India, like many other developing countries has not as yet eliminated the problem of under-nourishment in its poorer communities particularly with respect to protein. In these communities protein intakes tend to be low, and to exacerbate the problem Indian diets are generally cereal and legume based, the proteins of which tend to be more poorly digested than North American protein-based foods (57–75 % versus 88–94 %), largely due to the presence of high levels of insoluble fibre and anti-nutritional factors(Reference Gilani, Cockell and Sepehr1). Cereals tend to be limiting in lysine while legumes are limiting in sulphur containing amino acids.
Ensuring food and nutritional security is a challenge for India, given its large population and high levels of poverty and malnutrition. National Nutrition Monitoring Bureau of India (NNMB) data have revealed a gradual decline in daily per capita intake of protein from 62·9 g in 1975–1979 to 48·8 g in 2004–2005. In countries like India, where dietary amino acid supply may potentially limit protein metabolism, it is imperative to accurately monitor the dietary supply of ‘available amino acids’ in relation to the dietary requirement(Reference Rowan, Moughan and Wilson2). Consequently, it is essential to have a fundamental understanding of the digestible amino acid content of Indian foods and the extent to which the digestible amino acids meet the requirements of people in India. Protein digestibility-corrected amino acid score (PDCAAS), scores proteins based on their ability to meet the deemed amino acid requirement of humans for the first limiting amino acid and in the case of cereal-based diets that is likely to be lysine.
While the amino acid composition of many foods consumed in developing countries, including India, is known there is scant data about the digestibility of those amino acids and it is important that this gap in knowledge be addressed. Currently, PDCAAS uses faecal nitrogen digestibility values to correct amino acid scores to PDCAAS values. However, a single nitrogen digestibility value may not reflect the digestibility of all dietary amino acids(Reference Rowan, Moughan and Wilson2). Moreover, faecal amino acid digestibility values are often higher than ileal amino acid digestibility values, particularly for poorly digested protein sources(Reference Rowan, Moughan and Wilson2, Reference Ratriyanto, Mosenthin and Jezierny3). Consequently, PDCAAS values may be more accurate when derived using true ileal amino acid digestibility values as opposed to faecal nitrogen digestibility values(Reference Moughan4, Reference Fuller5).
Lysine is of particular interest since it is often first limiting in cereal-based diets and is susceptible to chemical modification during processing or cooking to form nutritionally unavailable derivatives(Reference Moughan4, Reference Hurrell and Carpenter6). Furthermore, lysine intakes have been shown to be marginal in low socio-economic group Indians(Reference Kurpad, Raj and El-Khoury7, Reference Kurpad, Regan and Raj8), which is of even greater concern given that the daily requirement for lysine has been shown to be approximately 30 mg kg− 1 day− 1 for healthy Indian men(Reference Kurpad, Raj and El-Khoury7) or higher (44 mg kg− 1 day− 1) where there is chronic under-nourishment(Reference Kurpad, Regan and Raj8). This value is more than double the requirement estimate laid out in 1985 by the WHO/FAO/UNU expert consultation group. Values for digestible lysine based on true ileal digestible total lysine are likely to overestimate the nutritional value of foods and food ingredients that have been processed or cooked. Instead true ileal digestible reactive lysine provides a more accurate measure of available lysine in such foods(Reference Moughan4). A method has been developed(Reference Moughan and Rutherfurd9, Reference Rutherfurd, Moughan and Morel10) that allows the accurate determination of the available lysine (true ileal digestible reactive lysine) content of processed foods (Biolysine™). The objective of this study was to determine true ileal amino acid digestibility and available (digestible reactive) lysine contents for a range of typical cereal- and legume-based foods from India.
