Introduction
Niobium-, tantalum- and wolfram-bearing minerals are considered strategic and critical materials because of their significance in several high-technology applications, for example the manufacturing of super-alloys for the aerospace industry, high-strength low-alloy steels for construction, low-temperature superconductor wires for medical equipment, and particle accelerators and nuclear fusion instruments (Sanchez-Segado et al., Reference Sanchez-Segado, Monti, Katrib, Kingman, Dodds and Jha2017 and references therein). Tantalum (Ta), one of these strategic materials recovered from oxide minerals as a minor constituent, is commonly associated with pegmatite-related deposits, rare-element-enriched granites and peralkaline granite complexes. It can be an important resource in weathered crusts and placer deposits, where the Ta may be a co-product of tin, whereas most niobium (Nb) resources are observed in carbonatite complex-related deposits and peralkaline intrusions and coexists with rare-earth-element mineralisation (Melcher et al., Reference Melcher, Graupner, Göbler, Sitnikova, Oberthür, Gerdes, Badanina and Chudy2017; Simandl et al., Reference Simandl, Burt, Trueman and Paradis2018).
The columbite-supergroup minerals have been established in five mineral groups including (questionable in italics): ixiolite [ixiolite-(Mn2+), ixiolite-(Fe2+), nioboixiolite-(Mn2+), nioboixiolite-(□), scrutinyite, seifertite and srilankite]; wolframite [ferberite, hübnerite, huanzalaite, sanmartinite, heftetjernite, nioboheftetjernite, rossovskyite, riesite and dmitryvarlamovite]; samarskite [samarskite-(Y), ekebergite, shakhdaraite-(Y), samarskite-(Yb), ishikawaite and calciosamarskite]; columbite [columbite-(Fe), columbite-(Mn), columbite-(Mg), tantalite-(Fe), tantalite-(Mn), tantalite-(Mg), fersmite, euxenite-(Y), tanteuxenite-(Y) and uranopolycrase]; and wodginite [wodginite, ferrowodginite, titanowodginite, ferrotitanowodginite, tantalowodginite, lithiowodginite and achalaite]. There are also four other questionable and insufficiently studied minerals [qitianlingite, yttrocolumbite-(Y), yttrotantalite-(Y), yttrocrasite-(Y)] and one ungrouped species [lithiotantite] in the general stoichiometry MO2 – crystal structures based on the hexagonal close packing of anions, the six-fold coordination number of M-type cations and the presence of zig-zag chains of edge-sharing M-centred polyhedra (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). All the species in the columbite supergroup have the same topology of their atomic nets with different schemes of cation ordering and unit-cell dimensions (Udoratina et al., Reference Udoratina, Panikorovskii, Chukanov, Voronin, Luteov, Agakhanov and Isaenko2024). There are quite a few mineral species that contain W, Mo, Nb, Ta, Sb, Ti, Sn, Si, Ge, Mn, Pb and Te as oxides with the stoichiometry MO2, related structurally to columbite. Although these species show significant common characteristics, they differ from each other in many respects such as symmetry, cation ordering, unit-cell dimensions and coordination numbers of cations (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). In the current columbite supergroup nomenclature scheme, topologically similar minerals with different cation ordering (i.e. with similar unit-cell dimensions and the same end-member formulae) have been taken into account as different mineral species. For example, srilankite with Pbcn, a = 4.71, b = 5.55, c = 5.02 Å and riesite with P2/b, a = 4.52, b = 5.50, c = 4.89 Å, β = 90.6°, both TiO2, are different minerals. Similarly, nioboixiolite-(Mn2+) [(Nb2/3Mn2+1/3)O2 = Mn2+Nb2O6)] with Pbcn, a = 4.756, b = 5.732, c = 5.134 Å and columbite-(Mn) (Mn2+Nb2O6) with Pbcn, a = 14.32, b = 5.74, c = 5.11 Å are also different species belonging to the ixiolite and columbite group, respectively (Udoratina et al., Reference Udoratina, Panikorovskii, Chukanov, Voronin, Luteov, Agakhanov and Isaenko2024).
