For several decades there has been much focus and research on the so-called Mediterranean diet and its demonstrated protective association with a range of non-communicable diseases and mortality( Reference Martinez-Gonzalez, Bes-Rastrollo and Serra-Majem 1 ). Recently there has also been growing interest in whether the same health benefits could be replicated with other regionally based diets; for example, a diet consisting largely of healthy foods native to a Nordic climate, like certain whole grains, wild fish and game, potatoes, root vegetables, cabbages, fruits and wild or cultivated berries( Reference Bere and Brug 2 , Reference Bere and Brug 3 ). The concept of a so-called New Nordic Diet (NND) additionally incorporates concern for environmental sustainability and planetary health( Reference Bere and Brug 3 , Reference Mithril, Dragsted and Meyer 4 ). Recent investigations into associations of healthy Nordic diets with measures of health indicate inverse relationships with several cardiovascular risk factors( Reference Adamsson, Reumark and Fredriksson 5 ), abdominal obesity( Reference Kanerva, Kaartinen and Schwab 6 , Reference Kanerva, Kaartinen and Ovaskainen 7 ), body fat( Reference Kanerva, Kaartinen and Ovaskainen 7 ), inflammatory markers and serum lipids( Reference Uusitupa, Hermansen and Savolainen 8 ), colorectal cancer risk( Reference Kyrø, Skeie and Loft 9 ) and total mortality( Reference Olsen, Egeberg and Halkjær 10 ). Associations of a Nordic diet with health-related pregnancy outcomes have not, however, been investigated so far.
Maternal nutritional status and diet during pregnancy may have long-lasting influences on offspring health( Reference Henriksen and Clausen 11 , Reference Barker 12 ). Excessive gestational weight gain (GWG) increases birth weight independently of genetic factors( Reference Ludwig and Currie 13 ). Animal studies indicate that in utero overnutrition has the potential of inducing permanent changes in key organs, disturbing appetite signalling and predisposing the infant to later overeating and obesity( Reference Alfaradhi and Ozanne 14 , Reference Heerwagen, Miller and Barbour 15 ). Macrosomia, or high infant birth weight, is associated with increased risk of complications for both mother and infant during delivery and in the perinatal period( Reference Boulet, Alexander and Salihu 16 ) and with increased risk of later obesity, insulin resistance and metabolic syndrome in the child( Reference Boney, Verma and Tucker 17 ). Fetal growth restriction, or being born small for gestational age (SGA), is similarly associated with future cardiovascular risk( Reference Henriksen and Clausen 11 , Reference Barker 12 ). Achieving maternal and intra-uterine conditions that optimize fetal growth and development is therefore an important public health concern.
GWG within the ranges recommended in the Institute of Medicine guidelines is associated with favourable health outcomes for both mother and infant( 18 ). At present, however, a large proportion of women are gaining weight outside their recommended range( 18 , Reference Moore, Garrison and Sullivan 19 ). Excessive GWG may contribute to excessive fetal growth, whereas inadequate GWG increases the risk of fetal growth restriction, both being associated with adverse short- and long-term health( 18 ). Documentation on successful ways of facilitating weight gain within optimal ranges is, however, scarce and research on this topic has been called for( Reference Moore, Garrison and Sullivan 19 , Reference Rasmussen, Catalano and Yaktine 20 ).
In a meta-analysis of three large cohort studies in non-pregnant adults, Mozaffarian et al. found that long-term weight gain was inversely associated with an increase in intake of vegetables, whole grains, fruits, nuts and yoghurt( Reference Mozaffarian, Hao and Rimm 21 ), most of which are also central constituents of the NND. In line with this and with the demonstrated effects of a healthy Nordic diet on various health parameters and satiety( Reference Adamsson, Reumark and Fredriksson 5 ), we hypothesize that adherence to the NND during pregnancy could facilitate optimal GWG and optimize fetal growth.
The purposes of the present study were to: (i) construct an NND score reflecting adherence to an environmentally sustainable and healthy Nordic diet; and (ii) investigate its association with adequacy of GWG and fetal growth in a large population-based pregnancy cohort.
