCHS Ethical Review Board. The methods of the survey are described in detail elsewhere( 15 – 17 ). The nutrition data used for the present analysis were collected in a household interview and two 24 h dietary recalls. The first recall was administered in person at the time of the physical examination and the second recall was administered 3 to 10 d later via telephone.
The NHANES data sets contain nutrient information for every food reported by each participant in the two 24 h dietary recalls, including folic acid. A daily total intake for each nutrient for each participant is also included in the data sets, which is a sum of the nutrient amounts from each individual food reported by that participant on that day. Food codes were assigned to each reported food, which allowed us to identify ready-to-eat cereals (RTEC; codes available upon request). RTEC and meal replacement products (MRP) are the only food sources of folic acid other than ECGP in the USA; MRP contribute only a minor amount to total folic acid intake in our data. For each participant, the amount of folic acid consumed from RTEC and MRP was defined as the sum of the folic acid intake from all reported foods identified as RTEC and MRP, respectively, consumed in the previous 24 h. We then estimated the amount of folic acid consumed from ECGP by subtracting the folic acid intake consumed from RTEC and MRP from the total folic acid intake from all foods consumed.
We obtained data on dietary supplement use during the household interview from questions in which the participants were asked whether they took a dietary supplement in the last 30 d and if so, how often they took the supplement and to show the supplement package to the interviewer. NCHS then obtained product information regarding the ingredients, amounts and serving sizes of the reported supplements and made these data available. We estimated the average daily amount of folic acid consumed from supplements by multiplying the number of days the participant reported taking the supplement over the past 30 d times the folic acid dose in one serving times the daily number he/she reported consuming (e.g. 2 tablets), divided by the amount in one serving (serving size, e.g. 2 tablets), times 30: i.e. (days taken in the last 30 d × nutrient dose per serving × number of supplements taken) ÷ (serving size × 30). We estimated the total folic acid consumed daily from all sources for each participant by adding the daily average folic acid from supplements to the total folic acid from foods on each day of the dietary recall. We considered anyone who reported consuming a supplement containing folic acid within the past 30 d to be a supplement user.
There were 16 665 adults aged 19 years and older in the 2003–2008 NHANES. We excluded 2312 participants based on: being pregnant at the time of the survey (n 658), not attending the mobile examination centre (n 728), having incomplete data for the day 1 (n 835) or day 2 (n 76) dietary recall, or having missing data on supplement use (n 15). Among the 5424 women of childbearing age (15 to 44 years; partial overlap with adult age range), exclusions were made for pregnancy (n 730), not attending the mobile examination centre (n 151), unreliable day 1 (n 251) or day 2 (n 15) dietary recall data, and missing dietary supplement (n 5) data.
Simulation scenarios
We varied the level of folic acid used in mandatory ECGP fortification, voluntary RTEC fortification and supplements (Table 1) for each individual in the populations we considered, regardless of their sources of folic acid consumed. If an individual did not consume folic acid from one of the sources then the modelled level of that source for that individual would not impact their estimate of folic acid intake. We included the daily intake of folic acid from MRP in the estimates of individuals’ total daily folic acid intake, but left the level unchanged in the models. We assessed five levels for mandatory ECGP fortification: multiples of 70 μg, from 70 μg to 350 μg/100 g flour, which include the levels considered during the development of the current folic acid fortification legislation in the USA( 4 ). We assessed four levels for the upper limit of folic acid per serving of RTEC: 400 μg (the current limit( 18 )), 200 μg, 100 μg (a limit considered by FDA in 1993( 4 )) and no folic acid in RTEC. We did not model any changes to RTEC that did not contain folic acid (i.e. those cereals without folic acid in the current scenario had no folic acid in any of the modelled scenarios). We also assessed the most common amount of folic acid in dietary supplements at 400 μg, 200 μg and no folic acid in supplements. In total, we evaluated thirty-five scenarios.
Table 1 Folic acid fortification and supplementation scenarios considered in simulation analyses
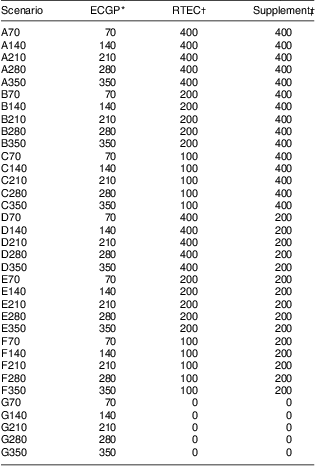
| Scenario | ECGP* | RTEC† | Supplement‡ |
| A70 | 70 | 400 | 400 |
| A140 | 140 | 400 | 400 |
| A210 | 210 | 400 | 400 |
| A280 | 280 | 400 | 400 |
| A350 | 350 | 400 | 400 |
| B70 | 70 | 200 | 400 |
| B140 | 140 | 200 | 400 |
| B210 | 210 | 200 | 400 |
| B280 | 280 | 200 | 400 |
| B350 | 350 | 200 | 400 |
| C70 | 70 | 100 | 400 |
| C140 | 140 | 100 | 400 |
| C210 | 210 | 100 | 400 |
| C280 | 280 | 100 | 400 |
| C350 | 350 | 100 | 400 |
| D70 | 70 | 400 | 200 |
| D140 | 140 | 400 | 200 |
| D210 | 210 | 400 | 200 |
| D280 | 280 | 400 | 200 |
| D350 | 350 | 400 | 200 |
| E70 | 70 | 200 | 200 |
| E140 | 140 | 200 | 200 |
| E210 | 210 | 200 | 200 |
| E280 | 280 | 200 | 200 |
| E350 | 350 | 200 | 200 |
| F70 | 70 | 100 | 200 |
| F140 | 140 | 100 | 200 |
| F210 | 210 | 100 | 200 |
| F280 | 280 | 100 | 200 |
| F350 | 350 | 100 | 200 |
| G70 | 70 | 0 | 0 |
| G140 | 140 | 0 | 0 |
| G210 | 210 | 0 | 0 |
| G280 | 280 | 0 | 0 |
| G350 | 350 | 0 | 0 |
ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal.
