Introduction
Myxozoans are microscopic, obligate, endoparasitic cnidarians with complex life cycles (Okamura et al., Reference Okamura, Gruhl, Bartholomew, Okamura, Gruhl and Bartholomew2015a). Transmission from host to host is achieved by multicellular spores whose morphologies have been used extensively for taxonomic purposes. However, as it became clear that convergence in spore morphotypes could be problematic, researchers have increasingly incorporated small subunit ribosomal DNA (SSU rDNA) sequences as additional data for the reliable identification of species (Kent et al., Reference Kent, Andree, Bartholomew, El-Matbouli, Desser, Devlin, Feist, Hedrick, Hoffmann, Khattra, Hallett, Lester, Longshaw, Palenzeula, Siddall and Xiao2001; Atkinson et al., Reference Atkinson, Bartošová-Sojková, Whipps, Bartholomew, Okamura, Gruhl and Bartholomew2015). Myxozoans are comprised of two lineages: the speciose Myxosporea and the species-poor Malacosporea. Collectively there are about 2500 described myxozoan species (Okamura et al., Reference Okamura, Hartigan and Naldoni2018). Myxosporean lifecycles involve annelids as definitive hosts and vertebrates, mainly fishes, as intermediate hosts. Malacosporeans use freshwater bryozoans as definitive hosts and fish as intermediate hosts.
To date only five malacosporean species have been described (Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016). There are two malacosporean genera: Tetracapsuloides and Buddenbrockia. Species in both genera develop as sac-like or vermiform (myxoworm) stages in the body cavity of their freshwater bryozoan hosts (Hartikainen et al., Reference Hartikainen, Gruhl and Okamura2014). Spores produced within sacs and myxoworms are infectious to fish. The only malacosporean whose life cycle has been resolved and whose development in both hosts has been characterized is Tetracapsuloides bryosalmonae, the causative agent of salmonid proliferative kidney disease (PKD). Tetracapsuloides bryosalmonae develops as sacs in the body cavity of freshwater bryozoans (Anderson et al., Reference Anderson, Canning and Okamura1999; Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura1999) and as pseudoplasmodia in kidney tubules of salmonid fish (Kent and Hedrick, Reference Kent and Hedrick1985). Spores released from bryozoans infect fish (Feist et al., Reference Feist, Longshaw, Canning and Okamura2001) and spores passed with fish urine infect bryozoans (Hedrick et al., Reference Hedrick, Baxa, de Kinkelin and Okamura2004; Morris and Adams, Reference Morris and Adams2006).
Recent studies provide evidence for a further 12 undescribed species of malacosporeans from a diversity of bryozoan and fish hosts (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014; Hartikainen et al., Reference Hartikainen, Gruhl and Okamura2014; Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016). These results suggest that there is substantially greater diversity of malacosporeans than is currently appreciated and that further investigations may link malacosporeans detected in fish with those detected in bryozoans, thereby resolving life cycles. The detection of undescribed malacosporeans in fish has, however, largely been gained by polymerase chain reaction (PCR) and sequencing of material from fish kidney without ascertaining spore development in the putative fish hosts. It is therefore possible that putative fish hosts may be accidental (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014; Hartikainen et al., Reference Hartikainen, Gruhl and Okamura2014). For example, larvae of some nematode parasites (e.g. Ancylostoma braziliense, Ancylostoma caninum, Toxocara canis and Gnathostoma spinigerum) may begin their development in humans but only develop to mature adult worms in their true mammal hosts (Rey, Reference Rey2008). Similarly, malacosporeans could invade fish as blood stages that are detected by PCR of fish kidney but fish may not support subsequent spore development. Indeed, this has been observed when local T. bryosalmonae strains infect exotic rainbow trout in the UK (Bucke et al., Reference Bucke, Feist and Clifton-Hadley1991; Morris et al., Reference Morris, Adams and Richards1997).
The aim of this study was to characterize malacosporeans in a diversity of fish kidney material, employing both molecular and ultrastructural methods. By using this combined approach, we are able to confirm that malacosporeans exploit fish hosts belonging to at least three families. In addition, we are able to resolve a second malacosporean life cycle by linking the vertebrate and invertebrate hosts. Extending our knowledge of malacosporean host diversity is of general importance for understanding biodiversity, ecology and co-evolutionary relationships in freshwater systems and could be relevant for diagnosis and control of emerging diseases in aquaculture or wild fish populations in our changing world.
Materials and methods
A total of 256 fish kidney were screened for the presence of malacosporean DNA. The material we studied was obtained by a mixture of general and targeted sampling. The former involved taking advantage of ongoing project work sampling fish in the River Stour (electrofishing) and in Blickling Lake (rod fishing), and screening for fish parasites in practical classes in Switzerland (caught by net). More targeted sampling included material collected during surveys for parasites in paddlefish in the USA and by specifically sampling fish on farms in Brazil. Nineteen fish species belonging to nine fish families (Salmonidae, Cyprinidae, Nemacheilidae, Esocidae, Percidae, Polyodontidae, Serrasalmidae, Cichlidae and Pimelodidae) were sampled from the UK, Switzerland, Brazil and the USA (Tables 1 and 2). We included archived samples of brown and rainbow trout that were known to be infected by T. bryosalmonae to provide comparative material because the development of T. bryosalmonae is well known. The fish were euthanized in Brazil by benzocaine overdose, in accordance with Brazilian law (Federal Law No. 11.794, dated 8 October 2008 and Federal Decree No. 6899, dated 15 July 2009), and in Europe by a blow to the head, followed by severance of the spinal cord. Approximately 27 mm3 of tissue was immediately dissected from the posterior portion of the kidney. One-half of the kidney material was fixed in 99% ethanol for the molecular analysis and the other half in 2.5% glutaraldehyde in 0.2 m sodium cacodylate buffer for the ultrastructural studies described below.