Experimental methods
The data for the present study were derived from the Norwegian Mother and Child Cohort Study (MoBa), which is a prospective, population-based, pregnancy cohort study conducted and maintained by the Norwegian Institute of Public Health( Reference Magnus, Irgens and Haug 22 ). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Norwegian Data Inspectorate and The Regional Committee for Ethics in Medical Research (reference S-97045 and S-95113). Written informed consent was obtained from all participants upon recruitment. Participants were recruited from all parts of Norway from 1999 to 2008, and 38·5 % of invited women consented to participate. The cohort now includes 108 000 children, 90 700 mothers and 71 500 fathers. Follow-up is conducted by questionnaires at regular intervals and by linkage to national health registries. The current study is based on version 7 of the quality-assured data released for research in July 2012. For the present study, data from the Medical Birth Registry of Norway (MBRN) and from three separate questionnaires were used: (i) a baseline questionnaire (Q1) that was completed around gestational week 17; (ii) an FFQ that was completed around gestational week 22 (Q2); and (iii) a follow-up questionnaire (Q4) completed 6 months postpartum.
Preparation of the data set
Prerequisites for study eligibility were carrying a single fetus, having completed Q1, Q2 and Q4, and contributing data from the MBRN. A total of 85 160 women fulfilled these criteria. Exclusion criteria comprised delivery at <37 or >42 weeks of gestation (n 4414), lack of information on length of gestation (n 344), more than one pregnancy contributed by the same mother (n 10 434), biologically implausible energy intakes (≤4500 kJ or >20 000 kJ; n 1181), diabetes mellitus diagnosed prior to pregnancy (n 362), maternal height <140 cm or ≥193 cm (n 1), lack of information on maternal pre-pregnancy weight or height (n 1791), lack of information on birth weight (n 33) and birth weight <1000 g at ≥37 weeks of pregnancy (n 3). The final study sample consisted of 66 597 mother–infant pairs.
Construction of the New Nordic Diet score
The NND score was constructed to reflect and quantify participant adherence with the fundamental guidelines of the NND, basically being the inclusion in the diet of: (i) more calories from plant foods and fewer from meat; (ii) more calories from the sea and lakes; and (iii) more calories from the wild countryside as compared with current diet( Reference Mithril, Dragsted and Meyer 4 ). In addition, the score aimed to cover nutritional aspects of importance in attaining healthy balanced diets. The rationale, dietary composition and nutrient content of the NND have been carefully described by Mithril et al.( Reference Mithril, Dragsted and Meyer 4 ).
Ten dietary indicators (subscales) were chosen to constitute the score, based on a combination of their potential Nordic identity, the possibility of the foods being caught, grown, cultivated or picked locally without extensive use of fertilizers, their tradition or importance as a food source within the Nordic countries, and their favourable nutritional or health-enhancing potential relative to similar foods within the same food group( Reference Bere and Brug 3 , Reference Mithril, Dragsted and Meyer 4 ). A brief description of the ten subscales is included below.
-
1. Meal pattern: frequency of eating breakfast, lunch, dinner and evening meal.
-
2. Nordic fruits: frequency of eating apples, pears, plums and strawberries.
-
3. Root vegetables: frequency of eating carrots, rutabaga and various types of onions.
-
4. Cabbages: frequency of eating kale, cauliflower, broccoli and Brussels sprouts.
-
5. Potatoes: frequency of eating potatoes relative to rice and pasta.
-
6. Whole grain breads: consumption of whole grain breads relative to refined breads.
-
7. Oatmeal porridge: frequency of eating oatmeal porridge.
-
8. Foods from the wild countryside: frequency of eating game, fish, seafood and native berries.
-
9. Milk: consumption of unsweetened milk relative to fruit juice.
-
10. Water: consumption of water relative to sweetened beverages.
The NND as defined by Mithril et al. additionally comprises guidelines on the consumption of free-range livestock, rapeseed oil, legumes, nuts and seeds, fresh herbs, seaweeds, wild plants and mushrooms( Reference Mithril, Dragsted and Meyer 4 ). These foods were not addressed in the MoBa FFQ and could therefore not be included in the score. Meal frequency was, however, included in the score as an indicator of meal regularity, the fact that high-quality food is more often served in prepared meals and that complying with main meals may prevent frequent snacks and overeating( Reference Larson, Nelson and Neumark-Sztainer 23 ). Wild fish, seafood, game and native berries were combined in a common subscale since they share much quality due to their complete reliance on soil and local vegetation. Salmon and trout were excluded from this subscale since a large proportion of their consumption represents cultivated seafood not necessarily being in agreement with the perspectives of the NND( Reference Bere and Brug 3 ).
