Asians and Pacific Islanders have higher circulating serum ferritin (SF) compared with Caucasians but the clinical significance of this is unclear. Both genetic( Reference Adams, Reboussin and Barton 1 , Reference Harris, McLaren and Reboussin 2 ) and non-genetic factors contribute to elevated ferritin concentrations in man. Genetic predisposition is the basic reason behind the condition of high SF concentration in Caucasians( Reference Adams, Reboussin and Barton 1 ). Mutations (e.g. C282Y and H63D) in the haemochromatosis gene are commonly associated with high ferritin levels in the liver and in the peripheral circulation. However, Asians and Pacific Islanders have the highest geometric mean levels of SF and mean transferrin saturation compared with white counterparts despite having the lowest prevalence of C282Y homozygotes( Reference Adams, Reboussin and Barton 1 ). Fe overload (indicated by high SF levels) can cause serious health problems through parenchymal damage to organs, but primary Fe overload appears to be rare in Asians and Pacific Islanders( Reference Adams, Reboussin and Barton 1 , Reference Harris, McLaren and Reboussin 2 ). A higher prevalence of thalassaemia trait may partly explain higher mean SF levels in Asian men( Reference Adams, Reboussin and Barton 1 ). The non-genetic causes of elevated SF levels may also contribute to the observed racial/ethnic differences in SF levels. Factors include chronic hepatitis, excessive Fe or alcohol intake, liver disorder, metabolic syndrome and neuron degenerative diseases. High levels of SF are significantly associated with chronic inflammation. Inflammatory markers such as C-reactive protein (CRP), adiponectin and IL-6 are positively correlated with SF concentrations( Reference Sun, Franco and Hu 3 ).
Elevated SF concentrations have recently been implicated in the pathogenesis of the metabolic syndrome (MetS) and type II diabetes( Reference Sun, Franco and Hu 3 – Reference Ryoo, Kim and Lee 7 ). Epidemiological studies showed that high SF levels are independently associated with risk of MetS for Caucasians( Reference Bozzini, Girelli and Olivieri 4 , Reference Jehn, Clark and Guallar 5 ), middle-aged and elderly Chinese( Reference Sun, Franco and Hu 3 ) and healthy Koreans( Reference Lee, Kim and Kim 8 ). This association is found in both genders in apparently healthy populations( Reference Rajpathak, Ma and Manson 6 , Reference Ryoo, Kim and Lee 7 ). Here, the physiological function of elevated SF remains uncertain. The underlying mechanisms responsible for the rising level of ferritin in circulation as well as its effect on the diseases remain unclear. The traditional view is that ferritin may protect against Fe-induced damage because of its function as a storage protein for Fe. Ferritin has the capacity to sequester large quantities of Fe in a soluble, non-toxic and biologically available form, which can store up to 4500 Fe atoms per ferritin protein complex. Free Fe is a potent oxidant and may cause tissue damage. This is attributed to the facts that: (i) no physiological mechanism of Fe excretion exists; (ii) Fe is an essential nutrient for all living organisms including man and pathogens; and (iii) Fe mediates the activation of reactive oxygen species. Activation of oxidative pathways causes damage to the host via several mechanisms: (i) Fe-mediated reactive oxygen species activation (e.g. DNA damage, lipid peroxidation and protein peroxidation); (ii) Fe-mediated activation of transcriptional mediators (e.g. AP1, NF-κB, endoplasmic reticulum stress mediators XBP1 and NFR1); and (iii) Fe-induced hypoxia-inducible factor 1 (HIF-1), an oxygen-sensitive transcriptional activator which promotes angiogenesis and Fe metabolism( Reference Huang 9 ).
Taiwan is an immigrant country and consists of four ethnic groups: Hoklo, Hakka, Mainlanders and Indigenous people, who are also known as the Mountainous( Reference Tsai 10 ). Each ethnic group has developed its own unique culture, language, dietary habits and a distinctive environment. Genetically, Taiwanese aborigines are related to Austronesians( Reference Friedlaender, Friedlaender and Reed 11 ) and three of the four ethnic groups on Taiwan are descendents of Han Chinese. It is believed that Pacific Islanders originated in Taiwanese aborigines about 5200 years ago( Reference Gray, Drummond and Greenhill 12 ).