Table 1. Fish sampled from the UK and Switzerland that were infected with malacosporeans

Details on collection sites, fish hosts, malacosporean species; prevalence of infection, SSU rDNA sequence lengths; sequences that provided the closest match in the GenBank, and bryozoans (*) and fishes previously identified to be infected by the respective parasites. Archived samples of brown and rainbow trout known to be infected by T. bryosalmonae (**) or infected by T. bryosalmonae through transmission studies (***). bp, base pairs.
Table 2. Fish sampled from the UK, Switzerland, the USA and Brazil in which infections were not detected

Data on collection sites, fish species and the number of fish sampled (No. sampled).
DNA extraction, PCR amplification, sequencing and species identification
The DNA was extracted using a DNeasy® Blood & Tissue kit (Qiagen, USA), following the manufacturer's instructions. Malacosporean specific mala-f and mala-r primers (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2010) were used in PCRs for all samples, amplifying approximately 680 bp of the SSU rDNA. Malacosporean-specific budd-f and budd-r primers (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2010) were then subsequently used to amplify almost complete length SSU rDNA giving a product that is approximately 1784 bp. General myxozoan primers such as MedlinA and MedlinB (Medlin et al., Reference Medlin, Elwood, Stickel and Sogin1988) were trialled in pilot work but did not amplify any malacosporeans.
PCRs were carried out in 25 µL reaction volumes using 100 ng of extracted DNA, 5× Go Taq Flexi buffer (Promega, Madison, WI, USA), 10 mm dNTP mix, 25 mm MgCl2, 10 mm for each primer and 1× GoTaq G2 Flexi DNA polymerase (Promega, Madison, WI, USA). The original cycling conditions were used for mala-f and mala-r primers as described by Grabner and El-Matbouli (Reference Grabner and El-Matbouli2010). For runs using budd-f and budd-r primers an initial denaturation stage at 95 °C for 5 min was followed by 40 cycles of denaturation at 95 °C for 45 s, annealing at 61 °C for 45 s, extension at 72 °C for 105 s, finishing with an extended elongation stage at 72 °C for 8 min. The cycling conditions were modified from Grabner and El-Matbouli (Reference Grabner and El-Matbouli2010) for budd-f and budd-r primers to increase primer specificity.
Ultrastructural investigation suggested the presence of sphaerosporid myxozoans in kidney of white fish and dace. To identify cases presenting simultaneous infections of both sphaerosporids and malacosporeans all kidney material was screened by nested PCR using the primers and conditions outlined in Patra et al. (Reference Patra, Bartošová-Sojková, Pecková, Fiala, Eszterbauer and Holzer2018) (Erib 1 and 10 primers for primary PCR [95 °C for 5 min, 35 cycles of 94 °C for 1 min, 60 °C for 1 min, extension 90 s] followed by final extension of 5 min and SphFWSSU1243F and SphFWSSU3418R for nested PCR primers [95 °C for 5 min, 35 cycles of 94 °C for 1 min, 56 °C for 1 min, extension 90 s] followed by final extension of 5 min).
PCR products were electrophoresed in 2.0% agarose gel, stained with gel red and analysed by a Syngene Transilluminator. PCR products were purified using a Gel/PCR DNA Fragment Extraction Kit (Geneaid Biotech Ltd., USA) and sequenced. This work was conducted in the Molecular Biology Unit of the Natural History Museum, London (NHM) using the Applied Biosystems 3730xl DNA Analyser for Sanger sequencing. OTUs were compared with SSU rDNA sequence data in the GenBank and species identity was based on >99% similarity (Whipps and Kent, Reference Whipps and Kent2006; Bartošová and Fiala, Reference Bartošová and Fiala2011; Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014). An alignment of the original SSU rDNA sequences obtained in this study and related species from the GenBank (see Table 1 for sequence length), was used to produce a pairwise dissimilarity matrix using MEGA 6.0 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011).
Electron microscopy
Pieces of kidney were fixed in 2.5% glutaraldehyde in 0.2 m sodium cacodylate buffer, pH 7.4 and post-fixed in 1% OsO4 in cacodylate buffer. Matching kidney material revealed to be positive for malacosporean infection (as identified by PCR and sequencing) was then dehydrated in a graded series of ethanol and embedded in Agar 100 resin (Agar Scientific, Stansted, UK) via propylene oxide. Semi-thin sections were stained with toluidine blue and ultrathin sections with uranyl acetate and lead citrate. Material was examined using a Hitachi H-7650 transmission electron microscope available at the NHMs sister institute, Jodrell Laboratory at Kew Gardens and a LEO 906 electron microscope available at the University of Campinas (UNICAMP), São Paulo, Brazil. We attempted to locate malacosporean infections in at least five kidneys that were identified as positive by PCR of each fish species. The number of kidneys analysed by ultrastructure was ultimately constrained by availability, suitability of material and time (see Table 3).
Table 3. The detection of malacosporean infections in fish kidney material from the UK and Switzerland (CF) according to PCR, sequencing and ultrastructural analysis

Included are fish species and locality data, the malacosporean species inferred by sequencing, the number (No.) of fish kidneys detected by PCR, the number of kidneys examined by ultrastructure and the number of kidneys in which spores were detected by ultrastructure.
a Degraded material.
Results
Malacosporean infections were detected in the kidney of fishes originating from the UK and Switzerland. Five species were identified: T. bryosalmonae; Tetracapsuloides sp. 4 (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014) (also referred to as Tetracapsuloides sp. 3 [Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016] and from here on called Tetracapsuloides sp. 4); Tetracapsuloides sp. 5 (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014) (also referred to as Tetracapsuloides sp. 2 [Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016] and from here on called Tetracapsuloides sp. 5); Buddenbrockia plumatellae and Buddenbrockia sp. 2 (Hartikainen et al., Reference Hartikainen, Gruhl and Okamura2014) (also referred to as Buddenbrockia sp. 4 [Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016] and from here on called Buddenbrockia sp. 2). The infection prevalences of these species ranged from 5.5 to 100% (Table 1).