The dietary information was collected from a self-administered, semi-quantitative FFQ that has been validated for use in MoBa( Reference Brantsaeter, Haugen and Alexander 24 ). The FFQ covers the period from conception until mid-pregnancy and comprises 255 food items that have been converted into daily energy and nutrient intakes by FoodCalc and the Norwegian food composition table( Reference Brantsaeter, Haugen and Alexander 24 , Reference Meltzer, Brantsaeter and Ydersbond 25 ). The FFQ can be downloaded from the MoBa website (http://www.fhi.no/morogbarn/). All subscales were constructed in a positive direction, so that increasing value indicated higher consumption of the relevant foods or more of the dietary behaviour in question. Each subscale was dichotomized using the median as cut-off and ‘0’ was assigned for a sum score below the median and ‘1’ for a sum score above the median. In brief, the approximate dietary behaviours associated with scoring in the respective subscales were: (i) eating at least 24 main meals/week; (ii) eating Nordic fruits at least 5 times/week; (iii) eating root vegetables at least 5 times/week; (iv) eating cabbage at least 2 times/week; (v) eating potatoes at least one-third of total occasions of eating potatoes, rice or pasta; (vi) choosing whole grain bread more often than refined bread; (vii) eating oatmeal at least monthly; (viii) eating fish/game/berries about 2 times/week; (ix) drinking milk more often than juice; and (x) drinking at least six times as much water as sugar-sweetened beverages. Similar methods have been extensively used in studies investigating associations of adherence to a Mediterranean diet with health parameters( Reference Bach, Serra-Majem and Carrasco 26 ). The NND score was computed by adding the ten dichotomized subscales, yielding a possible scoring range from 0 to 10. For description and analysis, the NND score was divided into ‘low’ (0–3 points), ‘medium’ (4–5 points) and ‘high’ (6–10 points) NND adherence, respectively.
Outcome variables
The pregnancy outcomes investigated were adequacy of GWG and fetal growth. GWG was calculated as the difference between pre-pregnancy body weight and body weight at birth as reported in Q1 and Q4. Pre-pregnancy BMI was calculated as weight divided by height squared (kg/m2). Absolute GWG was categorized as inadequate, optimal or excessive according to maternal pre-pregnancy BMI in line with the BMI-specific Institute of Medicine guidelines for weight gain during pregnancy( 18 ). Optimal weight gain was 12·5–18·0 kg if the mother was underweight (BMI<18·5 kg/m2), 11·5–16·0 kg if normal weight (BMI=18·5–24·9 kg/m2), 7·0–11·5 kg if overweight (BMI=25·0–29·9 kg/m2) and 5·0–9·0 kg if the mother was obese pre-pregnancy (BMI≥30·0 kg/m2)( 18 ).
To operationalize fetal growth, newborn birth weight was obtained from the MBRN and categorized according to gender-specific cut-off values as SGA, appropriate for gestational age (AGA) or large for gestational age (LGA). The cut-off values for SGA and LGA corresponded to the 10th and 90th birth-weight percentile, respectively, as measured in 60 000 newborns with gestational age of 37–42 weeks born to nulliparous mothers in MoBa.
Other variables
Information on maternal age (years), length of gestation (weeks), marital status and gestational diabetes was obtained from the MBRN. Data regarding smoking, maternal weight and height, gestational weight gain, exercise in pregnancy, educational attainment and parity were obtained from Q1. Since diet was the main exposure, maternal characteristics are presented according to NND adherence.
In building the regression models we investigated variables previously shown to be associated with both outcome and NND adherence, explored potential covariates in univariate models, and included those with significant associations in the multivariable models to reveal and assess potential confounding of the relationship between NND adherence and the outcomes( Reference Hosmer and Lemeshow 27 ). Covariates assessed for inclusion in the GWG model included age, parity, pre-pregnancy BMI, length of gestation, marital status, exercise during pregnancy, educational attainment, smoking habits during pregnancy and sex of the infant. For inclusion in the fetal growth model we additionally assessed the contribution of maternal height and gestational diabetes. Neither marital status nor sex of the child contributed significantly to the models and since their removal changed the parameter estimates by <10 %, they were not included in the final model. Offspring sex was not included in the fetal growth model since the measure of fetal growth was gender-specific.
Statistical analyses
All statistical analyses were performed with the statistical software package IBM SPSS Statistics version 19·0. Most food and nutrient intake variables were skewed and are therefore presented as median and interquartile range. Differences in energy and nutrient intakes across NND adherence categories were analysed with the Kruskal–Wallis test. The χ 2 test was used to assess differences in food frequency and categorical maternal and infant characteristics across NND adherence.