*ECGP, level of mandatory folic acid fortification (μg/100 g).
†RTEC, maximum allowable amount of folic acid (μg/serving).
‡Supplement, usual amount of folic acid in a dietary supplement (μg/dose).
Modelling sources of folic acid
Enriched cereal grain products
For each participant, we varied the amount of folic acid consumed from ECGP by multiplying his/her daily ECGP folic acid intake under the existing US scenario of 140 μg/100 g flour by a factor. The factor was calculated by dividing the ECGP fortification level for each scenario (70, 140, 210, 280, 350 μg) by 140 μg/100 g (factor = 0·5, 1·0, 1·5, 2·0 and 2·5, respectively).
Ready-to-eat cereals
Folic acid fortification of RTEC in the USA is not required, and the amount of folic acid added to RTEC varies by brand; many are not fortified with folic acid. The current US regulation regarding the upper limit for voluntary fortification is based on serving size( 18 ), which also varies among brands. We used the US Department of Agriculture's Food and Nutrient Database for Dietary Studies (FNDDS), which contains information on the weight of one cup of each brand of RTEC and the folic acid content of 100 g of each brand of RTEC( 19 , 20 ). The FNDDS database was linked to NHANES data using a standard food code. Information on serving size was not available in NHANES or FNDDS; we obtained serving size information from manufacturer and other websites (available upon request).
Using the serving size in cups of each RTEC, the weight (g) of a cup of each RTEC and the folic acid content of 100 g of each RTEC, we calculated the folic acid content of one serving of each RTEC.
If the per-serving content of the RTEC, which could be up to 400 μg/serving, was below the upper limit of the modelled scenario (0, 100, 200 μg/serving), the folic acid intake from that RTEC was left unchanged. If the per-serving content of the RTEC was above the upper limit of a given modelled scenario, the per-serving content was forced to equal that limit. In the latter case, amounts for folic acid consumed from that RTEC in the modelled scenarios were adjusted by multiplying by a proportional factor calculated as (modelled per-serving content) ÷ (actual per-serving content).
Supplements
The most commonly observed amount included in the dietary supplements reported by the participants in NHANES 2003–2008 was 400 μg of folic acid (data not shown). We considered scenarios in which the amount of folic acid in supplements equalling 400 μg was changed to 200 μg. In addition, we considered scenarios in which there was no folic acid intake from supplements.
Analysis
For descriptive analyses we used the SUDAAN statistical software package version 10 (2008; RTI, Research Triangle Park, NC, USA) to account for the complex survey design. We used the Software for Intake Distribution Estimation (PC-SIDE) version 1·02 (2003; Iowa State University, Ames, IA, USA)( Reference Nusser, Carriquiry and Dodd 21 ) to estimate the distribution of folic acid intake accounting for both between- and within-person variability in intake. We used the estimated distribution to quantify the median and interquartile range (IQR) of daily usual folic acid intake, the percentage of women of childbearing age who achieved the recommended daily usual folic acid intake of 400 μg or more and the percentage of adults with daily usual folic acid intake exceeding the UL of 1000 μg( 6 ) for each of the scenarios listed in Table 1. We accounted for the NHANES complex sampling design in PC-SIDE using ninety-two jack-knife replicate weights based on the 6-year combined sampling weights for the first day of dietary recall provided for NHANES, which account for differential non-response and non-coverage and adjust for designed oversampling. We adjusted for day of week of the dietary recall and interview method (in person or by telephone). We also conducted stratified analyses by race and ethnicity. We used χ 2 tests to identify differences in estimated intake from the current scenario.
Results
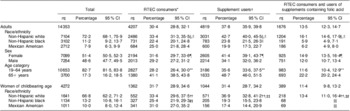
We analysed data on 14 353 adults and 4272 women of childbearing age (Table 2). Approximately a third of adults reported consuming RTEC containing folic acid (30·4 %, 95 % CI 28·8 %, 32·1 %); RTEC consumption was more common among non-Hispanic white (NHW) adults, women and older adults (Table 2). Among women of childbearing age, 31·7 % reported consumption of RTEC containing folic acid (95 % CI 28·9 %, 34·6 %), and consumption was more often reported by NHW and Mexican Americans (MA) than non-Hispanic blacks (NHB). Use of supplements containing folic acid was reported by 37·8 % of adults (95 % CI 35·9 %, 39·8 %) and 31·4 % of women of childbearing age (95 % CI 28·7 %, 34·2 %). For adults and women of childbearing age, significantly more NHW reported use of supplements containing folic acid than did NHB or MA (Table 2). Use of supplements containing folic acid was more common among female adults and older adults compared with male adults and younger adults, respectively.