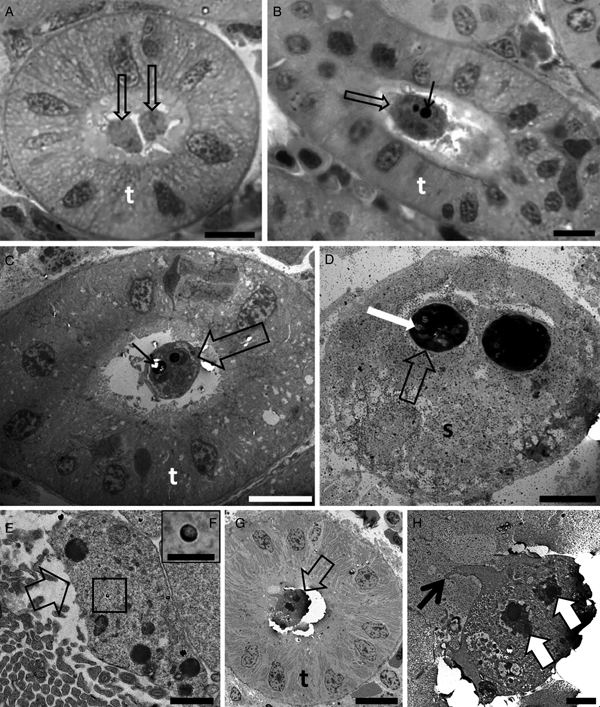
Tetracapsuloides bryosalmonae was identified to infect 85.7% of white fish from Lake Lucerne (n = 7), 77.7% of the brown trout specimens examined from the River Stour (n = 9), 89.0% of brown trout from the River Brubach (n = 18) and 100% of rainbow trout from the River Furtbach (n = 12) (Table 1). Young amorphous sporogonic stages (Fig. 1A) and mature spores (Fig. 1B–D) of T. bryosalmonae were found in kidney tubules of brown trout from the River Stour. Young amorphous sporogonic stages containing sporoplasmosomes with a lucent area (Fig. 1E, F) and a pseudoplasmodium connected to the kidney tubule wall via pseudopodia (Fig. 1G, H) were observed in white fish from Lake Lucerne. However, because PCR indicated co-infection of this material (see below), it is possible these are immature sporogonic stages of a sphaerosporid. Unfortunately, although five white fish kidneys were examined by ultrastructure, the only kidney that revealed developmental stages was this co-infected material (Table 3).
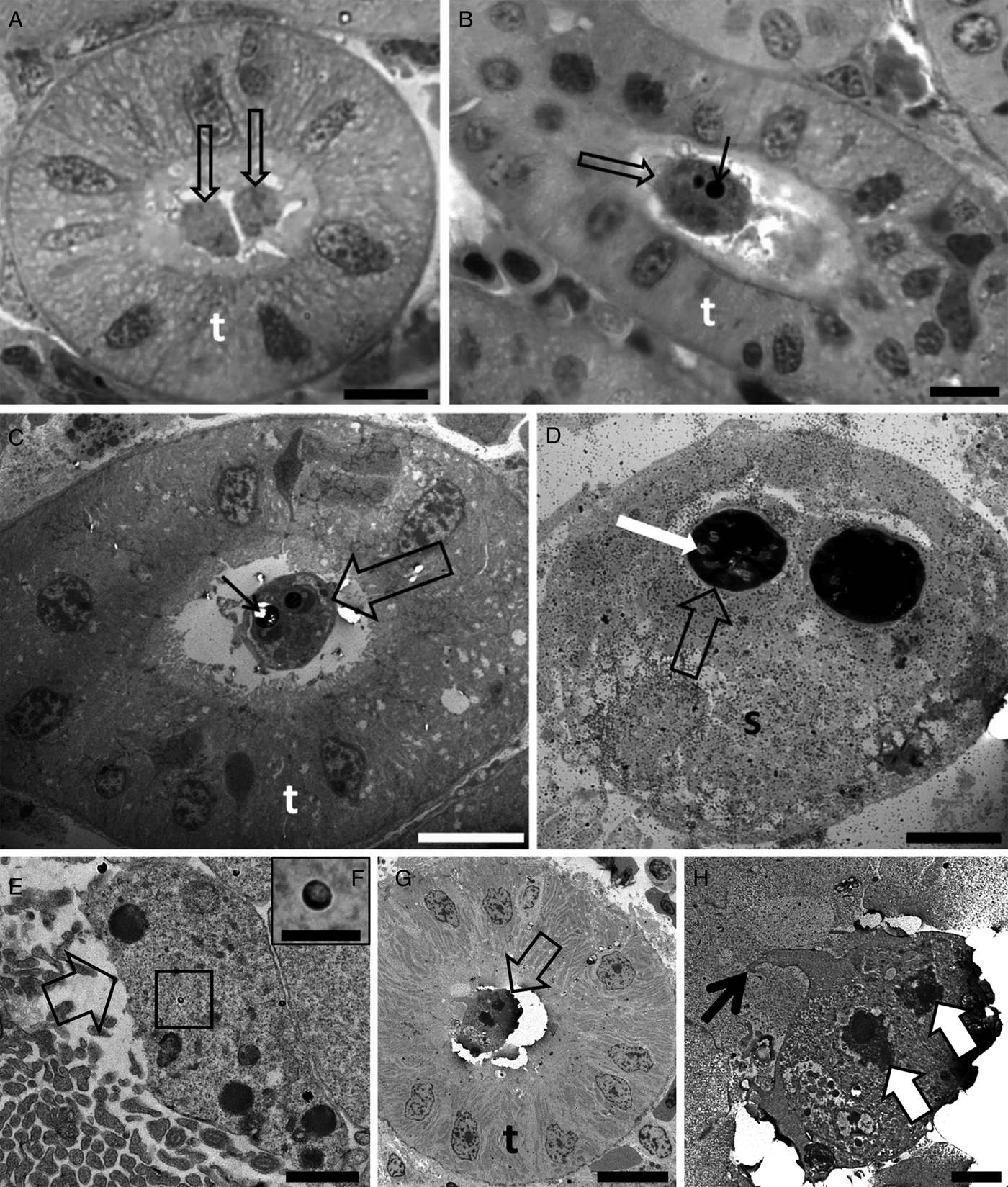
Fig. 1. Photomicrography of kidney tubules (t) of brown trout collected in the River Stour, Kent, UK, in semi-thin sections stained by toluidine blue (A, B). Transmission electron microscopy (C–H) showing the development of Tetracapsuloides bryosalmonae spores in the lumen of the kidney tubules (t) of brown trout (C and D) collected in the River Stour, Kent, UK, and myxozoan development (either malacosporean or sphaerosporid) in white fish (E–H), collected in the Lake Lucerne, Switzerland. (A) Note the presence of two early developmental stages (arrows) developing attached to the kidney tubule wall. Scale bar = 10 µm. (B and C) Advanced stage of spore development (empty arrows) showing polar capsule (thin black arrows). Scale bar = 10 µm. (D) Mature spore (s) showing polar capsule (empty arrow) with polar filaments (white arrow). Scale bar = 2 µm. (E) Primary cell (empty arrow) developing in the lumen of the kidney tubule. Note the presence of sporoplasmosomes (box). Scale bar = 1 µm. (F) High magnification of E showing the sporoplasmosomes with a lucent area. Scale bar = 200 nm. (G) Pseudoplasmodium (empty arrow) developing attached to the kidney tubule wall. Scale bar = 10 µm. (H) High magnification of G showing pseudoplasmodium connected to the kidney tubule wall via pseudopodia (thin black arrow). Note the two secondary cell nuclei (white arrows). Scale bar = 2 µm.