We used multinomial logistic regression to estimate crude and adjusted odds of attaining inadequate and excessive GWG (with optimal GWG as reference) with high NND as compared with low NND adherence. Likewise, we used multinomial logistic regression to estimate the odds of suboptimal fetal growth (SGA or LGA with AGA as reference), with high as compared with low NND adherence. Results are presented as crude and adjusted odds ratios with 95 % confidence intervals. All models were checked for violation of assumptions.
The covariates retained in the GWG model comprised maternal age at delivery (years), length of gestation (37–42 weeks), parity (0, 1, 2, ≥3), educational attainment (≤12 years, 13–16 years, ≥17 years), exercise in pregnancy (rarely, 1–2 times/week, ≥3 times/week), smoking during pregnancy (yes/no), pre-pregnancy BMI (<25·0 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2), and energy intake (kJ). Additional covariates retained in the fetal growth model were maternal height (140–164 cm, 165–168 cm, 169–172 cm, 173–193 cm) and gestational diabetes (yes/no).
A two-sided P-value of <0·05 was considered statistically significant.
Results
Dietary implications of higher adherence to the New Nordic Diet
The final study sample consisted of 66 597 pregnant women and their offspring, representing 78 % of the eligible group before exclusion. Mean maternal age was 30·1 (sd 4·6) years. Participants were categorized according to NND score into low (n 17 802), medium (n 23 558) or high (n 25 237) adherence to the NND, the groups representing 27 %, 35 % and 38 % of the sample, respectively. Table 1 describes the intakes of selected foods and drinks across NND adherence. Higher NND adherence implied higher intakes of fruits and vegetables, potatoes, whole grain bread, fish, game, milk and water. Women categorized with high NND adherence had a median daily intake of close to six portions of fruits and vegetables, almost twice as much as those with low NND adherence.
Table 1 Comparison of weekly food and drink consumption according to degree of adherence to the New Nordic Diet (NND) in the Norwegian Mother and Child Cohort Study (MoBa), presented as frequency, portions or units per week (n 66 597)

P25, 25th percentile; P75, 75th percentile.
* Kruskal–Wallis test across NND adherence categories.
† One portion of fruit is equivalent to one fruit or a similar amount (1/2 grapefruit, 8–10 grapes, etc.).
‡ One unit of drink is equivalent to 250 ml.
Higher NND adherence implied higher intakes of energy and nutrients (Table 2). There was a slightly lower proportion of energy (E%) from fat (30·8 v. 31·7 E%, P<0·001) and a higher proportion from protein (15·7 v. 14·9 E%, P<0·001) with high as compared with low NND adherence. Median proportion of energy from added sugar was lower (8·9 v. 11·2 E%, P<0·001) and fibre intake higher (35 v. 24 g/d, P<0·001) with high as compared with low NND adherence. Each one-unit increment between 0 and 10 in NND score corresponded with increased energy and nutrient intakes, higher nutrient density and improved macronutrient distribution (data not shown). Micronutrients associated with fruits, berries, vegetables and grains were especially abundant in the diet of those with higher NND adherence, as documented by the intake of β-carotene per 10 MJ that was almost doubled with high as compared with low NND adherence. Median fibre intake per 10 MJ was 50 % higher, and that of vitamin C, folic acid, Mg, K and Ca about 20 % higher, with high as compared with low NND adherence.
Table 2 Comparison of energy and nutrient intakes according to degree of adherence to the New Nordic Diet (NND) in the Norwegian Mother and Child Cohort Study (MoBa; n 66 597)
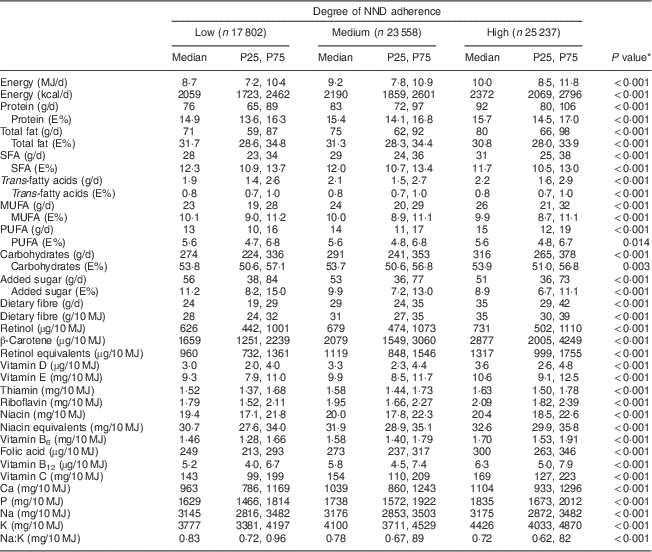
P25, 25th percentile; P75, 75th percentile; E%, macronutrient energy contribution.