Table 2 Population characteristics and prevalence of reported consumption of RTEC and supplements containing folic acid among US adults (age 19 years and older) and non-pregnant women of childbearing age (15 to 44 years), National Health and Nutrition Examination Survey, 2003–2008
| Total | RTEC consumers* | Supplement users† | RTEC consumers and users of supplements containing folic acid | |||||||||
| n‡ | Percentage | 95 % CI | n‡ | Percentage | 95 % CI | n‡ | Percentage | 95 % CI | n‡ | Percentage | 95 % CI | |
| Adults | 14 353 | 4207 | 30·4 | 28·8, 32·1 | 4819 | 37·8 | 35·9, 39·8 | 1676 | 13·5 | 12·3, 14·7 | ||
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 7104 | 72·2 | 68·1, 75·9 | 2486 | 33·4 | 31·3, 35·5§,∥ | 3031 | 42·7 | 40·0, 45·5§,∥ | 1204 | 16·1 | 14·6, 17·9§,∥ |
| Non-Hispanic black | 3102 | 11·2 | 9·2, 13·7 | 731 | 22·4 | 20·1, 24·8 | 783 | 23·8 | 21·5, 26·3∥ | 191 | 5·9 | 4·9, 7·1 |
| Mexican American | 2712 | 7·9 | 6·3, 9·9 | 684 | 25·0 | 21·8, 28·4 | 600 | 19·9 | 17·3, 22·7 | 186 | 6·2 | 4·9, 8·0 |
| Sex | ||||||||||||
| Female | 7099 | 51·4 | 50·5, 52·3 | 2194 | 31·6 | 29·7, 33·6¶ | 2605 | 41·4 | 39·1, 43·7¶ | 925 | 14·9 | 13·5, 16·4¶ |
| Male | 7254 | 48·6 | 47·7, 49·5 | 2013 | 29·2 | 27·2, 31·2 | 2214 | 34·1 | 32·0, 36·2 | 751 | 12·0 | 10·7, 13·4 |
| Age category | ||||||||||||
| 19–64 years | 10 653 | 82·7 | 81·5, 83·8 | 2827 | 28·2 | 26·4, 30·0** | 3186 | 35·6 | 33·6, 37·5** | 983 | 11·6 | 10·4, 12·9** |
| 65+ years | 3700 | 17·3 | 16·2, 18·5 | 1380 | 41·1 | 38·5, 43·8 | 1633 | 48·7 | 46·0, 51·5 | 693 | 22·2 | 20·2, 24·4 |
| Women of childbearing age | 4272 | 1362 | 31·7 | 28·9, 34·6 | 1044 | 31·4 | 28·7, 34·2 | 389 | 11·4 | 9·8, 13·2 | ||
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 1641 | 66·8 | 62·2, 71·2 | 552 | 33·4 | 29·6, 37·5†† | 571 | 36·9 | 32·8, 41·2††,‡‡ | 218 | 13·4 | 11·0, 16·4††,‡‡ |
| Non-Hispanic black | 1134 | 13·2 | 10·8, 16·1 | 327 | 25·4 | 21·9, 29·3‡‡ | 205 | 19·3 | 15·5, 23·8 | 68 | §§ | |
| Mexican American | 1011 | 10·0 | 8·0, 12·4 | 341 | 31·0 | 27·0, 35·2 | 156 | 17·4 | 14·4, 20·9 | 69 | §§ | |
RTEC, ready-to-eat cereal.
All P values are from χ 2 tests.
*RTEC consumer defined as anyone who reported consumption of an RTEC containing folic acid on either of the two 24 h dietary recalls.
†Supplement user defined as anyone who reported consuming a supplement containing folic acid in the past 30 d.
‡Unweighted.
§Significantly different from non-Hispanic black adults (P < 0·05).
∥Significantly different from Mexican American adults (P < 0·05).
¶Significantly different from males (P < 0·05).
**Significantly different from older adults (P < 0·05).
††Significantly different from non-Hispanic black women of childbearing age (P < 0·05).
‡‡Significantly different from Mexican-American women of childbearing age (P < 0·05).
§§Df<12; estimates unstable.
Median intake
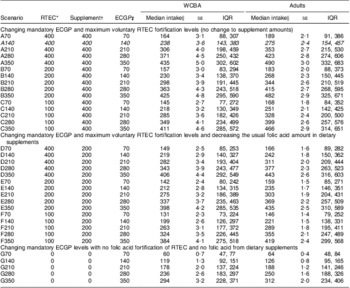
Under the current US scenario (A140) the median daily usual folic acid intake among women of childbearing age was 238 μg (IQR: 143 μg, 383 μg) and for US adults was 275 μg (IQR: 154 μg, 457 μg). As we increased the level of folic acid in ECGP within each scenario (A–G), the modelled median intake of folic acid also increased (Table 3). For example, under scenario A350, in which we increased the mandatory fortification level of ECGP to 350 μg/100 g and did not change the amount of folic acid in RTEC and supplements, the median usual intake of folic acid for women of childbearing age was estimated at 435 μg (IQR: 302 μg, 602 μg) and for adults at 490 μg (IQR: 332 μg, 683 μg). The only scenarios for which at least 50 % of women of childbearing age had daily usual folic acid intake above the recommended 400 μg were those in which ECGP were fortified at 350 μg/100 g (A350, B350, C350 and D350).