Materials and methods
Samples
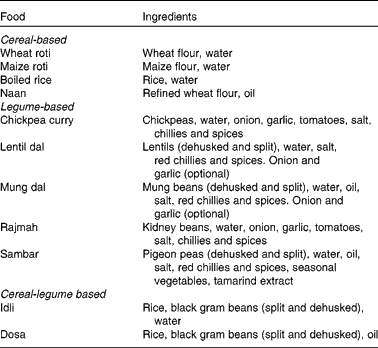
Ten raw ingredients (wheat flour, rice, maize flour, black gram beans, refined flour, mung beans, lentils, chickpeas, kidney beans and pigeon peas) and eleven prepared foods (wheat roti, cooked rice, maize roti, dosa, idli, naan, mung dal, lentil dal, sambar, chickpea curry and rajmah) were each collected from six households selected in the Punjab region of India. The raw ingredients and prepared foods were pooled across households, freeze dried and ground using a standard kitchen food processor. The dried samples were then air freighted to Massey University, New Zealand where they were further ground through a 1 mm mesh and stored at − 20°C prior to analysis. The ingredients used to prepare the food dish are shown in Table 1.
Table 1 Ingredients in the prepared foods.
Preparation of 0·6M O-methylisourea solution
A 0·6 M O-methylisourea solution was prepared as described by Moughan and Rutherfurd(Reference Moughan and Rutherfurd9).
Digestibility study
Ethics approval for the animal trial was obtained from the Animal Ethics Committee, Massey University, Palmerston North, New Zealand. Male Sprague-Dawley rats of approximately 200 g bodyweight were housed individually in stainless steel wire-bottomed cages in a room maintained at 22 ± 2°C with a 12 h light/dark cycle. Twenty one semi-synthetic test diets were formulated. The crude protein content of the raw ingredients and prepared foods ranged from 77 to 279 g kg− 1 DM. For samples that had a crude protein content less than 100 g kg− 1, the diets consisted of the raw ingredient or prepared food, a proprietary vitamin mix (50 g kg− 1), a proprietary mineral mix (50 g kg− 1) and an indigestible marker titanium dioxide (3 g kg− 1). For the remainder of the foods, diets were prepared by adding a proprietary vitamin mix, a proprietary mineral mix and an indigestible marker (titanium dioxide) to each of the foods and then diluting as appropriate with a mixture of soybean oil, purified cellulose, sugar and cornstarch in ratios of 10:5:15:70 to reduce the crude protein content to 100 g kg− 1. The concentrations of vitamin mix, mineral mix and titanium dioxide in the final diets were 50 g kg− 1, 50 g kg− 1, 3 g kg− 1 respectively. A basal diet containing 100 g kg− 1 protein was also formulated, using casein as the sole protein source, to meet the nutritional requirements for the growing rat for all nutrients except protein(11). The latter diet contained 118 g kg− 1 lactic casein, 50 g kg− 1 proprietary vitamin premix, 50 g kg− 1 proprietary mineral premix, 100 g kg− 1 soybean oil, 100 g kg− 1 sucrose, 50 g kg− 1 purified cellulose and 529 g kg− 1 cornstarch. Over the first eleven days of the 14-day experimental period, all the rats were fed the basal casein-based diet. The rats were then randomly allocated to the test diets such that there were five animals per diet and the animals were fed their respective test diets for a further three days. The test diets were not fed for the entire experimental period as they may not have met the rat's requirement for all nutrients. On each day, each rat had unrestricted access to its respective diet from 09.00 hours to 12.00 hours. Water was available at all times. On the final day of the study, between three and four hours after the start of feeding, the rats were asphyxiated using carbon dioxide gas and then decapitated. The stomach contents were checked for faecal matter and no sign of coprophagy was observed. The twenty centimetres of ileum immediately anterior to the ileo-caecal junction was dissected out. The dissected ileum was washed with distilled deionised water to remove any blood and hair and carefully dried on an absorbent paper towel. The digesta were gently flushed from the ileum section with distilled deionised water from a syringe. The digesta were then freeze-dried ready for chemical analysis.
Chemical analysis
Dry matter, ash, crude protein, crude fibre and total fat were determined according to the methods described by AOAC(12). Protein content was estimated from the nitrogen content using a nitrogen to protein conversion factor of 6·25.