* Kruskal–Wallis test across NND adherence categories.
Maternal and infant characteristics
Table 3 presents maternal and infant characteristics according to NND adherence. In short, women with high NND adherence were older, more educated and of higher parity than those with low NND adherence. They were also less likely to be smoking, less likely to be overweight or obese prior to pregnancy and more likely to exercise than women with low NND adherence.
Table 3 Maternal and infant characteristics in the whole sample and according to degree of adherence to the New Nordic Diet (NND) in the Norwegian Mother and Child Cohort Study (MoBa)
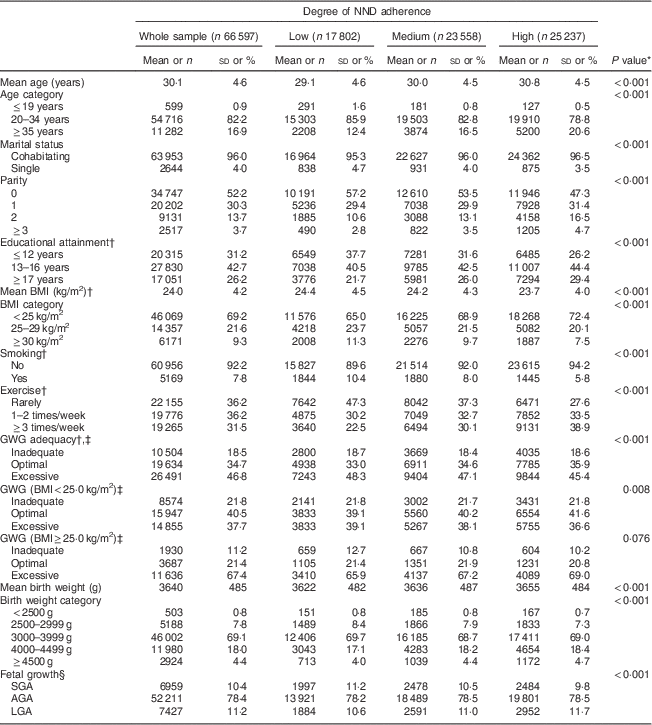
GWG, gestational weight gain; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
Continuous variables are presented as mean and standard deviation, categorical variables as number and percentage.
* χ 2 test for trend (categorical variables) and one-way ANOVA test for trend (continuous variables) across NND adherence categories.
† Number of missing observations: education, n 1401 (2·1 %); BMI, n 1687 (2·5 %); smoking. n 472 (0·7 %); exercise, n 5401 (8·1 %); GWG, n 9968 (15·0 %).
‡ Adequacy of GWG was defined according to maternal pre-pregnancy BMI in line with the BMI-specific Institute of Medicine guidelines for weight gain during pregnancy, with optimal weight gain constituting 12·5–18·0 kg if the mother was underweight (BMI<18·5 kg/m2), 11·5–16·0 kg if normal weight (BMI=18·5–24·9 kg/m2), 7·0–11·5 kg if overweight (BMI=25·0–29·9 kg/m2) and 5·0–9·0 kg if the mother was obese pre-pregnancy (BMI≥30·0 kg/m2)( 18 ).
§ Gender-specific cut-off values for SGA and LGA correspond to the 10th and 90th birth-weight percentile, respectively, as measured in 60 000 newborns with gestational age 37–42 weeks born to nulliparous mothers in MoBa.