Table 3 Median usual daily intake of folic acid among US non-pregnant women of childbearing age (15 to 44 years) and all adults (age 19 years and older) under various fortification and supplementation scenarios, National Health and Nutrition Examination Survey, 2003–2008
| WCBA | Adults | ||||||||
| Scenario | RTEC* | Supplement† | ECGP‡ | Median intake§ | se | IQR | Median intake§ | se | IQR |
| Changing mandatory ECGP and maximum voluntary RTEC fortification levels (no change to supplement amounts) | |||||||||
| A70 | 400 | 400 | 70 | 164 | 3·1 | 88, 307 | 189 | 2·1 | 91, 386 |
| A140 | 400 | 400 | 140 | 238 | 3·6 | 143, 383 | 275 | 2·4 | 154, 457 |
| A210 | 400 | 400 | 210 | 306 | 4·0 | 198, 459 | 353 | 2·7 | 215, 530 |
| A280 | 400 | 400 | 280 | 371 | 4·5 | 250, 432 | 423 | 2·8 | 274, 606 |
| A350 | 400 | 400 | 350 | 435 | 5·0 | 302, 602 | 490 | 3·0 | 332, 683 |
| B70 | 200 | 400 | 70 | 157 | 3·0 | 83, 294 | 183 | 2·0 | 88, 373 |
| B140 | 200 | 400 | 140 | 230 | 3·4 | 138, 370 | 268 | 2·3 | 150, 445 |
| B210 | 200 | 400 | 210 | 298 | 3·9 | 191, 445 | 344 | 2·6 | 210, 519 |
| B280 | 200 | 400 | 280 | 363 | 4·3 | 243, 518 | 415 | 2·7 | 268, 595 |
| B350 | 200 | 400 | 350 | 425 | 4·8 | 295, 590 | 482 | 2·9 | 325, 671 |
| C70 | 100 | 400 | 70 | 145 | 2·7 | 77, 272 | 168 | 1·8 | 84, 352 |
| C140 | 100 | 400 | 140 | 218 | 3·2 | 130, 349 | 251 | 2·1 | 142, 425 |
| C210 | 100 | 400 | 210 | 285 | 3·6 | 182, 426 | 328 | 2·4 | 200, 500 |
| C280 | 100 | 400 | 280 | 349 | 4·1 | 234, 499 | 399 | 2·6 | 257, 576 |
| C350 | 100 | 400 | 350 | 411 | 4·6 | 285, 572 | 466 | 2·9 | 314, 651 |
| Changing mandatory ECGP and maximum voluntary RTEC fortification levels and decreasing the usual folic acid amount in dietary supplements | |||||||||
| D70 | 400 | 200 | 70 | 149 | 2·5 | 85, 253 | 166 | 1·6 | 89, 282 |
| D140 | 400 | 200 | 140 | 219 | 2·9 | 140, 327 | 242 | 1·8 | 150, 362 |
| D210 | 400 | 200 | 210 | 282 | 3·4 | 193, 404 | 311 | 2·0 | 209, 444 |
| D280 | 400 | 200 | 280 | 343 | 3·9 | 243, 477 | 377 | 2·3 | 263, 523 |
| D350 | 400 | 200 | 350 | 406 | 4·4 | 292, 549 | 443 | 2·6 | 316, 603 |
| E70 | 200 | 200 | 70 | 142 | 2·4 | 80, 242 | 159 | 1·5 | 85, 271 |
| E140 | 200 | 200 | 140 | 212 | 2·8 | 134, 315 | 235 | 1·7 | 146, 351 |
| E210 | 200 | 200 | 210 | 275 | 3·2 | 186, 389 | 303 | 1·9 | 204, 431 |
| E280 | 200 | 200 | 280 | 337 | 3·7 | 235, 463 | 369 | 2·2 | 257, 509 |
| E350 | 200 | 200 | 350 | 398 | 4·2 | 285, 535 | 435 | 2·5 | 310, 589 |
| F70 | 100 | 200 | 70 | 131 | 2·3 | 73, 224 | 146 | 1·4 | 79, 252 |
| F140 | 100 | 200 | 140 | 199 | 2·6 | 126, 297 | 221 | 1·5 | 138, 331 |
| F210 | 100 | 200 | 210 | 263 | 3·1 | 177, 372 | 289 | 1·8 | 195, 411 |
| F280 | 100 | 200 | 280 | 324 | 3·5 | 226, 445 | 355 | 2·1 | 247, 489 |
| F350 | 100 | 200 | 350 | 384 | 4·1 | 275, 518 | 419 | 2·4 | 299, 568 |
| Changing mandatory ECGP levels with no folic acid fortification of RTEC and no folic acid from dietary supplements | |||||||||
| G70 | 0 | 0 | 70 | 60 | 0·7 | 47, 77 | 64 | 0·4 | 48, 84 |
| G140 | 0 | 0 | 140 | 119 | 1·3 | 92, 151 | 126 | 0·8 | 95, 165 |
| G210 | 0 | 0 | 210 | 178 | 2·0 | 137, 224 | 188 | 1·2 | 141, 246 |
| G280 | 0 | 0 | 280 | 236 | 2·6 | 183, 297 | 250 | 1·6 | 188, 326 |
| G350 | 0 | 0 | 350 | 294 | 3·2 | 228, 371 | 312 | 2·0 | 234, 406 |
ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal; WCBA, women of childbearing age; IQR, interquartile range.
Italics indicates current fortification/supplementation scenario; see Table 1 for details of others.
*RTEC levels indicate the maximum allowable voluntary fortification of RTEC under the given scenario (μg/serving).
†Supplement amounts indicate the usual amount of folic acid in dietary supplements (μg/dose).
‡ECGP levels indicate the mandatory fortification of ECGP under the given scenario (μg/100 g).
§μg/d.
The median usual daily folic acid intake was influenced more by changing the ECGP fortification level than by changing the typical amount in supplements or the maximum allowable level of RTEC fortification (Fig. 1; Supplementary materials). As an example, halving the ECGP fortification level relative to the current scenario (A140 to A70) resulted in an approximately 30 % reduction in the median daily usual folic acid intake (from 238 μg to 164 μg) among women of childbearing age, the target population for this public health intervention. By contrast, when we halved both the typical supplement dose and the maximum allowable fortification level for RTEC (E140) the median daily usual folic acid intake among this group was reduced by only 11 % (from 238 μg to 212 μg).
