Worldwide, bladder cancer is the most frequent malignant tumour of the urinary tract, with approximately 420 000 new cases each year with 4-fold higher incidence rates in men than in women( Reference Forman, Bray and Brewster 1 ). Although tobacco smoking is the major risk factor for the development of bladder cancer accounting for up to 30–50 % of cases( Reference Freedman, Silverman and Hollenbeck 2 ), hyperglycaemia and hyperinsulinaemia may play a role in initiation and progression.
Conditions characterised by long-term hyperinsulinaemia and hyperglycaemia, such as the metabolic syndrome and type 2 diabetes, have been identified as risk factors for bladder cancer incidence and mortality( Reference Esposito, Chiodini and Colao 3 – Reference Zhu, Zhang and Shen 7 ). In particular, a 10-year prospective study including more than a million people found significantly higher mortality for bladder cancer in men with blood glucose levels>6·9mmol/l, after adjusting for smoking, age and alcohol drinking( Reference Jee, Ohrr and Sull 4 ). Furthermore, poorly controlled glycaemia (glycated Hb (HbA1c)>53 mmol/mol or>7·0 %) in people with diabetes increased the risk of recurrence of specific types of bladder cancer compared with good glycaemic control (HbA1c<53 mmol/mol or <7·0 %)( Reference Hwang, Kim and Hwang 8 , Reference Tai, Chen and Huang 9 ). These data suggest that lifestyle factors promoting hyperglycaemia may play a role in bladder carcinogenesis. High glycaemic index (GI) and glycaemic load (GL) foods raise blood glucose levels even in the absence of diabetes, obesity and the metabolic syndrome. These foods increase blood glucose levels to a greater extent than the equivalent amount of carbohydrates consumed from low-GI foods( Reference Jenkins, Wolever and Taylor 10 ). High-GI diets have been associated with greater risk of cancer( Reference Barclay, Petocz and McMillan-Price 11 , Reference Choi, Giovannucci and Lee 12 ) including bladder cancer in North American populations( Reference George, Mayne and Leitzmann 13 , Reference Hu, La Vecchia and Augustin 14 ).
To provide further information on the association between dietary GI/GL and bladder cancer risk in a Mediterranean area, we examined data from an Italian case–control study.
Methods
Study subjects
Between 2003 and 2014, we conducted a case–control study on bladder cancer within an established Italian network of collaborating centres, including Aviano and Milan in northern Italy, and Naples and Catania in southern Italy( Reference Polesel, Bosetti and Di Maso 15 ).
Cases were 690 patients aged between 25 and 84 years (median age: 67 years) with incident bladder cancer diagnosis, admitted to major general hospitals in the catchment areas. Nearly all cases (n 642, 93·0 %) were confirmed by histological testing on tumour tissue specimen from biopsy or surgery and three additional cases were confirmed by cytology only. Patients with self-reported history of diabetes mellitus (n 112) may include diet modification to control hyperglycaemia; therefore, they were excluded from the present analysis, leaving 578 cases (median age: 67 years).
Controls were 690 cancer-free patients aged between 27 and 84 years admitted to the same network of hospitals as cases for a wide spectrum of acute conditions unrelated to tobacco smoking, alcohol consumption or long-term diet modification. Controls were frequency-matched to cases by study centre, sex and age (in 5-year groups). In all, twenty-five controls were excluded after enrolment because of inappropriate admission diagnosis. After excluding subjects with self-reported history of diabetes mellitus (n 57), the total number of eligible controls was 608 (median age: 66 years). Of these, 28·3 % were admitted for traumatic disorders, 22·5 % for non-traumatic orthopaedic disorders, 38·7 % for acute surgical conditions and 10·5 % for other various illnesses. Less than 5 % of cases and controls approached for interview during their hospital stay refused to participate for personal reasons. The power was adequate to detect 80 % risk difference for variables with a prevalence of at least 20 %, at the usual 95 % confidence limit. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and reported according to the STROBE guidelines( Reference von Elm, Altman and Egger 16 ). All procedures involving human subjects were approved by the Ethic Boards of each study centre (Centro di Riferimento Oncologico (CRO)-Aviano, University of Milan, National Cancer Institute Pascale). All study subjects signed an informed consent before interview.
Data collection
Trained interviewers administered a structured questionnaire to cases and controls during hospitalisation. It included information on age, education and other socio-demographic characteristics, anthropometric measures, selected lifestyle habits (e.g. tobacco smoking), personal medical history and family history of cancer. The presence of abdominal obesity was defined using the International Diabetes Federation cutoff points (waist circumference ≥94 cm for men and ≥80 cm for women). As the information on waist circumference could not be obtained for technical reasons in 135 cases and 173 controls, we considered the BMI≥30 kg/m2 as a proxy of abdominal obesity in patients missing waist circumference.