Amino acid contents were determined in duplicate five mg ingredient, prepared food and ileal digesta samples and quadruplicate five mg diet samples following hydrolysis in 6 M glass-distilled HCl containing 0·1 % phenol for 24 h at 110 ± 2°C in evacuated sealed tubes. The liberated amino acids were derivatised with o-phthalaldehyde (OPA) (proline was not detected in the assay as it does not react with OPA). The derivatives were then separated on an Agilent 1200SL HPLC system equipped with a C18 reverse-phase HPLC column and quantified using fluorescence detection (excitation λ 338 nm, emission λ 450 nm). Proline was detected using Accutag derivatisation (Waters Millipore, Milford, Ma) and fluorescence detection (excitation λ 245 nm, emission λ 395 nm). Cysteine and methionine were determined using performic acid oxidation followed by HCl acid hydrolysis as described above and quantified using Accutag derivatisation (Waters Millipore, Milford, Ma) and fluorescence detection. Tryptophan was determined using alkali hydrolysis in 4·5 M NaOH containing 5 % (w/v) maltodextrin at 110 ± 2°C for 20 h. Tryptophan was then quantified using absorbance at 280 nm. 5-methyl tryptophan was used as an internal standard. The weight of each amino acid was calculated using free amino acid molecular weights. It should be noted that for some rats there was insufficient digesta material for the determination of methionine, cysteine and tryptophan.
Reactive lysine contents were determined in duplicate five mg ingredient, prepared food, digesta and diet samples after guanidination by incubation for seven days in 0·6 M O-methylisourea, pH 10·6 (pH 11·0 for the digesta samples), at 21 ± 2°C in a shaking waterbath, with a reagent to lysine ratio greater than 1000(Reference Moughan and Rutherfurd9). After incubation, the samples were dried using a Speedvac concentrator (Savant Instruments, Inc, Farmingdale, NY, USA) and analysed for homoarginine content using a Waters ion-exchange HPLC system, utilising post-column OPA derivatisation and detection using fluorescence (excitation λ 338 nm, emission λ 450 nm), following hydrolysis as described above for the other amino acids.
The titanium contents of the diet and ileal digesta samples were determined in duplicate based on the method of Short et al. (Reference Short, Gorton and Wiseman13). Samples were ashed before being digested in 60 % (v/v) sulphuric acid and then incubated with 30 % hydrogen peroxide and the absorbance read at 405 nm.
Data Analysis
Ileal amino acid (AA) flows were calculated using the following equation (units are μg g− 1 dry matter (DM)):
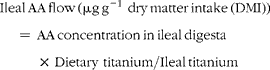 $$\begin{eqnarray} Ileal\,AA\,flow\,(\mu g\,g^{ - 1}\,\,dry\,matter\,intake\,(DMI)) = \,AA\,concentration\,in\,ileal\,digesta\,\times \,Dietary\,\,titanium/Ileal\,titanium \end{eqnarray}$$
$$\begin{eqnarray} Ileal\,AA\,flow\,(\mu g\,g^{ - 1}\,\,dry\,matter\,intake\,(DMI)) = \,AA\,concentration\,in\,ileal\,digesta\,\times \,Dietary\,\,titanium/Ileal\,titanium \end{eqnarray}$$
True ileal amino acid digestibility1 was calculated as follows (units are μg g− 1 DMI):
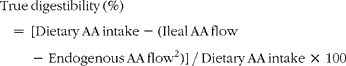 $$\begin{eqnarray} True\,digestibility\,(\%) = \,[Dietary\,AA\,intake - (Ileal\,AA\,flow\,-\,Endogenous\,AA\,flow^{2})]\,/\,Dietary\,AA\,intake\,\times \,100 \end{eqnarray}$$
$$\begin{eqnarray} True\,digestibility\,(\%) = \,[Dietary\,AA\,intake - (Ileal\,AA\,flow\,-\,Endogenous\,AA\,flow^{2})]\,/\,Dietary\,AA\,intake\,\times \,100 \end{eqnarray}$$
1True ileal glycine digestibility values were not calculated since the enzyme hydrolysed casein/ultrafiltration method underestimates endogenous ileal glycine losses.
2Based on endogenous amino acid flows for the growing rat as reported by Rutherfurd and Moughan(Reference Rutherfurd and Moughan14) and based on the enzyme hydrolysed casein/ultrafiltration method(Reference Moughan, Darragh and Smith15).