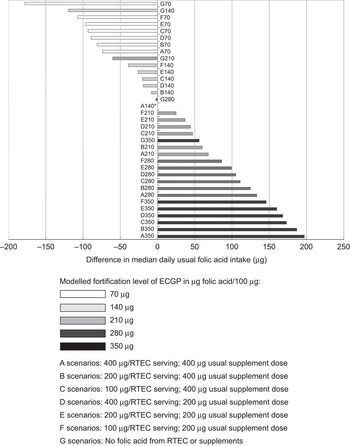
Fig. 1 Difference in median daily usual folic acid intake (μg) from current scenario (A140*), US non-pregnant women of childbearing age (15 to 44 years), National Health and Nutrition Examination Survey, 2003–2008 (ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal)
Impact on percentage of adults with intake above the tolerable upper intake level
The proportion of adults with usual folic acid intake above the UL was influenced most by changing the typical folic acid supplement dose, followed by the level of ECGP fortification, and least by the maximum allowable level of RTEC fortification (Fig. 2; Supplementary materials). For example, compared with the current scenario, halving the typical supplement dose (D140) resulted in a 63 % decrease in the percentage of adults with usual daily folic acid intake above the UL (from 2·4 % to 0·9 %). Halving the ECGP fortification level (A70) decreased the percentage of adults with usual intake exceeding the UL by 21 % (from 2·4 % to 1·9 %). When the maximum allowable level of RTEC fortification was halved (B140) the percentage of adults with daily usual folic acid intake exceeding the UL decreased by 17 % (from 2·4 % to 2·0 %).
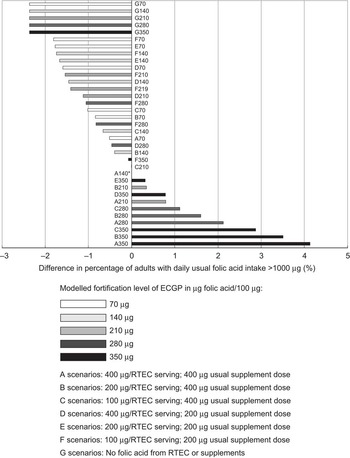
Fig. 2 Difference from current scenario (A140*) in percentage of US adults (age 19 years and older) with daily usual folic acid intake >1000 μg, National Health and Nutrition Examination Survey, 2003–2008 (ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal)
Distribution of intake
The current population distribution of daily usual folic acid intake in the USA is such that 23·0 % of women of childbearing age achieve the recommended level of intake and 2·4 % of adults have intake that exceeds the UL (Supplementary materials). Under the A scenarios, in which only the level of folic acid in ECGP was modified, these two levels decreased or increased together.
As shown in Fig. 3 (and Supplementary materials), at least two sources of folic acid need to be changed, relative to the current US scenario, to increase the percentage of women of childbearing age who achieve the recommended intake, while lowering or not changing the percentage of all adults with intake exceeding the UL. To illustrate, scenario C210, in which the level of folic acid in ECGP was raised to 210 μg/100 g and in RTEC was limited to 100 μg/serving, resulted in no change in the percentage of adults with intake exceeding the UL and a statistically significant 5·5 % absolute increase in women of childbearing age who achieve the recommended intake. The results from the simulation of scenarios B210, D210, D280, E280, E350, F280 and F350 also displayed this pattern.
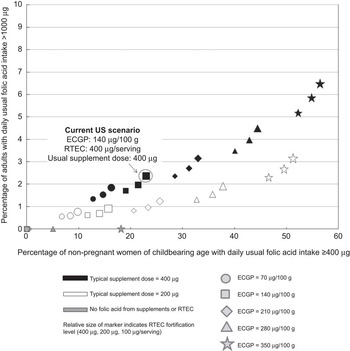
Fig. 3 Percentage of US non-pregnant women of childbearing age (15 to 44 years) with usual daily folic acid intake ≥400 μg and percentage of adults (age 19 years and older) with usual folic acid intake >1000 μg under different fortification and supplementation scenarios, National Health and Nutrition Examination Survey, 2003–2008 (ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal)
Our results in Fig. 2 (and Supplementary materials) suggest that if ECGP served as the only source of folic acid (G scenarios) no adults would have intake exceeding the UL. At ECGP fortification levels less than 210 μg/100 g (G70 and G140) no women of childbearing age would consume the recommended amount of folic acid. Under the highest level of ECGP fortification modelled, 350 μg/100 g (G350), significantly fewer women of childbearing age met the recommended intake than under the current US scenario.
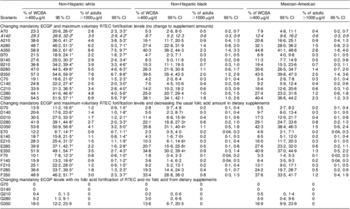
The distribution of daily usual folic acid intake was markedly different among US subpopulations of different races and ethnicities (Table 4). Under the current fortification and supplementation scenario, 29·3 % (95 % CI 26·6 %, 32·0 %) of NHW women of childbearing age achieve the recommended intake of folic acid intake, while only 8·7 % (95 % CI 5·2 %, 12·3 %) of NHB and 12·4 % (95 % CI 8·6 %, 16·2 %) of MA women of childbearing age achieve that amount of intake. NHW adults were almost six times more likely to have intake exceeding the UL under the current scenario compared with NHW and MA adults (3·5 %, 0·6 % and 0·6 %, for NHW, NHB and MA, respectively).