Gestational weight gain
Mean total GWG in the sample was 15·0 (sd 5·9) kg, averaging 6·8 (sd 3·9) kg in the inadequate GWG group, 12·8 (sd 2·5) kg in the optimal GWG group and 19·3 (sd 4·7) kg in the excessive GWG group. A total of 41 % of normal-weight women attained optimal GWG as compared with only 21 % of overweight/obese women (Table 3). Since the definition of what constitutes inadequate, optimal and excessive GWG depends on pre-pregnancy BMI, we ran the model stratified by BMI even though there was no formal interaction (P=0·133 for excessive GWG and P=0·497 for inadequate GWG). Because of observed effect modification by BMI on the association between NND adherence and adequacy of GWG, analyses were carried out and presented stratified by BMI, the categories hereby denoted normal weight (BMI<25·0 kg/m2) and overweight/obese (BMI≥25·0 kg/m2; Table 4). High as compared with low NND adherence implied lower adjusted odds of excessive GWG (OR=0·93; 95 % CI 0·87, 0·99; P=0·024) in normal-weight women. Degree of NND adherence was not significantly associated with GWG among overweight/obese women, but there was a trend towards higher odds of excessive GWG with high as compared with low NND adherence in this subset of participants (OR=1·11; 95 % CI 1·00, 1·23; P=0·061). Moving from low to medium NND adherence implied lower odds of inadequate GWG among overweight/obese women (OR=0·86; 95 % CI 0·74, 0·99; P=0·038). Otherwise there was no association between NND adherence and risk of inadequate GWG after adjustment for relevant confounding.
Table 4 Associations of adherence to the New Nordic Diet (NND) with adequacy of gestational weight gain (GWG) in the Norwegian Mother and Child Cohort Study (MoBa; n 56 629)
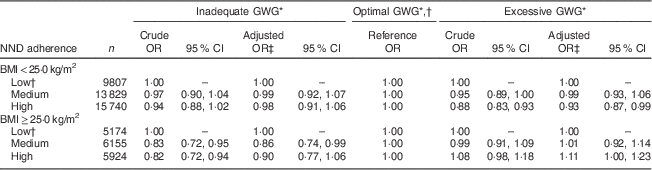
* Absolute GWG was categorized as inadequate, optimal or excessive according to maternal pre-pregnancy BMI in line with the BMI-specific Institute of Medicine guidelines for weight gain during pregnancy, with optimal weight gain constituting 12·5–18·0 kg if the mother was underweight (BMI<18·5 kg/m2), 11·5–16·0 kg if normal weight (BMI=18·5–24·9 kg/m2), 7·0–11·5 kg if overweight (BMI=25·0–29·9 kg/m2) and 5·0–9·0 kg if the mother was obese pre-pregnancy (BMI≥30·0 kg/m2)( 18 ).
† Low NND adherence is the reference category for diet and optimal GWG is the reference category for GWG in the multinomial model.
‡ Adjusted for parity, pre-pregnancy BMI, educational attainment, energy intake, smoking, exercise during pregnancy, length of gestation, maternal age at delivery and maternal age squared.
Fetal growth
Birth weight distribution and adequacy of fetal growth according to NND adherence are presented in Table 3. High as compared with low NND adherence was associated with reduced odds of the infant being born SGA (OR=0·92; 95 % CI 0·86, 0·99; P=0·025) and increased odds of the baby being born LGA (OR=1·07; 95 % CI 1·00, 1·15; P=0·048; Table 5). No formal interaction was observed between NND adherence and fetal growth (P=0·538 for SGA and P=0·578 for LGA). Since GWG could be in the causal pathway between NND adherence and fetal growth, GWG was not adjusted for in the multivariable model.
Table 5 Associations of adherence to the New Nordic Diet (NND) in pregnancy with adequacy of fetal growth in the Norwegian Mother and Child Cohort Study (MoBa; n 66 597)
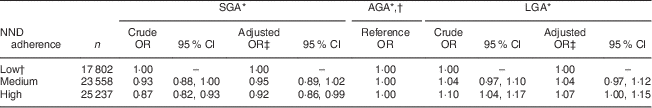
SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
* Gender-specific cut-off values for SGA and LGA correspond to the 10th and 90th birth-weight percentile, respectively, as measured in 60 000 newborns with gestational age 37–42 weeks born to nulliparous mothers in MoBa.
† Low NND adherence is the reference category for diet and AGA is the reference category for fetal growth in the multinomial model.
‡ Adjusted for maternal age at delivery, parity, pre-pregnancy BMI, maternal height, educational attainment, smoking, gestational diabetes, exercise during pregnancy and energy intake.
Discussion
In the present study we found that adherence to a regionally based diet including a large representation of fruits and vegetables, whole grains, potatoes, fish, game, milk and drinking water during pregnancy may facilitate optimal GWG in normal-weight women and improve fetal growth in general. Although the NND score may be seen as a fairly crude indicator of diet adherence, its dose-dependent association with nutrient density and a healthier macronutrient distribution confirms its ability to capture diet quality.