True ileal reactive lysine (RL) digestibility was calculated as follows (units are μg g− 1 DMI):
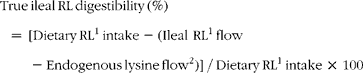 $$\begin{eqnarray} True\,ileal\,RL\,digestibility\,(\%) = \,[Dietary\,RL^{1}\,intake - (Ileal\,\,RL^{1}\,flow - Endogenous\,lysine\,flow^{2})]\,/\,Dietary\,RL^{1}\,intake\,\times \,100 \end{eqnarray}$$
$$\begin{eqnarray} True\,ileal\,RL\,digestibility\,(\%) = \,[Dietary\,RL^{1}\,intake - (Ileal\,\,RL^{1}\,flow - Endogenous\,lysine\,flow^{2})]\,/\,Dietary\,RL^{1}\,intake\,\times \,100 \end{eqnarray}$$
1Reactive lysine determined using the guanidination method.
2Based on the endogenous lysine flow reported by Rutherfurd and Moughan(Reference Rutherfurd and Moughan14) where for the enzyme hydrolysed casein/ultrafiltration method(Reference Moughan, Darragh and Smith15) endogenous reactive lysine is equivalent to endogenous total lysine.
True ileal digestible reactive lysine content of the foods was calculated as follows:
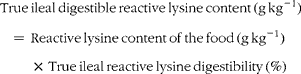 $$\begin{eqnarray} True\,ileal\,digestible\,reactive\,lysine\,content\,(g\,kg^{ - 1}) = \,Reactive\,lysine\,content\,of\,the\,food\,(g\,kg^{ - 1})\,\times \,True\,ileal\,reactive\,lysine\,digestibility\,(\%) \end{eqnarray}$$
$$\begin{eqnarray} True\,ileal\,digestible\,reactive\,lysine\,content\,(g\,kg^{ - 1}) = \,Reactive\,lysine\,content\,of\,the\,food\,(g\,kg^{ - 1})\,\times \,True\,ileal\,reactive\,lysine\,digestibility\,(\%) \end{eqnarray}$$
True ileal digestible amino acid content of the food was calculated as follows:
 $$\begin{eqnarray} True\,ileal\,digestible\,amino\,acid\,content\,of\,the\,food\,(g\,kg^{ - 1}) = \,Amino\,acid\,content\,of\,the\,food\,(g\,kg^{ - 1})\,\times \,True\,ileal\,amino\,acid\,digestibility\,(\%) \end{eqnarray}$$
$$\begin{eqnarray} True\,ileal\,digestible\,amino\,acid\,content\,of\,the\,food\,(g\,kg^{ - 1}) = \,Amino\,acid\,content\,of\,the\,food\,(g\,kg^{ - 1})\,\times \,True\,ileal\,amino\,acid\,digestibility\,(\%) \end{eqnarray}$$
The amino acid digestibility data were subjected to a one-way analysis of variance for each amino acid singly (GLM Procedure)(16).
Results
The determined proximate composition of the ten Indian food ingredients and eleven common Indian food dishes prepared from similar ingredients is presented in Table 2. Crude protein ranged from 77 to 279, crude fibre from 7 to 71, total fat from 11 to 154 and ash from 3 to 64 g kg− 1 DM across ingredients and prepared foods. The nitrogen free extractive (NFE) ranged from 576 to 884 g kg− 1 DM and demonstrated as expected, that carbohydrates were the main chemical component of the foods tested.
Table 2 Determined nutrient composition1 (g kg−1 DM) of the eleven prepared Indian foods and ten Indian food ingredients.