An interviewer-administered FFQ was used to assess dietary habits related to the 2 years preceding diagnosis/interview. This included seventy-eight foods, food groups or recipes (i.e. the most common ones in the Italian diet) structured into six sections: (i) bread, cereals and first courses; (ii) second courses (i.e. meat, fish and other main dishes); (iii) side dishes (i.e. vegetables); (iv) fruits; (v) desserts, sweets and soft drinks; (vi) milk, hot beverages and sweeteners. An additional section assessed the use of alcoholic beverages. Subjects were asked to indicate the average weekly frequency of consumption for each dietary item; intakes lower than once a week, but at least once a month, were coded as 0·5/week. For fruit and vegetables subject to seasonal variation, consumption in season and the corresponding duration were elicited. The serving size was defined in ‘natural’ unit (e.g. 1 egg) or as an average serving in the Italian diet (e.g. 50 g of salad, 150 g of tomatoes). The FFQ was successfully tested for validity( Reference Decarli, Franceschi and Ferraroni 17 ) and reproducibility( Reference Franceschi, Negri and Salvini 18 ). Total energy and carbohydrate intake was computed using an Italian food composition database( Reference Gnagnarella, Parpinel and Salvini 19 ).
Calculation of glycaemic index and glycaemic load
For each carbohydrate-containing food, we expressed GI as a percentage of the glycaemic response elicited using white bread as a standard food, using international GI tables( Reference Atkinson, Foster-Powell and Brand-Miller 20 ). The average daily GI of each subject was computed by summing the products of the GI value of one serving of each food times the average number of servings of that food consumed by the subject per week divided by the weekly available carbohydrate( Reference Atkinson, Foster-Powell and Brand-Miller 20 , Reference Wolever, Nguyen and Chiasson 21 ). In order to take into account Italian cooking habits (e.g. pasta ‘al dente’), Italian sources were used for a few local recipes( Reference Brighenti and Casiraghi 22 ). Food items for which a GI had not been determined were assigned the GI of the closest comparable food (e.g. tangerines were assigned the same GI of oranges). A score for the daily average GL was computed by summing the products of the GI value of one serving of each food times the average number of servings of that food consumed by the subject per week.
Statistical analysis
OR for bladder cancer and the corresponding 95 % CI were estimated using unconditional logistic regression models including terms for matching variables (study centre, sex, quinquennia of age), and potential confounders including years of education (<7, 7–11, ≥12 years), smoking habits (never, former, current smokers of <20 and of ≥20 cigarettes/d), alcohol drinking (<14, 14–20, ≥20 drinks/week), abdominal obesity (yes/no) and total energy intake (kJ/d). GI, GL, available carbohydrates, bread and pasta were entered in the models as quartiles based on the distribution of controls, using the lowest quartile as the reference category. Given the low consumption, legumes and whole-grain products were categorised as use v. abstinence. Tests for trend were based on the likelihood-ratio test between the models with and without a linear term for each variable of interest. Heterogeneity across strata was tested by comparing the models with and without an interaction term for such variable( Reference Breslow and Day 23 ).
Results
Table 1 shows the distribution of cases and controls according to socio-demographic characteristics, smoking habits, alcohol drinking, abdominal obesity and total energy intake. Compared with never smokers, heavy smokers (i.e.≥20 cigarettes/d) reported a 7-fold increase in bladder cancer risk (95 % CI 4·58, 11·04) with a significant risk trend for number of cigarettes (P<0·01). Patients with abdominal obesity showed a significantly higher risk of bladder cancer (OR 1·33; 95 % CI 1·05, 1·69) as compared with those without this condition. No significant association emerged for education, alcohol drinking and total energy intake.
Table 1 Distribution of 578 bladder cancer cases and 608 controls according to socio-demographic characteristics and selected variables (Numbers and percentages, odds ratios and 95 % confidence intervals)
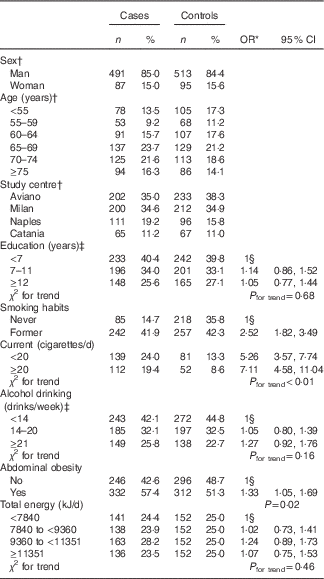
* Estimated by logistic regression model adjusted for sex, age and study centre.