There is considerable racial and/or geographical variation in the prevalence of obesity and obesity-related disease risks in Taiwan( Reference Pan, Lee and Chuang 13 , Reference Hsiao and Wka 14 ). Taiwanese Indigenous, who originally lived in the central mountainous regions, have significantly higher prevalence of MetS compared with the Han Chinese (32·1 % v. 20·2 % in men, 41·3 % v. 25·5 % in women)( Reference Pan, Lee and Chuang 13 ). Austronesia origin has significant independent effects on the presence of MetS (OR = 2·36, 95 % CI 1·45, 3·87 in men and OR = 3·49, 95 % CI 1·66, 7·31 in women) after multiple adjustment for covariates( Reference Pan, Lee and Chuang 13 ). The average life expectancy for Indigenous is 10 years lower than for the average Taiwanese population( Reference Ko, Liu and Hsieh 15 ). The life expectancy gap may contribute to the high prevalence of chronic inflammatory diseases( Reference Pan, Chang and Yeh 16 , Reference Lee, Wahlqvist and Yu 17 ) and MetS( Reference Hsiao and Wka 14 ) among Indigenous people. The present study explored the association between SF concentration and risk of MetS in ethnically/racially diverse adult populations by use of the Nutrition and Health Survey in Taiwan (NAHSIT) 2005–2008. The objectives of the study were as follows: (i) to describe the distribution of SF level among ethnically diverse healthy populations; and (ii) to investigate the association between SF concentration and risk of MetS.
Experimental methods
Study design and definition of ethnicity
The third national nutrition and health survey in Taiwan (NAHSIT 2005–2008) was funded by the Department of Health to provide continued assessment of the health and nutrition of the people in Taiwan. The nationwide survey was conducted using a multistage, stratified and clustered sampling scheme which included a wide range of age groups across the whole of Taiwan. The present study only analysed data on adults aged ≥19 years old. Four samples were collected and analysed. One sample (n 1582), which represents the Nation, with five geographical strata, was selected for inference to the whole of Taiwan. We also examined the influence of race/ethnicity and lifestyle variables (e.g. geographic isolation and dietary habits). For this reason, three additional strata were selected, including Hakka area (n 354), mountainous regions (n 330) and Penghu Island (n 372). Ethnicity was self-reported and/or defined based on the geographical location of the strata: mountainous are known as Indigenous, Hakka and Penghu Islanders refer to Han Chinese. Penghu Island represents the geographical isolation between Taiwan Island and other islands. The government of Taiwan officially recognizes distinct tribes among the Indigenous community based upon the qualifications drawn up by the Council of Indigenous Peoples (http://www.apc.gov.tw). Currently, a total of fourteen tribes have been officially recognized. The nationwide study selected thirty townships spread across the mountainous regions that are officially recognized by the Council of Indigenous Peoples. It also included Hua-lien and Tai-tung City/County in the East stratum of Taiwan. The East stratum is heavily populated with Amis and Puyuma tribes. Hakka were collected from eighteen representative townships acknowledged by the Council of Hakka people (http://www.hakka.gov.tw). Although no definitive definition of ethnicity/race exists, the NAHSIT selected a representative population representing each ethnic group according to the guidelines of each ethnic council. Details of the study design can be found elsewhere( Reference Tu, Chen and Hsieh 18 ). Informed consent was obtained from all participants. The study was approved by the Research Ethics Committee of Taipei Medical University (201203029) and Academia Sinica (AS-IRB01-07020) and was consistent with the World Medical Association Declaration of Helsinki (certificate of IRB approval: 201203029).
Sample inclusion and exclusion
Exclusion criteria were as follows: (i) individuals with missing data for clinical biochemistry, anthropometry and 24 h dietary recall; (ii) individuals with total energy intake ≥20 920 kJ/d (≥5000 kcal/d) or ≤2092 kJ/d (≤500 kcal/d); and (iii) individuals with abnormal SF >500 ng/ml (as a surrogate marker for chronic inflammation). As such, a total of 1659 participants, 801 male and 858 female, were selected for the Nation. A total of 354 participants, 177 male and 177 female, were selected for the Hakka. A total of 330 participants, 158 male and 172 female, were selected for the mountainous, which represents Taiwanese aborigines. A total of 372 participants, 188 male and 184 female, were selected for the Penghu Islanders, which represents Han Chinese living in a different environment from the Han Chinese living on Taiwan Island.