1 Analysis was conducted in duplicate.
2 Protein content was estimated from the nitrogen content using a nitrogen to protein conversion factor of 6·25.
3 Nitrogen free extractive was calculated as follows (units are g kg− 1): Nitrogen free extractive (g kg− 1 DM) = Total sample weight − (Moisture+Ash+Crude protein+Crude fibre+Ether extract)
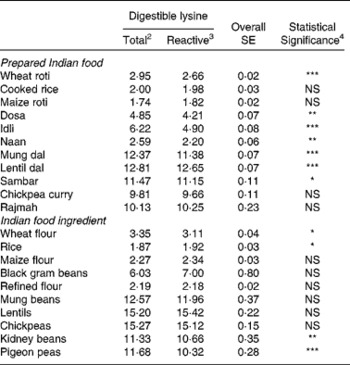
The true ileal digestible reactive (available) lysine content varied markedly across ingredients and prepared foods from 1·9 g kg− 1 DM for rice to 15·4 g kg− 1 DM for lentils for the ingredients and 1·8 g kg− 1 DM for maize roti to 12·7 g kg− 1 DM for lentil dal for the prepared foods (Table 3). For the cereal ingredients and cereal-based prepared foods the available lysine content ranged from 1·9 g kg− 1 DM for rice to 3·1 g kg− 1 DM for wheat flour for the ingredients and from 1·8 g kg− 1 DM for maize roti to 2·7 g kg− 1 DM for wheat roti for the prepared foods. For the legume ingredients and legume-based prepared foods the available lysine content ranged from 10·3 g kg− 1 DM for pigeon peas to 15·4 g kg− 1 DM for lentils for the ingredients and 9·7 g kg− 1 DM for chickpea curry to 12·7 g kg− 1 DM for lentil dal for the prepared foods. For the prepared foods containing both cereals and legumes the available lysine ranged from 4·2 g kg− 1 DM for dosa to 4·9 g kg− 1 DM for idli.
Table 3 Mean (n=5) true ileal digestible total and reactive (available) lysine contents1 (g kg−1 DM) for the eleven prepared Indian foods and ten Indian food ingredients.
1 The true ileal digestibility values used to calculate the true ileal digestible amino acid content were determined after correction for endogenous lysine flow determined using the enzyme hydrolysed casein/ultrafiltration method(Reference Moughan, Darragh and Smith15) and using values as reported by Rutherfurd and Moughan(Reference Rutherfurd and Moughan14).
2 Determined based on the analysis of total lysine in diet and digesta of rats fed the experimental diets.
3 Determined based on the analysis of reactive lysine (guanidination reaction) in diet and digesta of rats fed the experimental diets.
4 NS Not significant P>0·05, *0·05> P>0·01, **0·01> P>0·001, ***P < 0·001.
The true ileal digestible total lysine content was also determined and compared with the true ileal digestible reactive (available) lysine content (Table 3). For seven of the eleven prepared foods, true ileal digestible total lysine significantly (P < 0·05) overestimated true ileal digestible reactive (available) lysine content. This overestimation ranged from 1 % for lentil dal to 27 % for idli with the mean overestimation being 12 %. For the other prepared foods, cooked rice, maize roti, rajmah and chickpea curry there was no significant difference (P>0·05) between digestible total lysine and digestible reactive lysine contents. For the ingredients, there was no significant (P>0·05) difference between digestible total and reactive lysine contents for most of the ingredients. The exceptions were wheat flour, rice, kidney beans and pigeon peas where for wheat flour, kidney beans and pigeon peas digestible total lysine significantly (P < 0·05) overestimated digestible reactive (available) lysine by between 6 and 12 %.
The true ileal digestibilities of amino acids (including reactive lysine) for the eleven commonly prepared Indian foods and the ten Indian food ingredients are given in Table 4. The overall true ileal amino acid digestibility determined across all amino acids for each food ingredient ranged from 31 % for black gram beans to 96 % for wheat flour with an overall mean digestibility across all ingredients of 77 %. For the prepared foods, overall amino acid digestibility ranged from 67 % for rajmah to 95 % for lentil dal with a mean overall amino acid digestibility calculated across all foods of 86 %. The least digestible amino acid across all the prepared foods was cysteine (mean value of 69 %) and the most digestible amino acids were lysine and leucine (mean value of 92 %). For the ingredients, the least digestible amino acid was aspartic acid (mean value of 60 %) and the most digestible was methionine (mean value of 89 %).
Table 4 Mean (n=5) true ileal amino acid digestibility1 (%) for the eleven prepared Indian foods and ten raw Indian food ingredients1.

1 Values were corrected for endogenous amino acid flows determined using the enzyme hydrolysed casein/ultrafiltration method(Reference Moughan, Darragh and Smith15) and as reported by Rutherfurd and Moughan(Reference Rutherfurd and Moughan14).
2 Insufficient digesta material for the determination of cysteine, methionine, proline and tryptophan.
3 Based on reactive lysine determined using the guanidination method.
4 n = 5 for all ingredients except wheat flour (n = 4) and kidney beans (n = 2) and all prepared foods except wheat roti (n = 4), dosa (n = 1) and idli (n = 2) where there was insufficient material to analyse digesta from all the rats.