Table 4 Percentage of US non-pregnant women of childbearing age (15 to 44 years) with usual daily intake of folic acid ≥400 μg and percentage of adults (age 19 years and older) with usual daily intake of folic acid >1000 μg under various fortification and supplementation scenarios, National Health and Nutrition Examination Survey, 2003–2008, by race and ethnicity
| Non-Hispanic white | Non-Hispanic black | Mexican-American | ||||||||||
| Scenario | % of WCBA ≥400 μg/d | 95 % CI | % of adults >1000 μg/d | 95 % CI | % of WCBA ≥400 μg/d | 95 % CI | % of adults >1000 μg/d | 95 % CI | % of WCBA ≥400 μg/d | 95 % CI | % of adults >1000 μg/d | 95 % CI |
| Changing mandatory ECGP and maximum voluntary RTEC fortification levels (no change to supplement amounts) | ||||||||||||
| A70 | 23·3 | 20·6, 26·0* | 2·8 | 2·3, 3·3* | 5·3 | 2·6, 8·0 | 0·5 | 0·2, 0·7 | 7·9 | 4·8, 11·1 | 0·4 | 0·2, 0·7 |
| A140 | 29·3 | 26·6, 32·0* | 3·5 | 2·9, 4·2* | 8·7 | 5·2, 12·3 | 0·6 | 0·3, 0·9 | 12·4 | 8·6, 16·2 | 0·6 | 0·3, 0·9 |
| A210 | 38·6 | 36·0, 41·2* | 4·6 | 3·8, 5·3* | 16·1 | 11·7, 20·5 | 0·9 | 0·4, 1·3 | 20·8 | 16·6, 25·1 | 0·9 | 0·4, 1·3 |
| A280 | 48·7 | 46·2, 51·3* | 6·2 | 5·3, 7·1* | 27·4 | 22·8, 31·9 | 1·4 | 0·8, 2·0 | 32·1 | 28·0, 36·2 | 1·5 | 0·8, 2·3 |
| A350 | 58·9 | 56·2, 61·6* | 8·6 | 7·5, 9·7* | 40·3 | 36·2, 44·3 | 2·3 | 1·4, 3·3 | 44·8 | 41·1, 48·6 | 2·8 | 1·6, 4·0 |
| B70 | 21·5 | 18·9, 24·1* | 2·2 | 1·8, 2·7* | 4·7 | 2·3, 7·1 | 0·3 | 0·1, 0·5 | 6·9 | 4·0, 9·8 | 0·3 | 0·1, 0·5 |
| B140 | 27·6 | 25·0, 30·3* | 2·9 | 2·4, 3·4* | 8·3 | 5·0, 11·6 | 0·5 | 0·2, 0·8 | 11·3 | 7·7, 14·9 | 0·5 | 0·2, 0·8 |
| B210 | 36·8 | 34·2, 39·4* | 3·9 | 3·3, 4·6* | 15·3 | 11·1, 19·5 | 0·7 | 0·3, 1·0 | 19·4 | 15·3, 23·6 | 0·7 | 0·3, 1·1 |
| B280 | 47·0 | 44·5, 49·6* | 5·5 | 4·6, 6·3* | 26·3 | 21·9, 30·8 | 1·1 | 0·6, 1·7 | 30·6 | 26·5, 34·7 | 1·4 | 0·7, 2·0 |
| B350 | 57·3 | 54·6, 59·9* | 7·8 | 6·7, 8·8* | 39·5 | 35·4, 43·5 | 2·0 | 1·2, 2·9 | 43·5 | 39·8, 47·3 | 2·5 | 1·4, 3·6 |
| C70 | 19·1 | 16·6, 21·6* | 1·9 | 1·5, 2·3* | 4·2 | 2·0, 6·4 | 0·3 | 0·1, 0·4 | 5·4 | 2·9, 7·8 | 0·3 | 0·1, 0·4 |
| C140 | 24·8 | 22·2, 27·4* | 2·5 | 2·0, 3·0* | 7·5 | 4·5, 10·6 | 0·4 | 0·2, 0·6 | 9·2 | 5·9, 12·4 | 0·4 | 0·2, 0·6 |
| C210 | 33·9 | 31·3, 36·5* | 3·4 | 2·8, 4·0* | 14·2 | 10·2, 18·2 | 0·6 | 0·3, 0·9 | 16·6 | 12·6, 20·6 | 0·6 | 0·3, 1·0 |
| C280 | 44·1 | 41·6, 46·6* | 4·8 | 4·0, 5·6* | 25·0 | 20·7, 29·4 | 1·0 | 0·5, 1·5 | 27·4 | 23·2, 31·6 | 1·2 | 0·6, 1·8 |
| C350 | 54·5 | 51·9, 57·1* | 6·9 | 5·9, 7·9* | 38·1 | 34·1, 42·1 | 1·8 | 1·0, 2·6 | 40·4 | 36·6, 44·2 | 2·3 | 1·2, 3·3 |
| Changing mandatory ECGP and maximum voluntary RTEC fortification levels and decreasing the usual folic acid amount in dietary supplements | ||||||||||||
| D70 | 13·9 | 11·2, 16·6* | 1·2 | 0·9, 1·5* | 2·8 | 0·7, 4·8 | 0·2 | 0·1, 0·4 | 5·5 | 2·7, 8·2 | 0·2 | 0·1, 0·4 |
| D140 | 20·6 | 17·6, 23·6* | 1·4 | 1·0, 1·8* | 5·5 | 2·3, 8·7† | 0·3 | 0·1, 0·4 | 9·7 | 6·0, 13·4 | 0·3 | 0·1, 0·5 |
| D210 | 30·5 | 27·5, 33·5* | 1·7 | 1·2, 2·1* | 11·4 | 6·8, 15·9† | 0·4 | 0·1, 0·6 | 17·3 | 12·9, 21·7 | 0·4 | 0·1, 0·8 |
| D280 | 41·9 | 39·1, 44·8* | 2·7 | 2·0, 3·3* | 22·1 | 16·8, 27·3† | 0·6 | 0·2, 1·0 | 29·1 | 24·7, 33·6 | 0·8 | 0·2, 1·3 |