Buddenbrockia plumatellae infected 33.3% (n = 16) of the dace specimens from the River Stour, 53.8% of the roach from Blickling Lake (n = 13), and 5.5% of the roach from Lake Lucerne (n = 18) (Table 1). Unfortunately, ultrastructure was uninformative, being compromised by degeneration of dace material.
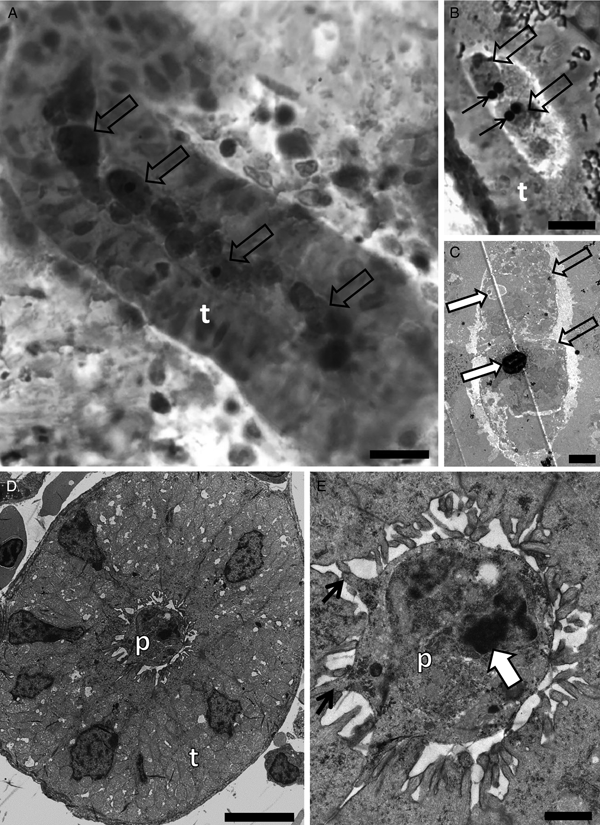
Tetracapsuloides sp. 4 was detected in the kidney of roach sampled from the River Stour at a prevalence of 6.3% (n = 16) (Table 1). Advanced developmental stages anchored to the kidney tubule wall via pseudopodia showed polar capsules and sporoplasmosomes with the characteristic lucent area (Fig. 2A–D).

Fig. 2. Photomicrography of kidney tubules (t) of roach collected in the River Stour, Kent, UK, in semi-thin sections stained by toluidine blue (A), and by transmission electron microscopy (B–D) showing spore development of Tetracapsuloides sp. 4. (A) Advanced stage of spore development (empty arrow) with polar capsules (white arrow). Note a stage connected to the kidney tubule wall (thin black arrow). Scale bar = 20 µm. (B) Two pseudoplasmodia (p) showing secondary cell nuclei (empty arrows) and a polar capsule (white arrow). Note pseudopodia anchoring the parasite to the kidney tubule wall via pseudopodia (thin black arrows). Scale bar = 2 µm. (C) Primary cell (p) with scattered sporoplasmosomes (box). Scale bar = 2 µm. (D) High magnification of box in panel (C) showing details of sporoplasmosomes, each with a lucent area. Scale bar = 500 nm.
Tetracapsuloides sp. 5 was detected in the kidney of gudgeon from the River Stour at a prevalence of 42.9% (n = 21) (Table 1). Clear developmental stages of spores were not observed in kidney tubules.
Buddenbrockia sp. 2 was detected in 100% (n = 20) of the stone loach specimens sampled from the River Stour (Table 1). Ultrastructural analysis revealed sporogonic stages and mature spores in kidney tubules (Fig. 3A–E).