5 Insufficient digesta material for the determination of tryptophan.
The true ileal digestible amino acid contents of the materials is presented in Table 5. There was considerable variation in the digestible amino acid content across both the prepared foods and food ingredients, with on average a 4·2- and 5·0-fold range in digestible amino acid contents across foods and amino acids for the prepared foods and ingredients respectively.
Table 5 Mean (n=5) true ileal digestible amino acid contents1 (g kg−1 DM) for the eleven prepared Indian foods and ten Indian food ingredients.

1 True ileal digestible lysine content is presented in Table 3 as the true ileal digestible reactive lysine content.
2 Insufficient digesta material for the determination of cysteine, methionine, proline and tryptophan.
3 n = 5 for all ingredients except wheat flour (n = 4) and kidney beans (n = 2) and all prepared foods except wheat roti (n = 4), dosa (n = 1) and idli (n = 2) where there was insufficient material to analyse digesta from all the rats.
4 Insufficient digesta material for the determination of tryptophan.
Discussion
The lysine intake of people in India, particularly children, is likely to be marginal, because firstly, food intake is generally low (Reference Brahmam17). Secondly, cereals and legumes tend to be staple foods for many Indians(Reference Brahmam17) and cereals are low in lysine while legume intake relative to cereals tends to be low(Reference Ghatak18). Thirdly, lysine is prone to undergo chemical modification when foods undergo heat processing (such as cooking) to form Maillard-type products which are generally nutritionally unavailable and will therefore further reduce the available lysine content. In this study, the available content of lysine and the ileal digestible amounts of other amino acids were determined in eleven commonly prepared Indian foods and ten Indian food ingredients.
Overall, true ileal digestible total lysine overestimated available lysine (true ileal digestible reactive lysine) for more than half the prepared foods and just under half of the food ingredients and in many cases this overestimation was large (>8 % for seven of the prepared foods and ingredients). This is consistent with previous results(Reference Rutherfurd, Torbatinejad and Moughan19–Reference Rutherfurd, Moughan and van Osch21) and given that the available lysine assay (Biolysine™) only requires a different step in the chemical analysis rather than a separate bioassay, this method should be the preferred one for determining lysine availability in foods. The traditional (total lysine based) ileal digestibility assay will lead to considerable error for some foods.
There was considerable variation in the available lysine content of the Indian food ingredients and prepared foods with 8-fold differences in the available lysine content across the ingredients and prepared foods. As expected, the available lysine content was lowest for the cereal-based prepared foods (1·8–2·7 g kg− 1 DM), intermediate in the prepared foods containing cereals and legumes (4·2–4·9 g kg− 1 DM) and highest in the legume-based prepared foods (9·7–12·7 g kg− 1 DM). For the cereals and legume ingredients respectively, the range in available lysine content was small with a 1·6-fold and 1·5-fold difference in the available lysine content across cereal ingredients and cereal-based prepared foods respectively and a 1·4-fold and 1·3-fold difference in available lysine content across legume ingredients and prepared foods respectively. The inclusion of legumes into cereal-based diets increased the available lysine content considerably (approximately double) and clearly fortification of cereal-based diets with legumes is a useful approach for increasing the lysine intakes of the Indian population.
Overall true ileal amino acid digestibility varied markedly across both ingredients and prepared foods and was often relatively low. It is clear that for even the most digestible ingredients and prepared foods amino acid digestion and absorption was far from complete and therefore must be taken into account when determining available amino acid content. Amino acid digestibility also varied considerably across amino acids within each ingredient and prepared food, with the difference between the lowest and highest digestibilities across amino acids within ingredients or prepared foods ranging from 12 % units (88–100 %) for wheat flour to 85 % units (0 –85 %) for black gram beans for the ingredients and 15 % units (82–97 %) for lentil dal to 49 % units (33–82 %) for rajmah. This highlights the potential error in using a single digestibility value (eg. crude protein digestibility) to predict amino acid digestibility in general. Protein digestibility-corrected amino acid scores (PDCAAS) are commonly calculated using a single digestibility factor (true faecal nitrogen digestibility). Depending on the limiting amino acid in each ingredient or prepared food, the use of true faecal nitrogen digestibility values could result in an inaccurate assessment of protein quality.