Data collection
Information on sociodemographic variables, self-reported family health histories, 24 h dietary recall and lifestyle factors were obtained using a standardized questionnaire. Smoking status was divided into three categories: current smoker, past smoker and non-smoker. Questions about alcohol intake included the frequency of alcohol consumption on a weekly basis and the amount of alcohol consumed was categorized into four groups: non-drinker, light drinker (1–20 g/d), moderate drinker (≥21–40 g/d) and heavy drinker (≥41 g/d). Measurements of body weight and height, waist circumference (WC) and blood pressures are described elsewhere( Reference Pan, Lee and Chuang 13 ). WC measurements were taken at the midpoint between the lower edge of the rib cage and the top of the iliac crest( Reference Pan, Lee and Chuang 13 ). Dietary intake was estimated by the 24 h dietary recall which includes measurement of household recipes, the individual dietary recall and validation of individual dietary recall by food models. Dietary data on total energy intake, total Fe intake, type of Fe (haem Fe and non-haem Fe) consumed, intakes of carbohydrates, protein, fats and oils, dairy products, fruit and vegetables, and use of animal or vegetable oil during cooking were obtained from 24 h dietary recall. Details of the data collection and data analysis have been described elsewhere( Reference Wu, Chang and Wei 19 ).
Laboratory measurements
Biochemistry data were obtained from 8 h fasting blood samples. Heparinized whole blood was collected for on-site measurement of Hb. Peripheral venous blood samples were collected in tubes containing EDTA, centrifuged at 4°C and serum stored at −80°C until analysis. Clinical biochemistry included: serum cholesterol (including total cholesterol, LDL-cholesterol and HDL-cholesterol (HDL-C)), TAG, fasting blood glucose, uric acid (UA), CRP, creatinine, homocysteine, liver function tests (glutamic–oxoacetic transaminase (GOT), glutamic–pyruvate transaminase (GPT)), amylase, blood urea nitrogen (BUN), alkaline phosphatase and Fe parameters (serum Fe, SF, total iron binding capacity (TIBC)).
Definitions of Fe-deficiency anaemia and Fe overload
Fe status was evaluated by serum Fe, transferrin saturation and SF concentrations( Reference Looker, Dallman and Carroll 20 ). SF was measured using a commercially available electrochemiluminescence immunoassay and was quantified by the Roche Modular P800 analyser. Hb was measured by the cyanomethaemoglobin method (Merckotest; Merck) using a portable filter photometer calibrated with haemoglobin cyanide standard solution (Merck). Serum Fe and TIBC were measured by the ferrozine-based colorimetric method. Percentage transferrin saturation (%TS) was calculated as serum Fe/TIBC × 100 %. Criteria for anaemia were based on the WHO cut-off values of Hb <12 g/dl for adult females and <13 g/dl for adult males( 21 ). Fe deficiency and Fe-deficiency anaemia were defined by use of a combination of several Fe indicators as originally proposed by Cook et al.( Reference Looker, Dallman and Carroll 20 , Reference Cook, Finch and Smith 22 , Reference Wang and Shaw 23 ). Fe deficiency was considered if any two of the three indicators of Fe status showed abnormal values: SF <12 ng/ml, %TS <15 % and Hb <13 mg/dl in men and <12 mg/dl in women( Reference Looker, Dallman and Carroll 20 , Reference Cook, Finch and Smith 22 , Reference Wang and Shaw 23 ). Fe-deficiency anaemia was considered if all three of the Fe indicators showed abnormal values. Fe overload was defined as SF >300 ng/ml for men and >200 ng/ml for women( Reference Sun, Franco and Hu 3 ).
Definition of obesity and metabolic syndrome
Obesity and overweight were defined based on definitions used by the Department of Health in Taiwan( 24 , Reference Tan, Ma and Wai 25 ). Overweight was defined as a BMI ≥24 kg/m2 and <27 kg/m2, and obesity was defined as a BMI of ≥27 kg/m2. This definition differs from the WHO Asians’ criteria which define overweight as BMI ≥23 kg/m2 and obese as BMI ≥25 kg/m2( 26 ). Central obesity was defined as a WC ≥90 cm in men and ≥80 cm in women. Hypertension was defined according to criteria in the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure( Reference Chobanian, Bakris and Black 27 ).
MetS was defined based on the modified National Cholesterol Education Program Adult Treatment Panel III criteria for Asia Pacific( 24 , 26 ). Individuals with the presence of three or more of the criteria listed below were classified as having MetS( Reference Tan, Ma and Wai 25 ): (i) WC ≥90 cm in men and ≥80 cm in women; (ii) TAG ≥150 mg/dl; (iii) HDL-C <40 mg/dl for men and <50 mg/dl for women; (iv) systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or current use of antihypertensive drugs; and (v) fasting blood glucose ≥110 mg/dl or current use of antihyperglycaemic drugs.