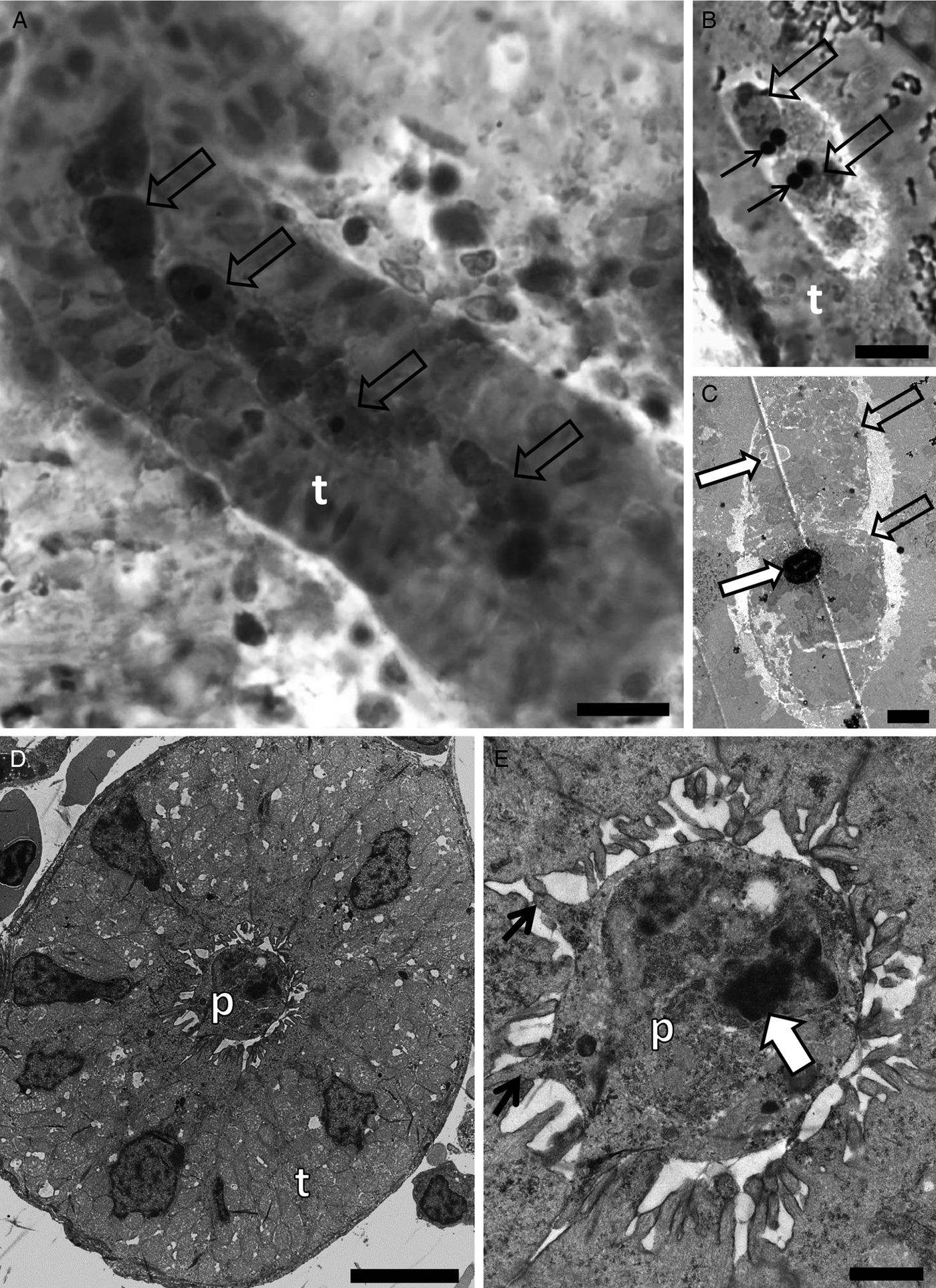
Fig. 3. Photomicrography of kidney tubules (t) of stone loach collected in the River collected in the River Stour, Kent, UK, in semi-thin sections stained by toluidine blue (A and B), and by transmission electron microscopy (C–E) showing spore development of Buddenbrockia sp. 2. (A) Note the different stages of development of spores (arrows) as well as earlier developmental stages. Scale bar = 10 µm. (B) Two spores in advanced developmental stages (empty arrows) with polar capsules (black arrows). Scale bar = 10 µm. (C) Note two young developmental stages of spores (empty arrows), with a polar capsule in development (large white arrows). Scale bar = 2 µm. (D) Pseudoplasmodium (p) in the lumen of the kidney tubule. Scale bar = 5 µm. (E) High magnification of D showing pseudoplasmodium connected to the kidney tubule wall via pseudopodia (thin black arrow). Note the secondary cell nucleus (white arrow). Scale bar = 1 µm.
There was low divergence between the SSU rDNA sequences of the malacosporean species found in this study and the most similar sequences available in the GenBank (ranging from 0.1 to 0.3%) (Table 4).
Table 4. Dissimilarity matrix for SSU rDNA of malacosporean species found in this study and their closest matches in the GenBank

The upper triangle shows nucleotide differences in relation to the number of bases compared. The lower triangle shows % pairwise distances. SSU rDNA = 18 small subunit ribosomal DNA; UK: United Kingdom, CF: Switzerland. BS = Bartošová-Sojková et al. (Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014); H = Hartikainen et al. (Reference Hartikainen, Gruhl and Okamura2014).
Sphaerosporid co-infections were identified in four individuals, two dace from Kent and two white fish from Zurich. Both host species have previously been reported with sphaerosporid infections in Europe (El-Matbouli and Hoffman, Reference El-Matbouli and Hoffman1996; Patra et al., Reference Patra, Bartošová-Sojková, Pecková, Fiala, Eszterbauer and Holzer2018). Rounded sporogonic stages (Fig. 4A and D) with an electron-dense material surrounding each early developmental spore stage (black arrows in Fig. 4A, B, E, F) were observed in white fish and dace. The same electron-dense material was observed forming the hard valves of a mature spore in white fish (Fig. 4C).