For the foods containing legumes, true ileal amino acid digestibility determined across all amino acids was lower for the food ingredients than for the prepared foods, which is most likely due to the presence of anti-nutritional factors(Reference Gilani, Cockell and Sepehr1). For example, the overall mean amino acid digestibilities for mung dal, sambar, and rajmah were markedly higher than for their main ingredients (mung beans, pigeon peas and kidney beans). Similarly, Wu et al. (Reference Wu, Williams and Kunkel22) reported true faecal protein digestibility values of 16 and 79 % respectively for raw kidney beans and kidney beans cooked at 100oC for 2 h. The increase in the mean amino acid digestibility is most likely due to the destruction of anti-nutritional factors present in the legume ingredients during cooking(Reference Uebersax, Ruengsakulrach and Occena23). For the cereal-based ingredients and prepared foods, wheat roti, cooked rice, maize roti, naan were more poorly digested than their respective major ingredients wheat flour, rice, maize flour, wheat flour. Kubota et al. (Reference Kubota, Saito and Masumura24) have also reported that cooking reduces the digestion of prolamin, a major storage protein in rice. The decrease in mean amino acid digestibility observed for the cereal-based prepared foods is possibly due to Maillard-type reactions occurring during cooking(Reference Hurrell and Carpenter6) and the subsequent formation of indigestible limit peptides(Reference Moughan, Gall and Rutherfurd25). For lentil dal and chickpea curry each made from lentils and chickpeas respectively, the overall mean amino acid digestibility of the prepared food was similar to that of the respective major ingredients.
There was considerable variation in the true ileal digestible amino acid contents for each amino acid, including lysine, across both the prepared foods (on average a 4·6-fold difference) and food ingredients (on average a 5·4-fold difference). As expected the amino acid profile of the prepared foods generally reflected that observed for the main ingredients included in each of the prepared foods.
Diet surveys conducted by the National Nutrition Monitoring Bureau (NNMB) of India during 2001–2002(Reference Brahmam17) revealed that among 1–6 year-old children, the consumption of various foods like cereals and pulses was less than the recommended levels. The intake among the adults was higher, where the recommended intake of cereals was met, but the intake of legumes was still inadequate. Furthermore, while adults received 80–90 % of the recommended dietary protein intake only 30 % of children consumed protein adequate diets. The net availability (per capita per day) of cereals in India has increased from 394·9 g in 1951 to 439·3 g in 2007 but at the same time the net availability of legumes which are a significant source of lysine for Indians has declined from 60·7 g in 1951 to 29·4 g in 2007(Reference Ghatak18). In an attempt to put the results of the present study into context, the available lysine intake of the Indian population for a range of commonly consumed prepared food combinations based on the prepared foods tested in the present study was estimated and is presented in Table 6. The daily lysine intakes were estimated in two ways, firstly, based on the reported average daily energy intake of the Indian population (2034 kcal day− 1)(Reference Deaton and Drèze26) and secondly based on cereal and legume supply data(Reference Ghatak18) and assuming 10 % food wastage. In both cases it was assumed that for each day each meal consisted of a legume-based prepared food and a cereal-based prepared food (for example, cooked rice with sambar) the proportions of which were assumed to be between 10 % to 20 % legume-based prepared food and correspondingly between 90 % to 80 % cereal-based prepared food (these figures were based on the relative legume and cereal availability in India(Reference Ghatak18)). A similar analysis (data not shown) was conducted for the sulphur amino acids (methionine plus cysteine) which tend to be first limiting in legumes. For a seventy kilogram adult human, the lysine requirement is 2·12 g day− 1(27); and the methionine plus cysteine requirement 0·98 g day− 1(27); however, the estimated daily lysine intake for all of the prepared food combinations, with the exception of sambar/idli was lower than 2·12 g day− 1 ranging from 0·94 g day− 1 for rajmah/maize roti (10:90) to 2·04 g day− 1 for sambar/dosa (20:80) when based on cereal and legume availability per capita and ranging from 1·02 g day− 1 for rajmah/maize roti (10:90) to 2·1 g day− 1 for sambar/dosa (20:80) when based on daily energy intake. In contrast, the daily intake of sulphur amino acids estimated based on the daily energy intake of the Indian population ranged from 1·30 g day− 1 for sambar/cooked rice (10:90) to 2·88 g day− 1 for chickpea curry/wheat roti (10:90) and would be sufficient to meet the daily methionine plus cysteine requirement for a 70 kg man for all of the prepared food combinations examined. Although it is recognised that it is unlikely that Indians will consume the same prepared food combination for each meal in a day, consumption of any of the prepared food combinations, excluding sambar/idli, would lead to an insufficient daily lysine intake and in many cases supplying less than three quarters of the daily lysine requirement. The similar outcome, as to whether analysis was based on food supply data or reported energy intakes, gives some confidence in the data. For the scenario above both protein and energy would be limiting. However, even if food intakes were sufficient to meet energy requirements it was calculated that lysine would still be limiting for between 40–80 % of the food combinations evaluated as part of this study.