Statistical analyses
Statistical analyses were performed using the statistical software package SAS version 9·22. Categorical data were presented as number and percentage. Continuous data were presented as mean and standard deviation or median and interquartile range. Due to smoothing the trend, the plot in Fig. 1 refers only to those subjects with SF level lower than 500 ng/ml. Also we replace the mean by median in the figures to resolve the unstable prevalence of MetS due to small sample size in each subgroup. SF concentrations were divided into tertiles. One-way ANOVA and the χ 2 test were used to compare the differences among tertile groups of SF. Multiple logistic regression models were used to estimate the odds ratios and 95 % confidence intervals for MetS and its components. The dependent variables were the presence of MetS or components of MetS. The independent covariates which may, directly or indirectly, modulate the distribution of SF levels were included in our analysis. These include: (i) dietary variables; (ii) lifestyle factors; (iii) self-reported family health history; (iv) inflammatory markers; (v) Fe parameters; and (vi) age, gender and ethnicity/race. The covariates for the adjusted OR calculation for the Nation were age, sex, BMI, UA, CRP, GOT, GPT, past smoker, hypertension, diabetes mellitus (DM) and hyperlipidaemia. The covariates for the adjusted OR calculation for the Hakka were age, sex, BMI, UA, CRP, GOT, GPT, hypertension, DM and hyperlipidaemia. The covariates for the adjusted OR calculation for the Taiwanese aborigines were age, sex, UA, CRP, GOT, GPT, hypertension, DM and hyperlipidaemia. The covariates for the adjusted OR calculation for the Penghu Islanders were age, sex, BMI, UA, CRP, GOT, GPT, hypertension, DM and hyperlipidaemia. All nutrient intakes were adjusted by total energy using the residual method( Reference Willett and Stampfer 28 ). We calculated the prevalence rate ratio as a sensitivity analysis. However, as our data did not obey the rare event assumption (having a very large variance-to-mean ratio) of Poisson regression, the over-dispersion problem would not be avoided and the estimation would be biased. An alternative, Breslow–Cox regression with assigned equal time, was used in this case( Reference Barros and Hirakata 29 ). This method works well if data have moderate sample size and continuous predictors( Reference Lee and Chia 30 ). A P value <0·05 was considered statistically significant.

Fig. 1 Distribution of serum ferritin (SF) level by decade of age for healthy Taiwanese men and women aged ≥19 years (n 2638) stratified by ethnicity (Nation: ![]() (men),
(men), ![]() (women); Hakka:
(women); Hakka: ![]() (men),
(men), ![]() (women); Indigenous:
(women); Indigenous: ![]() (men),
(men), ![]() (women); Penghu Islander:
(women); Penghu Islander: ![]() (men),
(men), ![]() (women))
(women))
Results
Baseline characteristics
Mean ages were similar among Nation (54·3 (sd 17·8) years) and the three ethnic groups (54·4 (sd 17·6) years for Hakka; 56·7 (sd 17·8) years for Indigenous; 54·0 (sd 19·3) years for Penghu Islanders). No significant difference was found in the prevalence of Fe-deficiency anaemia (Nation: 5·2 %, Hakka: 6·6 %, Indigenous: 5·4 %, Penghu Islanders: 4·2 %). Indigenous (26·3 %) had the highest prevalence of Fe overload and Penghu Islanders (15·7 %) the lowest. Prevalence of obesity was higher in Indigenous (42·4 %) followed by Penghu Islanders (23·7 %), Nation (20·6 %) and Hakka (20·4 %). Similarly, Indigenous had the highest prevalence of MetS (53·3 %) followed by Penghu Islanders (35·5 %), Hakka (34·8 %) and Nation (30·9 %). Dietary Fe consumption was similar among ethnic groups (Nation: 15·8 (sd 17·4) mg/d, Hakka: 15·3 (sd 14·8) mg/d, Indigenous: 11·2 (sd 8·9) mg/d, Penghu Islanders: 13·1 (sd 10·5) mg/d). Amount of haem Fe and non-haem Fe intake did not differ among ethnic/racial groups.