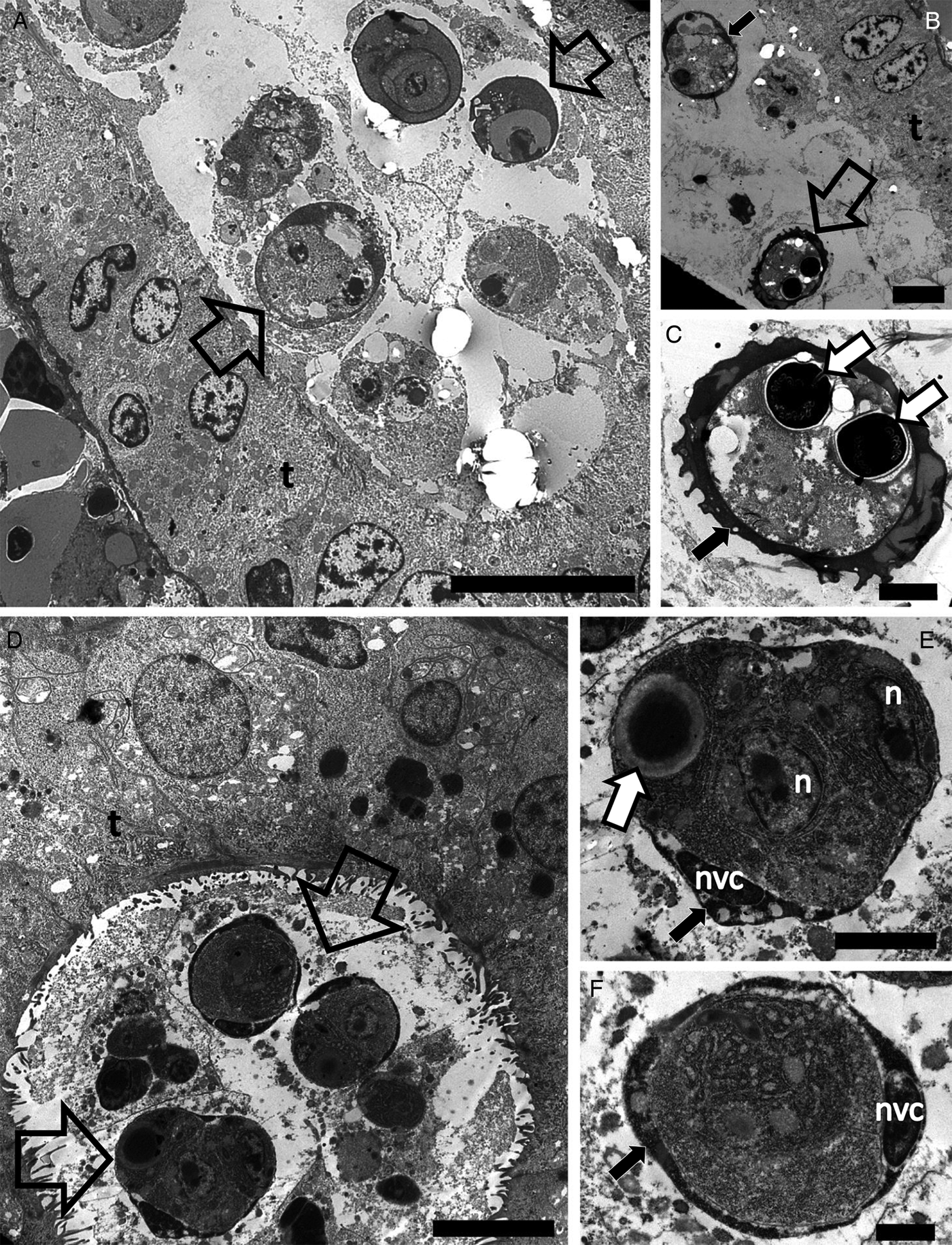
Fig. 4. Transmission electron microscopy showing the development of Sphaerospora spp. spores in the lumen of kidney tubules of white fish (A–C) collected in the Lake Lucerne, Switzerland and dace (D–F) collected in the River Stour, Kent, UK. (A) Sporogonic stages (empty arrows) developing in the lumen of the kidney tubule of white fish. Scale bar = 10 µm. (B) Early sporogonic stage with electron dense material in valvogenic cells (black arrow) and a mature spore (empty arrow) in the lumen of the kidney tubule. Scale bar = 5 µm. (C) High magnification of C showing the mature spore with electron dense material forming the hard valves (black arrow) and two polar capsules (white arrows). Scale bar = 2 µm. (D) Sporogonic stages (empty arrows) developing in the lumen of the kidney tubule of dace. Scale bar = 5 µm. (E) High magnification of (D) showing a sporogonic stage with electron dense material in the valvogenic cells (black arrow), the nucleus of the valvogenic cell (nvc), other nuclei (n) and a polar capsule (white arrow). Scale bar = 2 µm. (F) A sporogonic stage showing electron dense material in the valvogenic cells (black arrow) and the nucleus of the valvogenic cell (nvc). Scale bar = 1 µm.
Malacosporean infections were detected by ultrastructure for material that was positive by PCR in 4 of 33 cases that were examined (see Table 3).
Discussion
Malacosporeans exploit a diversity of fish hosts
Our results demonstrate that a range of fish hosts belonging to different families are used by the two currently recognized malacosporean genera, Tetracapsuloides and Buddenbrockia. Infection of trout by T. bryosalmonae has been known for decades (Kent and Hedrick, Reference Kent and Hedrick1985), with many studies demonstrating development in kidney tubules of brown and rainbow trout in the UK and the USA (Kent and Hedrick, Reference Kent and Hedrick1986; Clifton-Hadley and Feist, Reference Clifton-Hadley and Feist1989; Hedrick et al., Reference Hedrick, MacConnell and Kinkelin1993; Morris et al., Reference Morris, Adams and Richards2000). Tetracapsuloides bryosalmonae has been suggested to infect all salmonid species (Hedrick et al., Reference Hedrick, MacConnell and Kinkelin1993) but whether all species serve as effective hosts is unclear. The consistent lack of sporogony in exotic rainbow trout in Europe (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2008; Hartikainen and Okamura, Reference Hartikainen, Okamura, Okamura, Gruhl and Bartholomew2015) demonstrates that, although some salmonids are susceptible to infection, they are accidental hosts. Our results suggest that white fish in Switzerland may also serve as hosts of T. bryosalmonae but we were unable to definitively confirm spore production in white fish that were not also infected with sphaerosporids. The prevalences of T. bryosalmonae infections were similar in white fish, brown trout and rainbow trout, although it should be stressed that this observation is based on relatively low sample sizes.