| D350 | 53·8 | 50·9, 56·6* | 4·1 | 3·2, 5·0* | 35·8 | 31·1, 40·4† | 1·1 | 0·5, 1·8 | 42·3 | 38·4, 46·3 | 1·5 | 0·6, 2·4 |
| E70 | 12·2 | 9·7, 14·7* | 0·9 | 0·7, 1·1* | 2·3 | 0·5, 4·0 | 0·2 | 0·04, 0·3 | 4·6 | 2·1, 7·1 | 0·2 | 0·04, 0·3 |
| E140 | 18·7 | 15·8, 21·5* | 1·1 | 0·8, 1·4* | 4·3 | 1·6, 7·0† | 0·2 | 0·1, 0·3 | 8·5 | 5·1, 12·0 | 0·2 | 0·1, 0·4 |
| E210 | 28·3 | 25·4, 31·3* | 1·5 | 1·1, 1·9* | 10·3 | 6·1, 14·6† | 0·3 | 0·1, 0·4 | 16·0 | 11·7, 20·3 | 0·3 | 0·1, 0·5 |
| E280 | 39·9 | 37·1, 42·7* | 2·2 | 1·6, 2·8* | 20·7 | 15·6, 25·8† | 0·5 | 0·1, 0·8 | 27·6 | 23·2, 32·0 | 0·6 | 0·2, 1·1 |
| E350 | 51·9 | 49·1, 54·7* | 3·5 | 2·7, 4·3* | 34·8 | 30·2, 39·4† | 0·9 | 0·3, 1·4 | 40·9 | 37·0, 44·9 | 1·3 | 0·5, 2·2 |
| F70 | 10·1 | 7·8, 12·3* | 0·8 | 0·6, 1·0* | 1·8 | 0·4, 3·3 | 0·1 | 0·04, 0·2 | 3·3 | 1·4, 5·3 | 0·1 | 0·03, 0·3 |
| F140 | 15·9 | 13·2, 18·6* | 0·9 | 0·7, 1·2* | 3·8 | 1·4, 6·2 | 0·2 | 0·04, 0·3 | 6·4 | 3·5, 9·3 | 0·2 | 0·04, 0·3 |
| F210 | 25·1 | 22·2, 28·0* | 1·2 | 0·9, 1·5* | 9·2 | 5·2, 13·1 | 0·2 | 0·1, 0·4 | 13·1 | 9·0, 17·1 | 0·3 | 0·1, 0·5 |
| F280 | 36·6 | 33·7, 39·5* | 1·8 | 1·3, 2·3* | 19·3 | 14·4, 24·3 | 0·4 | 0·1, 0·7 | 24·2 | 19·7, 28·7 | 0·5 | 0·2, 0·9 |
| F350 | 48·9 | 46·2, 51·7* | 3·0 | 2·3, 3·7* | 33·4 | 28·8, 38·0 | 0·8 | 0·3, 1·3 | 37·6 | 33·5, 41·7 | 1·2 | 0·4, 1·9 |
| Changing mandatory ECGP levels with no folic acid fortification of RTEC and no folic acid from dietary supplements | ||||||||||||
| G70 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| G140 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| G210 | 0·4 | 0, 1·3 | 0 | 0·2 | 0, 0·6 | 0 | 0·5 | 0, 1·4 | 0 | |||
| G280 | 5·2 | 1·3, 9·1 | 0 | 3·0 | 0, 6·6 | 0 | 5·0 | 0·4, 9·6 | 0 | |||
| G350 | 18·0 | 12·2, 23·9 | 0 | 13·6 | 6·7, 20·5 | 0 | 16·9 | 9·9, 23·9 | 0 | |||
WCBA, women of childbearing age; ECGP, enriched cereal grain product; RTEC, ready-to-eat cereal.
Italics indicates current fortification/supplementation scenario; see Table 1 for details of others.
All P values are from χ 2 tests.
*Statistically significantly (P < 0·05) different from non-Hispanic blacks and Mexican Americans.
†Statistically significantly (P < 0·05) different from Mexican Americans.
The absolute change in the percentage of women of childbearing age who achieve the recommended intake, compared with the current scenario, was generally similar across scenarios for NHW, NHB, and MA. However, the relative percentage change was much higher for NHB and MA than for NHW. As an example, doubling the folic acid in ECGP (A280) increased the percentage of women of childbearing age with recommended intake by an absolute value of 19·4 %, 18·7 % and 19·7 %, and the relative percentage change was an increase of 66·2 %, 214·9 % and 158·9 %, for NHW, NHB and MA, respectively. In none of the scenarios examined were more than 50 % of NHB or MA women of childbearing age able to achieve the recommended intake. For all scenarios, the relative percentage change for the percentage of adults exceeding the UL was similar for the racial/ethnic groups at the lower ECGP levels and higher for NHB and MA compared with NHW at the higher ECGP levels.