† Matching variable.
‡ The sum does not add up to total because of missing values, one case for education, one case and one control for alcohol.
§ Reference category.
Table 2 gives the OR for bladder cancer according to quartiles of available carbohydrates, GI, GL and selected carbohydrate-rich food groups. No significant associations emerged for total available carbohydrates (OR 1·52; 95 % CI 0·85, 2·69) and for GI (OR 1·18; 95 % CI 0·83, 1·67), whereas a significant direct association was observed for GL, with an OR of 1·96 (95 % CI 1·16, 3·31; P for trend<0·01). Bread was directly associated with bladder cancer risk with OR of 1·64 (95 % CI 1·14, 2·35) and 1·92 (95 % CI 1·28, 2·86) for the third and fourth quartiles, respectively, compared with the lowest quartile (P for trend<0·01). Similarly, elevated pasta consumption was associated with increased bladder cancer (OR 1·58; 95 % CI 1·09, 2·29 in the highest v. the lowest quartile of intake; P for trend=0·03). The association with pasta was significant in men only (OR 1·81; 95 % CI 1·20, 2·73; P for trend<0·01), but no heterogeneity was observed between men and women (P=0·26). Although not significant, high regular use of whole-grain products and legumes showed an inverse association with bladder cancer risk (OR 0·82; 95 % CI 0·63, 1·08 and OR 0·78; 95 % CI 0·60, 1·00, respectively), as compared with abstainers. No heterogeneity emerged according to sex (P for heterogeneity≥0·05).
Table 2 Distribution of 578 bladder cancer cases (Ca) and 608 controls (Co), according to available carbohydrates, glycaemic index, glycaemic load, bread, pasta, legumes and whole-grain products (Odds ratios and 95 % confidence intervals)
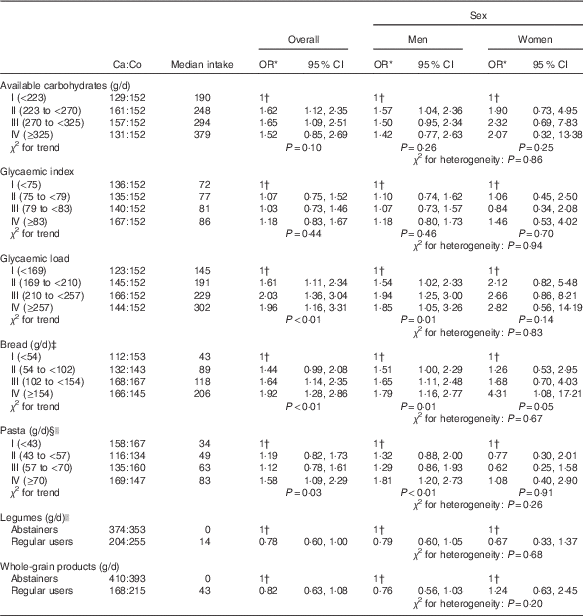
* Estimated by logistic regression model, adjusted for sex, age, study centre, education, smoking habits, alcohol drinking, abdominal obesity and total energy.
† Reference category.
‡ Includes also crackers and bread sticks.
§ Includes also rice.
|| Uncooked.
As vegetables and related seasoning, particularly olive oil, may counteract the glycaemic response of food, the associations between bladder cancer risk and available carbohydrates, GI, GL and intake of bread and pasta were further analysed in separate strata of vegetable intake (Table 3). Although no significant heterogeneity emerged for all the considered food items, results were suggestive that the effect for elevated GL was stronger among people with vegetable consumption below the median (i.e. 160 g/d) than among those above this level of consumption (OR 2·41 and 1·74, respectively).
Table 3 Distribution of 578 bladder cancer cases and 608 controls, according to quartiles of available carbohydrates, glycaemic index, glycaemic load, consumption of bread and pasta, in strata of vegetable intake (Odds ratios and 95 % confidence intervals)
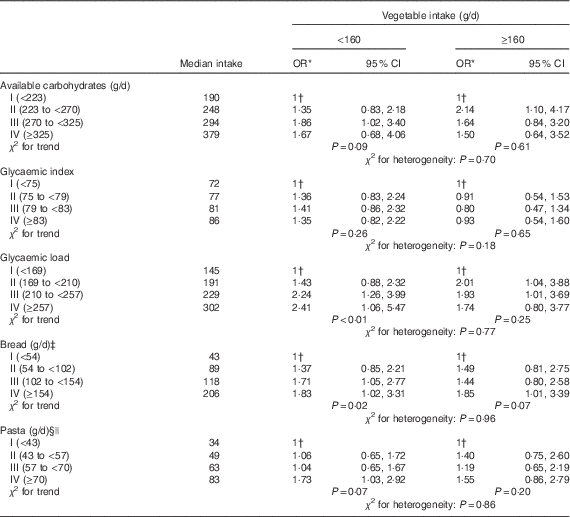
* Estimated by logistic regression model, adjusted for sex, age, study centre, education, smoking habits, alcohol drinking, abdominal obesity and total energy.