A comparison of baseline characteristics between racial/ethnic groups according to SF tertiles is shown in Table 1. SF tertiles were positively correlated with age and WC among ethnic groups (Table 1). SF tertiles were also significantly correlated with male gender and other Fe parameters such as TIBC, %TS and Hb (data not shown). A positive correlation between SF tertiles and disease history among ethnic groups was also observed. Except hypertension, SF tertiles were positively correlated with hyperlipidaemia, DM, hepatitis, fatty liver and cirrhosis in Hakka. For the Indigenous, we found positive correlations between SF tertiles and hypertension and between SF tertiles and hyperlipidaemia. SF tertiles were positively correlated with disease history of hyperlipidaemia, DM and fatty liver in the Penghu Islanders. Liver function indices such as GOT and GPT were significantly correlated with SF tertiles in Hakka, Indigenous and Penghu Islanders (Table 1). We also measured dietary intake by 24 h dietary record. Except for Penghu Islanders, no difference between SF tertiles and dietary Fe intake was found (Table 1). SF tertiles were positively correlated with Fe consumed from animal sources and protein intake in Penghu Islanders (P < 0·05). In Hakka, SF tertiles were positively correlated with vitamin B6, protein and fat intakes (all P < 0·05). Indigenous showed a positive correlation between thiamin and SF tertiles (P < 0·05; Table 1).
Table 1 Comparison of baseline characteristics between racial/ethnic groups in relation to SF tertiles; healthy Taiwanese men and women aged ≥19 years (n 2638)

SF, serum ferritin; WC, waist circumference; DM, diabetes mellitus; CRP, C-reactive protein; GOT, glutamic–oxoacetic transaminase; GPT, glutamic–pyruvate transaminase.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Distribution of serum ferritin levels stratified by age, sex and race
Mean levels of SF (Nation: 170 (sd 311) ng/ml, Hakka: 172 (sd 188) ng/ml, Indigenous: 214 (sd 322) ng/ml, Penghu Islanders: 153 (sd 133) ng/ml) and %TS (Nation: 34·5 (sd 14·1) %, Hakka: 33·4 (sd 12·8) %, Indigenous: 32·0 (sd 16·0) %, Penghu Islanders: 33·8 (sd 13·2) %) were similar. We next examined the distribution of SF by age and sex stratified by ethnicity. We found that distributions of SF level were strongly associated with age and gender but not with ethnicity (Fig. 1). SF levels reached a maximum in men aged 30–49 years; by contrast, maximum levels of SF were observed in women after menopause (Fig. 1). SF levels were comparable among women of ethnic groups during the premenopausal years. After menopause, SF rose gradually and approached the levels found in males (Fig. 1).
A close association between SF distribution and prevalence of MetS was found in Indigenous men and women aged 19–59 years (Fig. 2). At ages 19–39 years, Indigenous (18·9 % of males and 11·6 % of females) had the highest and Penghu Islanders (8·9 % of males and 1·9 % of females) had the lowest prevalence of MetS. Prevalence of MetS was closely related to distribution of SF levels in young and middle-aged Indigenous males and females (Fig. 2). Overall, the prevalence of MetS remained relatively low among ethnic groups until after the fifth decade of life, after which all groups exhibited a steep rise in MetS and a concomitant decline in SF levels (Fig. 2).