Discussion
Our results indicate that the distribution of usual intake of folic acid intake among US adults is affected by the levels of both mandatory and voluntary fortification of foods and the typical amount of folic acid in supplements. Assuming the proportion of US adults who use supplements and consume RTEC with folic acid remains constant, modifying the level of folic acid in ECGP would have the greatest impact on increasing the percentage of women of childbearing age who meet the IOM recommendation. In contrast, modifying the typical dose of folic acid in dietary supplements would have the greatest impact on the percentage of adults with usual daily intake exceeding the UL. Taken together these results suggest that, in the USA, usual folic acid intake exceeding the UL is largely a function of the amount of folic acid in supplements. Conversely, more than one strategy is needed to meet the recommendations for folic acid intake among women of childbearing age. Although increasing the level of folic acid in ECGP increases overall intake, it is more effective if a large proportion of US women of childbearing age also consume supplements or RTEC with folic acid.
Our results add to those of modelling exercises prior to fortification( 4 , Reference Crane, Wilson and Cook 22 ). Direct comparison is difficult as we used updated data and focused on the percentage of women of childbearing age meeting the USPHS( 3 ), IOM( 6 ) and US Preventive Services Task Force recommendations( 23 ) for daily intake of at least 400 μg of folic acid, rather than overall folate intake. As in previous studies( 4 , Reference Crane, Wilson and Cook 22 ), we found that fortification of ECGP would increase total folic acid intake among all consumers across the entire distribution of intakes. Our new finding is that increasing the level of folic acid fortification in ECGP would not substantially increase the percentage of US adults with intake above the UL but could increase the percentage of women meeting recommended folic acid intake. We found that modifying the amount of folic acid in supplements and RTEC influences intake of consumers at the high end of the distribution rather than the low end. This is because individuals with high consumption of folic acid are also the most likely to consume RTEC and supplements( Reference Tinker, Cogswell and Devine 24 , Reference Yang, Cogswell and Hamner 25 ).
Our modelling exercise also illustrates the importance of considering subpopulations when assessing various folic acid fortification scenarios. While goals could be set at a national level, our results indicate that approaches targeted to specific racial/ethnic groups might be needed for subpopulations. For example, other modelling exercises have looked at the potential impact of fortifying corn masa flour with folic acid in an attempt to selectively increase intake among Mexican Americans( Reference Hamner, Mulinare and Cogswell 26 ).
Although current recommendations exist for the global fortification of flour with folic acid( 27 ), it is also recommended that countries consider the dietary patterns of their population( 28 ). Modelling usual intake under different fortification scenarios can provide valuable information in the development of fortification policies. Although we used six years of US nationally representative data on dietary intake, data of this level of sophistication are not necessary for using the principles of modelling to inform decisions regarding fortification in other countries. Modelling can be useful even for countries with only one primary source of folic acid, and for countries attempting to identify which product or products to fortify and at what level. For example, ECGP are relatively self-limiting in terms of the maximum amount that a typical person would eat in one day (e.g. an adult who consumed only ECGP fortified at 140 μg would have to consume more than 680 g (1·5 lb) of ECGP daily to exceed the UL of 1000 μg). However, other potential fortification vehicles, such as RTEC, could have greater potential to be consumed in quantities higher than intended. With no folic acid in RTEC or supplements (i.e. G scenarios), which approximates the current situation in the developing world, the highest level of fortification in our model (350 μg/100 g) resulted in 18 % of women of childbearing age consuming at least the recommended 400 μg/d with virtually no adults whose intake exceeded the UL. Therefore countries with no other sources of folic acid might decide to fortify at a higher level than the USA, such as was done in Chile, which chose to fortify ECGP at a level of 220 μg/100 g.
Strengths of our modelling exercise include the use of a large, nationally representative sample of US adults and the ability to examine differences by race and ethnicity due to oversampling. The addition of folic acid to the nutrient database and the ability to take into account day-to-day variation allowed us to make more precise estimates than were possible in the past. The estimates in the current report are subject to several limitations. Actual folic acid in foods might be higher or lower than that estimated in the nutrient database( Reference Raper, Perloff and Ingwersen 13 ). The amount of folic acid indicated on supplement labels could be an underestimate( 29 ). Dietary data are self-reported. It has been previously reported that the 24 h dietary recall used in NHANES underestimates energy intake by 11 %, but how this translates to estimates of micronutrient intake such as folic acid is unclear( 30 , Reference Moshfegh, Rhodes and Baer 31 ).
Our modelling assumes that the dietary patterns of the population that consumes products that contain folic acid would not change if the folic acid content of the product was changed. It is also probable that the number of products voluntarily fortified with folic acid will change over time. Therefore, the actual impact of any changes in fortification is difficult to predict.
Our results demonstrate the value of considering the contribution of all folic acid sources among population subgroups when assessing the implications of policies and initiatives to increase folic acid consumption. Public health efforts should continue to increase consumption of folic acid among women of childbearing potential and thereby reduce the prevalence of NTD.
Acknowledgements
The research was funded by the US Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors report no conflicts of interest. Each author contributed to the development of this work. S.C.T., M.E.C., H.C.H. and R.J.B. designed the research; S.C.T. analysed the data and performed statistical analysis; S.C.T., M.E.C., H.C.H. and R.J.B. wrote the paper. S.C.T. had primary responsibility for final content. The authors would like to gratefully acknowledge Alicia Carriquiry for her assistance in using PC-SIDE to estimate usual intake.
Supplementary materials
For Supplementary materials for this article, please visit http://dx.doi.org/10.1017/S1368980012000638