† Reference category.
‡ Includes also crackers and bread sticks.
§ Includes also rice
|| Uncooked.
Discussion
Our study found that high dietary GL, an indicator of both quality and quantity of carbohydrate foods, and high intakes of bread and pasta were directly associated with bladder cancer risk. Interestingly, these associations were less strong in people with elevated vegetable consumption.
Two other studies, one conducted in the USA and one in Canada, investigated the association of dietary GI and GL and bladder cancer risk and found an increased risk with higher GI but no association with GL( Reference George, Mayne and Leitzmann 13 , Reference Hu, La Vecchia and Augustin 14 ). The apparent discrepancy in results may be because of different dietary habits in different countries. In the US study, the first quantile of GL started at very low values compared with the present Italian population, where diets are very rich in total carbohydrates. The dietary GL is the product of each food’s GI (derived from GI testing of foods in human subjects) by the amount of total available carbohydrates present in the same food (excluding unavailable carbohydrate such as fibre). A low-GL diet can be achieved by either consuming low-GI foods or by consuming small amounts of carbohydrate foods with any GI value or by consuming both low-GI foods and small quantity of carbohydrate foods. Considering that the Mediterranean–Italian diet is characterised by frequent (e.g. daily) consumption of pasta (a medium–low GI food), a high GL in Italy may be the result of very large quantities of carbohydrates. High-carbohydrate diets, as typically seen in Italy, may need to include very low-GI foods to show an impact on glycaemia. With the shift of the Mediterranean diet towards more convenient and fast food choices, lower-GI foods such as legumes, have become less frequent on the plates of many Italians( Reference Giampaoli, Krogh and Grioni 24 ). It is possible that in bladder cancer a high carbohydrate intake may confound any potential association with GI. Diets with low GI (<69 on a bread scale) and low GL are considered more beneficial to overall health, especially in environments characterised by sedentary behaviour, excess energy intake and excess body weight( Reference Augustin, Kendall and Jenkins 25 ). Reducing the glycaemic impact of the overall diet with low-GI/GL foods has been shown to decrease concentrations of fasting blood glucose, glycosylated proteins, insulin and inflammatory markers, and to reduce the risk of developing type 2 diabetes and some cancers( Reference Hu, La Vecchia and Augustin 14 , Reference Augustin, Kendall and Jenkins 25 ). Fasting hyperglycaemia (>7·0mmol/l) has been found to significantly increase bladder cancer risk in an Asian study including 1·3 million people aged 30–95 years( Reference Jee, Ohrr and Sull 4 ). Insulin can activate epidermal growth factors and protein kinases and in in vitro studies insulin increased bladder cancer cell proliferation in a time- and dose-dependent manner( Reference Liu, Li and Lin 26 , Reference Ornskov, Nexo and Sorensen 27 ). The diabetic medication metformin, which helps to control both glycaemia and insulin resistance, has been shown to reduce growth of malignant cell types including bladder cancer cells( Reference Zhang, Guo and Zhang 28 , Reference Soranna, Scotti and Zambon 29 ). It is also possible that diets promoting large increases in blood glucose may promote urinary tract infections and hence increase bladder cancer risk( Reference Brown, Wessells and Chancellor 30 ).
Two main staple carbohydrate foods of the Italian Mediterranean diet are bread (high GI) and pasta (medium–low GI), representing 24 and 16 % of total carbohydrate intake, respectively( Reference Sette, Le Donne and Piccinelli 31 ). Generally, the associations with cancer risk have been stronger for bread than for pasta( Reference Augustin, Malerba and Lugo 32 , Reference Farvid, Cho and Eliassen 33 ) and with a difference between sexes. In a previous Italian study investigating the association of pasta or bread and colorectal or breast cancer, the associations with bread were stronger in women than in men( Reference Augustin, Malerba and Lugo 32 ). In the current study, bread, pasta and GL were directly and significantly associated with bladder cancer particularly in men. Men are generally affected by bladder cancer three to four times more often than women( Reference Hartge, Harvey and Linehan 34 , Reference Torre, Siegel and Ward 35 ), and several in vitro and animal studies suggest a potential involvement of sex steroid hormonal pathways( Reference Godoy, Gakis and Smith 36 ). Indeed, recent evidence suggests that androgen and androgen receptor may contribute to bladder carcinogenesis both through hormonal pathways and by other mechanisms such as reduced expression of detoxifying enzymes in the bladder( Reference Izumi, Zheng and Hsu 37 , Reference Miyamoto, Yang and Chen 38 ). It is possible that diets that increase blood glucose the most may favour higher testosterone bioavailability by reducing its binding protein( Reference Boering, van Dijk and Logtenberg 39 ).