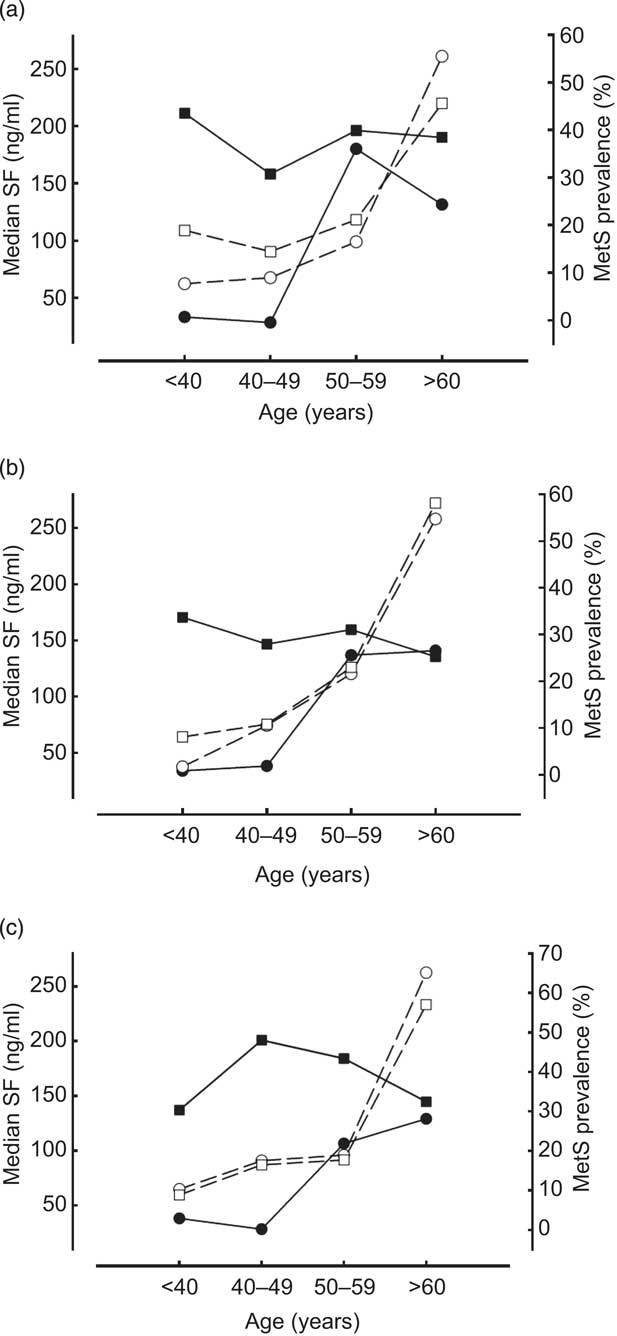
Fig. 2 Association between serum ferritin (SF) level (![]() , men;
, men; ![]() , women) and metabolic syndrome (MetS) prevalence (
, women) and metabolic syndrome (MetS) prevalence (![]() , men;
, men; ![]() , women) by decade of age for healthy Taiwanese men and women aged ≥19 years (n 2638) according to ethnic group: (a) Indigenous; (b) Hakka; (c) Penghu Islanders
, women) by decade of age for healthy Taiwanese men and women aged ≥19 years (n 2638) according to ethnic group: (a) Indigenous; (b) Hakka; (c) Penghu Islanders
Association between serum ferritin and metabolic syndrome and its components
A best-fit multivariable model was used to assess the association between SF and risk of MetS among ethnic groups. The adjusted odds for MetS was OR = 1·92 (95 % CI 1·31, 2·81) for the Nation for individuals in the highest SF tertile compared with those in the lowest, after adjusting for the age, sex, BMI, inflammatory markers (amylase, UA, CRP, GOT, GPT), lifestyle factors (smoking, betel-nut consumption) and family history of chronic diseases (hypertension, DM, hyperlipidaemia). The univariate logistic regression model identified Austronesia origin as independently associated with risk of MetS (OR = 2·61, 95 % CI 2·02, 3·36). Multiple logistic regression analysis showed the odds was substantially higher for Indigenous people (OR = 2·11, 95 % CI 1·02, 4·36; Table 2). By contrast, Hakka and Penghu Islanders yield the lowest risks (OR = 0·87, 95 % CI 0·34, 2·21 and OR = 1·85, 95 % CI 0·84, 4·09, respectively). We also show the prevalence rate ratio in Table 2 as sensitivity analysis. The estimation ratio was similar and the confidence interval was smaller (Table 2). For Indigenous, being in the highest SF tertile was significantly correlated with fasting glucose (OR = 2·56, 95 % CI 1·35, 4·86), serum TAG (OR = 2·17, 95 % CI 1·22, 3·85) and serum HDL-C (OR = 2·08, 95 % CI 1·15, 3·75; Table 3).
Table 2 Risk for MetS of racial/ethnic groups in relation to SF tertiles; healthy Taiwanese men and women aged ≥19 years (n 2638)

MetS, metabolic syndrome; SF, serum ferritin; Ref., reference category; PRR, prevalence rate ratio; UA, uric acid; CRP, C-reactive protein; GOT, glutamic–oxoacetic transaminase; GPT, glutamic–pyruvate transaminase; DM, diabetes mellitus.
†Hakka SF tertile cut-off values by gender: 110·3 ng/ml, 199·6 ng/ml for males; 43·3 ng/ml, 139·1 ng/ml for females.
‡Hakka adjusted for age, sex, BMI, inflammation (UA, CRP, GOT, GPT) and self-reported disease history (DM, hypertension, hyperlipidaemia, fatty liver disease).