Our further studies of Tetracapsuloides spp. were also informative. The presence of sporogonic stages including advanced developmental stages of spores of Tetracapsuloides sp. 4 in kidney tubules in roach from the River Stour, imply that roach is a true host. The prevalence of infection (6.3%) was lower than that reported in a study based on molecular analyses of roach kidney material from the Czech Republic (100%; Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014). However, the high prevalence reported by Bartošová-Sojková et al. (Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014) is very likely biased by low sample size (n = 2). Tetracapsuloides sp. 5 was detected in the kidney of gudgeon from the River Stour where the prevalence of infection was 43.0%. Previous molecular investigation detected this species in gudgeon in the Czech Republic, with prevalences of 33.0 and 91.0% (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014). We did not observe sporogonic stages in kidney tubules and thus cannot confirm the host status of gudgeon. Nevertheless, recurrent detection of this species in gudgeon (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014; Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016) often at substantial prevalences suggests that infection by Tetracapsuloides sp. 5 is common. Further work is required to clarify the host status of gudgeon.
Bartošová-Sojková et al. (Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014) found B. plumatellae infections in dace, roach and bleak (Alburnus alburnus) in the Czech Republic at 100, 60.0 and 46.0% infection prevalences, respectively. We detected this species in dace from the River Stour (infection prevalence = 33.3%) and in roach from Blickling Lake (infection prevalence = 53.8%) and Lake Lucerne (infection prevalence = 5.5%). Grabner and El-Matbouli (Reference Grabner and El-Matbouli2010) showed, in a cohabitation study, that B. plumatellae was transmitted from bryozoans to carp and minnow. The collective evidence thus suggests that of B. plumatellae is able to exploit a range of cyprinid hosts but it remains to be confirmed whether roach and dace support sporogony.
Our detection of Buddenbrockia sp. 2 in stone loach is the first time this malacosporean has been linked with a fish host. Hartikainen et al. (Reference Hartikainen, Gruhl and Okamura2014) reported infections of Buddenbrockia sp. 2 which develops as myxoworms in the bryozoan Fredericella sultana sampled in the UK, Switzerland and Germany. Our ultrastructural analyses revealed sporogonic stages and mature spores in kidney tubules, identifying stone loach as a true host. Thus, the life cycle of Buddenbrockia sp. 2 appears to be resolved, with the parasite exploiting F. sultana as an invertebrate host and Barbatula barbatula as a vertebrate host. It is of course conceivable that further fish and bryozoan hosts may be used.
It should be noted that no signals of kidney infection were observed when fish were dissected to collect material for study, an observation in keeping with the general view that many myxozoan infections are innocuous and/or have little impact on fish hosts (Shul'man, Reference Shul'man1990; Lom and Dyková, Reference Lom, Dyková, Lom and Dyková1992). It is also consistent with the weak or absent immune response, typically observed in natural fish hosts of malacosporeans (Grabner and El-Matbouli, Reference Grabner and El-Matbouli2008). Such inapparent infections almost certainly contribute to the general lack of investigation of malacosporean infections in fish. Notably, inapparency also characterized the infections of brown trout and white fish by T. bryosalmonae, suggesting that environmental conditions and/or fish health status were not conducive for PKD development. However, the high infection prevalences (78.0% in the River Stour and 87.0% in Lake Lucerne) suggest that many fish may have the potential to develop disease.
We should also note that in only about 12% of cases (4/33) where we obtained positive PCR results were able to detect malacosporean stages by ultrastructure in the paired kidney material (Table 3). In some cases this was due to degraded material (e.g. dace). In other cases, this could reflect little proliferation and development in kidney, which then made detection by ultrastructure very difficult and eventually we ceased searching.
The challenge of identifying fish hosts
The confirmation of malacosporean fish host status is variously challenging. For example, the lack of detection of malacosporeans in material from Brazil may reflect seasonality, low sample sizes, lack of examination of appropriate fish age classes or absence of bryozoan hosts where the fish were sampled. We anticipate that malacosporeans are present in Brazil in view of observations by Marcus (Reference Marcus1941) of Buddenbrockia infections in bryozoans in São Paulo State. In addition, ultrastructural detection of sporogonic stages of malacosporeans in the vertebrate host is complicated. It requires extensive sectioning of embedded material to search for small developmental stages, and spores that may be patchily distributed in kidney tubules. The failure to detect sporogonic stages in some cases may simply arise from a limited number of parasites or because the infection has not yet matured. This is compounded by the inapparency of many infections at the macroscopic level.
As shown here, a targeted approach employing associated SSU rDNA sequencing to confirm infection status will at least identify what material to investigate. The alternative approach of conducting transmission trials to confirm that infection is transmitted from fish to bryozoans requires fish husbandry, and permits are often required for such work. We suggest a potential alternative molecular approach for future identification of fish hosts by determining whether genes specifically involved in polar capsule development (e.g. minicollagens and nematogalactins: Holland et al., Reference Holland, Okamura, Hartikainen and Secombes2011; Shpirer et al., Reference Shpirer, Chang, Diamant, Rubinstein, Cartwright and Huchon2014, NSPs 1–7: Shpirer et al., Reference Shpirer, Diamant, Cartwright and Huchon2018) are expressed in infected kidney. The rationale is that polar capsules are only present in malacosporean spores and thus the detection of such expressed genes would indicate spore development. In practical terms this would involve preservation of kidney material in e.g. RNAlater and confirmation that these genes are not expressed in pre-sporogonic developmental stages.