Furthermore, the results of our study suggest that the associations between bladder cancer risk and GL may be stronger among people with a lower vegetable intake (<160 g/d) than in those with higher intakes (>160 g/d), possibly because of components present in vegetables (e.g. fibre and antioxidants) or consumed with vegetables (e.g. olive oil) that may modify the glycaemic response by various mechanisms (e.g. slowing carbohydrate absorption or reducing glucose toxicity to the beta cells)( Reference Augustin, Franceschi and Jenkins 40 , Reference Satija, Bhupathiraju and Rimm 41 ).
As for most case–control studies, information and selection biases were potential limitations of this study. Information bias, however, was minimised by the administration of the questionnaire to both cases and controls by the same trained interviewers, under similar conditions in a hospital setting. Differential recall in cases and controls in our study may be unlikely as the dietary hypothesis of GI and GL in bladder cancer risk was not known during the study and the questionnaire was satisfactorily reproducible( Reference D’Avanzo, La Vecchia and Katsouyanni 42 ). However, as the information was collected after diagnosis, it is possible that early symptoms of the disease may have caused changes in the diet. To avoid these potential sources of bias, any relevant dietary change in the 2 years preceding interview were recorded and we excluded from the control group all subjects with diagnoses that might have implicated long-term dietary modifications (e.g. diabetes). Furthermore, a possible limiting factor in our analyses was for the calculation of abdominal obesity where waist circumference was missing in approximately 25 % of cases and controls and BMI≥30 kg/m2 was used as a proxy. BMI≥30 kg/m2 is considered a good proxy of abdominal obesity, and analyses conducted excluding patients with missing waist circumference did not show appreciable variation in risk magnitude. In addition, GI and GL values may have some variability according to specific foods and cooking methods. In particular, although pasta and rice have different glycaemic responses, they were assessed together in our FFQ. However, this information bias is unlikely to have an impact on risk estimates, as rice accounts for<20 % in this food group of this study population( Reference Turrini, Saba and Perrone 43 ). The almost complete participation of identified cases and controls, and the use of a validated and reproducible questionnaire, contributed to strengthen our findings. Generalisability of our study results has the limitations discussed above, typical of a hospital case–control study.
In conclusion, this study showed that bladder cancer risk is directly associated with high dietary GL and with consumption of high quantity of refined carbohydrates. These associations were stronger in subjects reporting low vegetable consumption.
Acknowledgements
This work was partially supported by the Italian Foundation for Cancer Research (FIRC).
L. S. A. A.: compiled the glycaemic index database; L. S. A. A. and J. P.: interpreted the data; M. T., M. L., C. L. V., A. T., A. C., M. G., G. F. and J. P.: collected the data; M. T. and J. P.: analysed the data; L. S. A. A. and M. T.: wrote main sections of the manuscript; M. M.: coordinated the work and reviewed the manuscript; M. M., M. L., C. L. V., A. T., A. C., M. G., G. F., D. J. A. J., G. B. and D. S.: revised the analysis; M. L., M. M., C. L. V., A. T., A. C., M. G., G. F., D. J. A. J., G. B., D. S. and J. P.: revised the manuscript.
L. S. A. A. is the cofounder and coordinator of the International Carbohydrate Quality Consortium (ICQC), has received honoraria from the Nutrition Foundation of Italy (NFI, Milan) and from LegaItaliana per la LottaContro i Tumori (a non-profit organization for the fight against cancer). C. L. V. is Member of the Advisory Board of NFI (honorary), and expert consultant to MSL Italy and Sorematec. D. J. A. J. has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd, Unilever, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd, Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institute of Health Research, the Canada Foundation for Innovation and the Ontario Research Fund. He has received in-kind supplies for the trial as research support from the Almond Board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), FareWell, Verywell, True Health Initiative, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca-Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the NFI, Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society, the American Society of Nutrition, Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the US Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association. He is a cofounder and member of the ICQC. His wife is a director and partner of Glycemic Index Laboratories, Inc., and his sister received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. All other authors declare that there are no conflicts of interest.