§Indigenous SF tertile cut-off values by gender: 123·2 ng/ml, 260·4 ng/ml for males; 52·4 ng/ml, 147·6 ng/ml for females.
∥Indigenous adjusted for age, sex, inflammation (CRP, GOT, GPT) and self-reported disease history (DM, hypertension, hyperlipidaemia).
¶Penghu Islanders SF tertile cut-off values by gender: 114·9 ng/ml, 234·8 ng/ml for males; 47·2 ng/ml, 115·4 ng/ml for females.
††Penghu Islanders: adjusted for age, sex, BMI, inflammation (CRP, GOT, GPT) and self-reported disease history (DM, hypertension, hyperlipidaemia).
Table 3 Adjusted odds ratios and 95 % confidence intervals for the individual components of MetS by SF tertile among Indigenous people; healthy Taiwanese men and women aged ≥19 years (n 330)

MetS, metabolic syndrome; SF, serum ferritin; Ref., reference category; HDL-C, HDL-cholesterol.
†Age- and gender-adjusted.
‡Indigenous SF tertile cut-off values by gender: 123·2 ng/ml, 260·4 ng/ml for males; 52·4 ng/ml, 147·6 ng/ml for females.
Discussion
Our study raises the possibility that racial differences in SF tolerance may contribute to racial or geographic disparities in MetS. Fe overload and MetS are both chronic process that are known to be closely related to lifestyle and the life cycle. It has been known for some time that the prevalences of obesity and MetS are higher in Indigenous people than in Han Chinese in Taiwan( Reference Pan, Lee and Chuang 13 ). This motivated us to evaluate the effect of SF on MetS in relation to ethnicity. Although restricted to a small sample size, our study demonstrated that the odds for MetS was substantially higher for Indigenous people in the highest tertile of SF than for those in the lowest (OR = 2·11, 95 % CI 1·02, 4·36). By contrast, Hakka and Penghu islanders yielded the lowest risks (OR = 0·87, 95 % CI 0·34, 2·21 and OR = 1·85, 95 % CI 0·84, 4·09, respectively). This difference is not explained by the SF concentrations per se because both Han Chinese and Indigenous people had similar crude and adjusted SF concentrations. A 4-year follow-up study in 1038 Finnish men aged 42–60 years by Salonen et al. demonstrated that even mildly increased body Fe stores predict the development of non-insulin dependent diabetes( Reference Salonen, Tuomainen and Nyyssonen 31 ). A recent study showed that SF or ferritin L-chain/H-chain induces pro-inflammatory cytokine secretions via NF-κB pathways in rat hepatic stellate cells( Reference Ruddell, Hoang-Le and Barwood 32 ). Our in vitro data confirmed the pro-inflammatory activity of SF even in physiological concentrations (JS Chang, unpublished results). These data raise the possibility that SF per se acts as a signalling molecule and long-term exposure to elevated SF, even mild elevation, may have a profound effect on MetS. Our study showed that Austronesian origin did not predispose Indigenous people to high SF levels compared with Han Chinese. However, we noticed that SF concentrations were slightly higher in young Indigenous men aged >19 to <40 years compared with Hakka and Penghu Islander men, although it did not reach statistical significance (Fig. 2). Accordingly, young Indigenous men aged >19 to <40 years had higher prevalence of MetS than Hakka and Penghu Islanders (Fig. 2). These data imply that perhaps earlier exposure to mildly elevated SF levels may sensitize young Indigenous men to MetS. A follow-up study is required in the young Indigenous population to clarify the cause-and-effect relationship between SF and MetS and the racial disparities in MetS.
By the studying association between SF levels and DM in six racial/ethnic groups, Acton et al. showed a positive association between SF levels and DM risk in women across all racial groups( Reference Acton, Barton and Passmore 33 ). A notable exception was in Asian and Pacific Islander men. Asian and Pacific Islander men with SF concentrations in quintile 2 had increased risk for DM compared with those with the lowest SF quintile. The relationship between SF levels and DM in Asian/Pacific Islander men was not linear but bimodal, indicating that Asian/Pacific Islander men were more susceptible to SF. Although we were unable to conduct gender-specific examination due to the limited sample size, our data were partially in agreement with Acton et al.'s findings( Reference Acton, Barton and Passmore 33 ).