Ultrastructural distinction of malacosporean and myxosporean sporogonic stages in fish kidney tubules
We found co-infections of T. bryosalmonae and a sphaerosporid species in white fish and of B. plumatellae and a sphaerosporid species in dace. The most apparent morphological difference between myxosporean and malacosporean spores is the composition of their valves, which are hardened in myxosporeans but remain soft in malacosporeans (Anderson et al., Reference Anderson, Canning and Okamura1999; Canning and Okamura, Reference Canning and Okamura2004). The hardening of myxosporean spore valves is achieved by secretion of chitin (Mñnoz et al., Reference Muñoz, Palenzuela, Alvarez-Pellitero and Sitjà-Bobadilla1999, Reference Muñoz, Sitjà-Bobadilla and Álvarez-Pellitero2000; Liu et al., Reference Liu, Zhou, Miao, Zhang, Cao, He, Bai and Yao2011), which may also be associated with internal organelles (Lukeš et al., Reference Lukeš, Volf and Lom1993; Muñoz et al., Reference Muñoz, Sitjà-Bobadilla and Álvarez-Pellitero2000; Redondo et al., Reference Redondo, Cortadellas, Palenzuela and Alvarez-Pellitero2008). This glycoprotein likely protects myxosporean spores from environmental degradation and maintains spore shape (Mñnoz et al., Reference Muñoz, Palenzuela, Alvarez-Pellitero and Sitjà-Bobadilla1999; Kaltner et al., Reference Kaltner, Stippl, Knaus and El-Matbouli2007; Estensoro et al., Reference Estensoro, Álvarez-Pellitero and Sijtà-Bobadilla2013). Unprotected malacosporean spores degrade relatively quickly upon release from fish (in <24 h) (de Kinkelin et al., Reference de Kinkelin, Gay and Forman2002) compared to the chitin-protected spores of myxosporeans. Accordingly, electron microscopy of mature myxosporean spores reveals electron dense valves, and in immature spores an accumulation of electron dense material (inferred to be valve forming material) is observed in the cytoplasm of valvogenic cells (Adriano et al., Reference Adriano, Arana, Carriero, Naldoni, Ceccarelli and Maia2009; Moreira et al., Reference Moreira, Adriano, Silva, Ceccarelli and Maia2014; Morsy et al., Reference Morsy, Semmler, Al-Olayan and Mehlhorn2016). Fibrillar electron dense material reported in valvogenic cells of Sphaerospora jiroveci forms a continuous layer enclosing the developing spore (Dyková and Lom, Reference Dyková and Lom1997). Further studies are required to confirm whether this electron dense material is chitin and whether its presence is characteristic of sphaerosporids (Fig. 4B–F).
At least at present, there appear to be no other reliable morphological features that could be used to distinguish between malacosporean and myxosporean developmental stages. For example, although the lucent area of sporoplasmosomes has been highlighted as a malacosporean feature and is evident in the sporoplasmosomes in roach (Fig. 2C and D) and white fish (Fig. 1E and F), such lucent areas are also occasionally observed in sporoplasmosomes of myxosporeans (Lom et al., Reference Lom, Feist, Dyková and Kepr1989; Alvarez-Pellitero et al., Reference Alvarez-Pellitero, Molnár, Sitjà-Bobadilla and Székely2002; Morris and Freeman, Reference Morris and Freeman2009; Naldoni, pers. obs.). In addition, the noted tendency for a peripheral distribution of sporoplasmosomes in primary cells of malacosporeans (Canning et al., Reference Canning, Curry, Feist, Longshaw and Okamura2000; Canning et al., Reference Canning, Curry and Okamura2009) is also sometimes occasionally observed in the primary cytoplasm of myxosporeans (Supamattaya et al., Reference Supamattaya, Fischer-Scherl, Hoffmann and Boonyaratpalin1993). We would note that in our experience malacosporean sporogonic stages in kidney are difficult to find relative to myxosporean stages. Thus, frequent detection of developmental stages in histological sections could suggest the presence of myxosporeans.
Final comments
Our work has revealed five malacosporeans infecting a variety of fish hosts in the UK and Switzerland, contributing to the growing evidence of a hidden diversity of vertebrate hosts that are exploited by this myxozoan lineage. Further study is necessary to formally describe some of these malacosporean species, to determine if gudgeon act as true hosts of Tetracapsuloides sp. 5, to confirm that white fish are true hosts of T. bryosalmonae, and to ascertain whether B. plumatellae is able to utilize both dace and roach as hosts. It is also clear that the two malacosporean genera are not restricted to exploiting single fish families with Tetracapsuloides spp. exploiting members of Salmonidae and Cyprinidae and Buddenbrockia spp. exploiting members of Cyprinidae and Nemacheilidae (Table 1).
Screening both bryozoans and fish has provided vital information about malacosporean diversities and distributions (Bartošová-Sojková et al., Reference Bartošová-Sojková, Hrabcová, Pecková, Patra, Kodádková, Jurajda, Tyml and Holzer2014; Hartikainen et al., Reference Hartikainen, Gruhl and Okamura2014; Patra et al., Reference Patra, Hartigan, Morris, Kodádková and Holzer2016) and may lead to the resolution of life cycles (this study). Further studies should aim to determine whether malacosporeans generally show broad fish host use or whether some may be specialists. In addition, resolution of accidental hosts and the consequences of dead-end infections could lead to future studies on host–parasite interactions, effective immune responses and the potential dilution of infectious stages when non-permissive hosts are abundant. Finally, resolving malacosporean hosts may help us to understand the range of early hosts that were exploited as cnidarians evolved endoparasitism in view of the primitive nature of malacosporeans (Okamura et al., Reference Okamura, Gruhl, Reft, Okamura, Gruhl and Bartholomew2015b).
Author ORCIDs
Juliana Naldoni, 0000-0002-6764-448X
Acknowledgements
The authors thank Hanna Hartikainen (EAWAG, Zurich) and Heike Schmidt-Posthaus (University of Bern) for help with access to or provision of fish kidney material from the River Furtbach, Lake Lucerne and the River Brubach, and Joe Huddart and Guy Woodward (Imperial College, London) for enabling sampling kidney of fish from the River Stour, UK. We also thank Pavla Bartošová-Sojková for drawing our attention to the presence of a sphaerosporid infection in kidney material and advising on sphaerosporid development.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and by the grant #2013/21374-6, São Paulo Research Foundation (FAPESP) to E. A. Adriano. E. A. Adriano received a research productivity grant from the Brazilian Fostering Agency CNPq (grant #301886/2016-4). J. Naldoni was supported by the scholarship #2016/08831-7, São Paulo Research Foundation (FAPESP).
Conflict of interest
None.
Ethical standards
Not applicable.