Normal-weight women with high as compared with low NND adherence had an estimated 7 % lower odds of excessive GWG. Albeit small, this risk difference may be relevant in a public health perspective given the high prevalence of excessive GWG and its documented association with offspring macrosomia, perinatal morbidity, obstetric complications and later maternal and child health( Reference Moore, Garrison and Sullivan 19 , Reference Nohr, Vaeth and Baker 28 , Reference Muktabhant, Lumbiganon and Ngamjarus 29 ). The fact that NND adherence was differently (although not significantly) associated with risk of excessive GWG in overweight/obese women is difficult to explain, but could simply reflect that improved diet quality per se may not be sufficient to limit GWG to the low weight range recommended for these groups (7·0–11·5 kg and 5·0–9·0 kg, respectively). Whether the NND can positively influence GWG gain in overweight/obese women who, unlike the present participants, are aware of their weight gain goals can only be answered in a properly conducted randomized intervention study.
Few studies have explored dietary patterns in relation to GWG. Uusitalo et al. reported a dose–response association of a ‘fast-food pattern’ with rate of GWG, whereas an inversely associated ‘healthy pattern’ was not statistically significant( Reference Uusitalo, Arkkola and Ovaskainen 30 ). Stuebe et al. investigated associations of several dietary and nutritional aspects with risk for excessive GWG in 1388 pregnant women and found total energy intake, dairy and fried foods to be directly associated with risk of excessive GWG, whereas a vegetarian diet in the first trimester was inversely associated with excessive GWG( Reference Stuebe, Oken and Gillman 31 ). Olafsdottir et al. reported in a study in 495 pregnant women that eating more sweets in early pregnancy, drinking more milk and eating more food in general during late pregnancy increased the risk of excessive GWG( Reference Olafsdottir, Skuladottir and Thorsdottir 32 ). Normal-weight women with high NND adherence in our study had lower risk of excessive GWG despite higher milk intake, higher reported intakes of cakes and desserts and higher total energy intake than those with lower NND adherence, possibly pointing to the importance of taking into account the complexity of the diet when investigating associations of diet with health outcomes.
The estimated 9 % reduced odds of giving birth to an SGA infant with high as compared with low NND adherence may also be of public health relevance given the well-known risk of adverse fetal programming resulting from compromised nutrient supply that potentially can lead to increased cardiovascular risk later in life( Reference Leon 33 , Reference Ross and Beall 34 ). The fact that high as compared with low NND adherence also implied an estimated 7 % higher adjusted odds of the infant being born LGA indicates that factors associated with better diet quality may improve fetal substrate availability and enhance fetal growth in general. Boney et al. demonstrated that the risk of developing metabolic syndrome in childhood was associated with the intra-uterine environment conferred by maternal obesity and diabetes in addition to the risk conferred by being born LGA( Reference Boney, Verma and Tucker 17 ). Whether being born LGA as a result of a healthy and nutritious maternal diet is differently associated with future health than being born LGA due to maternal obesity or diabetes has not been investigated to our knowledge.
Mechanisms relating diet quality to fetal growth comprise direct effects through fetal nutrient availability as well as indirect effects related to modulation of placental gene expression affecting placental function( Reference Cetin, Mandò and Calabrese 35 ). Several studies have reported a healthier and more nutritious maternal diet to be inversely associated with the risk of fetal growth restriction. Knudsen et al. found that women with a ‘health conscious’ dietary pattern characterized by higher intake of fruits and vegetables, fish and poultry had 24 % reduced odds of delivering an SGA infant compared with women with a ‘Western’ dietary pattern characterized by a higher intakes of red and processed meat, high-fat dairy and energy( Reference Knudsen, Orozova-Bekkevold and Mikkelsen 36 ). Olsen et al. demonstrated reduced odds of SGA with increasing maternal milk consumption as compared with no milk intake, as well as higher odds of LGA with milk intakes exceeding 2–3 glasses/d( Reference Olsen, Halldorsson and Willett 37 ). Brantsæter et al. found reduced risk of SGA but no impact on risk of LGA with higher fish and seafood intake( Reference Brantsæter, Birgisdottir and Meltzer 38 ). Chatzi et al. found that high v. low adherence to a Mediterranean diet modified the detrimental effect of smoking on birth size( Reference Chatzi, Mendez and Garcia 39 ). Higher milk and fish consumptions were also among the characteristics of higher NND adherence in the present study.