To our knowledge, few studies have investigated the relationship between SF levels and MetS in a racially/ethnically diverse population. Blacks( Reference Zacharski, Ornstein and Woloshin 34 ), Asians and Pacific Islanders( Reference Adams, Reboussin and Barton 1 , Reference Harris, McLaren and Reboussin 2 ) are known to have higher SF concentrations than whites. However, a series of questions are also raised by these observations. Is genetic predisposition the basic reason behind the condition of high SF concentrations among Asians, blacks and Pacific Islanders compared with whites? Or are lifestyle-associated factors (e.g. dietary Fe intake, obesity, hepatic health) responsible for the racial disparities in hyperferritinaemia? Will high levels of SF predispose Asians to MetS? Zacharski et al. showed that different patterns of SF level exist according to age, sex and race (white/Hispanic v. black)( Reference Zacharski, Ornstein and Woloshin 34 ). However, we did not observe a similar association between SF concentrations and race/ethnicity (Han Chinese v. Austronesia origin). By contrast, an age-associated increase in body Fe stores was noted in all racial groups in our study.
SF concentration normally reflects body Fe stores in healthy individuals. However, SF is also an acute-phase protein and abnormal SF levels are commonly associated with chronic inflammation. Our first attempt was to understand whether SF distribution differed among ethnic groups; particularly, before the onset of MetS. We found there were no differences in crude mean SF concentrations among ethnic groups by decade of age for women and men (data not shown). We next attempted to minimize the potential confounding factor by excluding those individuals with SF >500 ng/ml (as a surrogate marker of chronic inflammation) or SF >500 ng/ml and family health history for liver diseases and diabetes. Both exclusion criteria did not change the distribution of SF by age, sex and stratified by race. Due to the restricted small sample size among ethnic groups, we decided not to exclude individuals with self-reported family health histories or those on medications for further analysis. However, we cannot rule out residual confounding effects due to failure to adjust for inflammatory conditions.
Our study is limited by the small sample size, particularly for the three ethnic groups, and confined by its cross-sectional nature. In order to understand the casual relationship between SF levels and MetS, a longitudinal study is necessary in order to understand if changes in Fe stores over time predict disease susceptibility in an apparently healthy population. Alternatively, future work should investigate the association between SF concentrations and risk of MetS at younger ages. Obese children and teenagers have relatively low grade of inflammation compared with obese adults. Thus, such a study will allow us to clarify the relevant contribution of certain environmental factors to the exaggerated Fe accumulation and disease risk. In addition, we rely on geographical location for the category of ethnicity, which may not be as accurate as the self-report of identity. However, previous data showed that >80 % of people living in the mountainous strata had aboriginal ancestry and ∼70–80 % of people living in Hakka strata were of Hakka origin. Such variations should lead the association towards the null. Also, the use of a 24 h dietary record may be not of sufficient length to obtain reliable data on Fe intakes.
Conclusion
Our study showed that the distribution of SF levels was comparable among ethnic groups; however, Indigenous people in the highest SF tertile were at highest risk for developing MetS than those in the lowest SF tertile. This association was limited to the Austronesia origins but not to Hakka and Penghu Islanders. Persons of Austronesia origin may be more sensitive to the change in levels of SF compared with Han Chinese. Future studies investigating the ‘cause-and-effect’ relationship between changes of SF distributions and risk of MetS among young ethnic groups are warranted.
Acknowledgements
Sources of funding: The Third Nutrition and Health Survey in Taiwan (NAHSIT 2005–2008, Adults) was sponsored by the Department of Health in Taiwan (DOH94-FS-6-4). J.-S.C. was supported by grant TMU100-AE1-B09. Conflicts of interest: The authors have no financial relationships relevant to this article to disclose. Authors’ contributions: J.-S.C. conceptualized and designed the study, drafted the initial manuscript and approved the final manuscript as submitted. S.-M.L. participated in Fe biochemistry analysis. J.C.-J.C. supervised data collection and carried out the initial data analyses. Y.-C.C. participated in data collection and pro-inflammatory cytokines analysis. C.-M.W. participated in biochemistry analysis of MetS components. N.-H.C. participated in data collection and analysis of 24 h dietary records. W.-H.P. conceptualized the National Nutrition and Health Survey and approved the final manuscript as submitted. C.-W.B. carried out the initial analyses and critically reviewed the manuscript. Acknowledgements: The authors express their sincere appreciation to the study participants. They also wish to thank staff from the Research Center for Humanities and Social Sciences, Center for Survey Research, Academia Sinica and Dr Wen-Han Pan and Dr Su-Hao Tu (Directors).