Table 6 Estimated daily lysine intake (g day−1) and lysine adequacy for the Indian population receiving a diet derived from selected foods tested in this study.

1 Based on a daily food intake of 468·7 g day− 1 (439·3 g day− 1 of cereals and 29·4 g day− 1 of legumes)(Reference Ghatak18) and assuming a DM content of 90 % calculated as follows: Daily lysine intake (g day1) = Avlys(legume-based food)x Daily DM intake x Percentage (legume-based food) + Avlys((cereal-based food)x Daily DM intake x Percentage (cereal-based food) Where Avlys is the determined available lysine content (g g− 1) of the prepared food, percentage is the percentage of either the legume-based prepared food or cereal-based prepared food in the combined food and daily DM intake = 421·8 g DM day− 1 (468·7 g day1x 90 % DM).
2 Based on the reported daily energy intake of 2034 kcal day− 1(Reference Deaton and Drèze26) and calculated as follows: Daily lysine intake = Avlys(legume-based food) x Daily DM intake x Percentage(legume-based food) + Avlys(cereal-based food) x Daily DM intake x Percentage(cereal-based food) Where Avlys is the determined available lysine content (g g− 1) of the prepared food, percentage is the percentage of either the legume-based prepared food or cereal-based prepared food in the combined food and daily DM intake is calculated as follows: Daily DM intake = Daily energy intake (kcal day− 1) (Reference Deaton and Drèze26) / Energy content of the combined food (kcal g− 1DM)
3 Adequacy was calculated for a 70 kg adult based on a lysine requirement of 30 mg kg− 1 day− 1(27) as follows: Adequacy = Lysine intake (g day− 1) / Lysine requirement (g day− 1)
4 Percentage of each prepared food in the combined food and based on the wet weight.
Conclusion
The available lysine content was highly variable across ingredients and prepared foods. In addition, digestible total lysine overestimated available lysine (digestible reactive lysine) for many of the ingredients and prepared foods. This has important implications for dietary protein quality assessment. Available (true ileal digestible reactive) lysine values are generally likely to be lower than true faecal lysine digestibility values because faecal lysine digestibility usually overestimates ileal lysine digestibility and digestible total lysine often over estimates digestible reactive (available) lysine. True ileal amino acid digestibility varied widely both across ingredients and prepared foods for each amino acid and also across amino acids within each ingredient and prepared food. True ileal nitrogen digestibility was a poor predictor of amino acid digestibility for many amino acids in the ingredients and prepared foods tested in this study. Amino acid digestibility was often far less than complete and consequently amino acid digestibility must be taken into account when assessing the protein quality of poorer quality foods and ingredients such as those often consumed in developing countries such as India.
Acknowledgements
We acknowledge the support and advice of Dr G Sarwar-Gilani in the conduct of this work. We also acknowledge the Riddet Institute, Massey University, Palmerston North for financial support.
SR co-designed the study, ran the animal trials and laboratory analysis and co-wrote the manuscript. KB co-designed the study and arranged for the collection and preparation of the samples and co-wrote the manuscript. PM co-designed the study and co-wrote the manuscript. The authors state that there are no conflicts of interest. Funding for the work was provided by the Riddet Institute.