Strengths to our study are its prospective design, the large size of the cohort, detailed and validated information on dietary intake from conception to mid-pregnancy and detailed information on a range of covariates. The use of a comprehensive diet assessment tool rather than investigating single foods as exposure in relation to health outcomes has the advantage of capturing a larger part of whole diet complexity with potential inherent interactive or synergistic effects of foods eaten in combination( Reference Moeller, Reedy and Millen 40 , Reference Kant 41 ).
Some limitations should, however, be addressed. Generalizability can be questioned since young and single mothers, smokers and mothers with more than two children are under-represented in the MoBa compared with the Norwegian background population( Reference Northstone, Emmett and Rogers 42 ). This implies a more homogeneous sample regarding health-related characteristics and may have influenced the estimates of the associations between NND adherence and the outcomes. Some misclassification of NND adherence and BMI category is likely because these data were self-reported. Gross under- and over-reporting of dietary intake was eliminated by the exclusion of participants with biologically implausible energy intakes( Reference Meltzer, Brantsaeter and Ydersbond 25 ). However, bias introduced by selective over-reporting of healthy dietary behaviours or under-reporting of perceived unhealthy dietary behaviours cannot be excluded( Reference Meltzer, Brantsaeter and Ydersbond 25 ). This could influence cut-off levels in the subscales that constitute the NND score and lead to misclassification of NND adherence. If randomly distributed, misreporting of diet would only tend to blur associations, but not change the direction of the findings. If misreporting should be correlated with specific participant traits or characteristics, however, the resulting misclassification of NND adherence could potentially diminish, conceal or even change the apparent direction of the estimated associations of NND adherence with the outcomes in participants with these traits. In the present study this kind of misclassification would tend to attenuate associations, thus potentially resulting in underestimation of the true associations between NND adherence and the outcomes.
Increasing NND score was reflected in higher absolute energy intake. This seems to be a common feature of healthy dietary indices( Reference Olsen, Halldorsson and Willett 37 , Reference Chatzi, Mendez and Garcia 39 , Reference Kant 41 , Reference Northstone, Emmett and Rogers 43 ) and may bias associations of dietary patterns with health outcomes closely related to energy intake. To account for this we adjusted for energy intake in the multivariable regression models even though energy intake could be in the causal pathway between NND adherence and the outcomes.
Importantly, the NND score was not constructed to capture the healthiest or most efficient weight-controlling diet possible, but rather to be an indicator of a generally healthy, realistic and sustainable regional diet. The fact that by this fairly crude classification of diet we were able to detect associations with adequacy of GWG and fetal growth confirms the ability of the NND score to capture aspects of diet quality.
Conclusion
The implication of the present study is that adherence to a regionally based diet including a large representation of fruits and vegetables, whole grain bread, potatoes, fish, game, milk, and drinking water during pregnancy may facilitate attainment of optimal GWG in normal-weight women and improve fetal growth in general. The NND score captures diet quality and will be used to investigate associations of NND adherence with other pregnancy outcomes in further studies.
Acknowledgements
Acknowledgements: The authors are grateful to all the participating families in Norway who take part in the MoBa ongoing cohort study. Financial support: MoBa was supported by the Norwegian Ministry of Health and the Ministry of Education and Research; the National Institutes of Health (NIH)/National Institute of Environmental Health Sciences (NIEHS) (contract no N01-ES-75558); the NIH/National Institute of Neurological Disorders and Stroke (NINDS) (grant numbers UO1 NS 047537-01 and UO1 NS 047537-06A1); and the Norwegian Research Council/FUGE (grant number 151918/S10). The present study was funded by the University of Agder. None of the funders had any role in the design, analysis or writing of this article. Conflicts of interest: None. Authorship: E.R.H., E.B. and N.C.Ø. conceived the project and designed the present study; M.H. was involved in the development and calculations of the MoBa FFQ; E.R.H. and M.H. prepared the data set; E.R.H. analysed data and wrote the paper; E.R.H., E.B. and N.C.Ø. had primary responsibility for final content. All authors have seen and approved the submitted manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Norwegian Data Inspectorate and The Regional Committee for Ethics in Medical Research (reference S-97045 and S-95113). Written informed consent was obtained from all MoBa participants upon recruitment.