Many studies have shown that the investigation of paragenetic assemblages, together with the chemical composition of some accessory minerals (e.g. columbite–tantalite, tourmaline and gahnite) provides a useful knowledge about the magmatic evolution of the granitic pegmatite and pegmatite melts (Tindle and Breaks, Reference Tindle and Breaks2000; Tindle et al., Reference Tindle, Breaks and Selway2002; López de Azarevich et al., Reference López de Azarevich, Fulignati, Gioncada and Azarevich2021). The root-name columbite within the columbite-group minerals is the oldest species among all of the mineral names and also important in numerous petrological and geochemical studies. For example, columbite crystals may show partial cation disorder depending on their chemical compositions as well as formation conditions. The compositional variations in columbite-group minerals (e.g. Nb–Ta and Fe–Mn pairs) are sensitive indicators of magmatic to subsolidus evolution of the parental rock. Hence, the columbite-group minerals are, actually, potential indicators in the evolutionary history of their parental rocks (Beurlen et al., Reference Beurlen, Da Silva, Thomas, Soares and Oliver2008; Novak et al., Reference Novák, Chládek, Uher and Gadas2018). Consequently, the columbite-group minerals are both economically and petrologically important in our understanding the regional and internal geochemical variations and specification of fractionation and crystallisation conditions of rare-element-enriched granitic pegmatites and fertile granite as well as for U–Pb age determination to obtain emplacement and rare-element mineralisation ages of granitic pegmatites due to their high U and low Pb common contents (Černý and Ercit, Reference Černý and Ercit1985; Reference Černý, Ercit, Moeller, Černý and Saupé1989; Černý et al., Reference Černý, Goad, Hawthorne and Chapman1986; Ercit et al., Reference Ercit, Wise and Černý1995; Ercit, Reference Ercit1994; Tindle and Breaks, Reference Tindle and Breaks2000; Novák et al., Reference Novák, Chládek, Uher and Gadas2018; Zhou et al., Reference Zhou, Qin and Tang2021 and references therein; Ryznar et al., Reference Ryznar, Pršek, Wlodek and Uher2023).
Although various computer programs applicable to the calculation and classification of rock-forming silicate minerals have been developed over the past two decades (e.g. Yavuz, Reference Yavuz1999; Reference Yavuz2001a; Reference Yavuz2003; Reference Yavuz2007; Reference Yavuz2013; Yavuz et al., Reference Yavuz, Karakaya, Yıldırım, Karakaya and Kumral2014; Reference Yavuz, Kumral, Karakaya, Karakaya and Yıldırım2015; Yavuz and Yıldırım, Reference Yavuz and Yıldırım2020; Yavuz and Yavuz, Reference Yavuz and Yavuz2023a, Reference Yavuz and Yavuz2023b; Reference Yavuz and Yavuz2024), none useful for columbite-supergroup minerals, according to the current IMA report, has yet appeared in the literature, except for a program specially focused on columbite-group minerals (Yavuz Reference Yavuz2001b), possibly in part owing to the lack of classification scheme until the recent work by Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). Taking this situation into consideration, a computer program called WinClbclas has been developed using the Microsoft® Visual Basic programming language. It can be used to calculate the chemical formulae from up to 200 analyses obtained from both wet-chemical and electron-microprobe techniques. The program estimates and classifies mineral analyses of the columbite-supergroup minerals on the basis of 24 O and 12 cations. However, options for different normalisation procedures [e.g. columbite group (M1M22O6) to total cations = 3.00 atoms per formula unit (apfu)] can be applied to each group in the columbite supergroup from the pull-down menu of Normalization in the Start-up Screen. The calculation and classification procedures applied to columbite-supergroup minerals by WinClbclas are carried out based on the currently accepted IMA nomenclature scheme, but also take into account new species that post-date the IMA report. The program is capable of estimating the FeO and Fe2O3 (wt.%) contents from a microprobe-derived total FeO (wt.%) analysis using the stoichiometric constraints proposed by Droop (Reference Droop1987).
Columbite-supergroup minerals nomenclature
Using the available data on minerals with the stoichiometry MO2 that are topologically related to columbite and constitute the columbite supergroup, Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a) proposed a nomenclature and classification scheme for the columbite-supergroup minerals which has been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) for columbite-supergroup minerals (Miyawaki et al., Reference Miyawaki, Hatert, Pasero and Mills2022). In this context, the 36 valid minerals of the columbite supergroup have been divided into five groups by Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a, see Table 1) on the basis of the following criteria: (1) the general stoichiometry MO2 is required; (2) the crystal structure based on the hexagonal close packing (hcp) of anions is considered; (3) only octahedral voids of hcp are occupied; and (4) the presence of zig-zag chains of edge-shared octahedra is taken into account. The mineral nomenclature procedure is carried out according to the dominant cation in each site(s) of the ixiolite (MO2), wolframite (M1M2O4), samarskite (ABM 2O8), columbite (M1M22O6) and wodginite (M1M2M32O8) group.
Table 1. A list of the IMA-approved, currently questionable and insufficiently studied species in the columbite supergroup (from Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a).

A = Approved by the IMA; QMS = Questionable mineral species; □ = Vacancy; † = new columbite-supergroup species approved by the IMA subsequent to the subcommittee report by Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a): nioboixiolite-(Mn2+) (Chukanov et al., Reference Chukanov, Pekov, Zubkova, Yapaskurt, Shelukhina, Britvin and Pushcharovsky2023b), nioboixiolite-(□) (Li et al., Reference Li, Ke, Wang, Chen, Li, Wang, Li, Hu, Yu and Zhao2023) and dmitryvarlamovite (Udoratina et al., Reference Udoratina, Panikorovskii, Chukanov, Voronin, Luteov, Agakhanov and Isaenko2024). Insufficiently studied minerals are italicised.
According to Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a), all minerals in the ixiolite group have the general formula M1O2 (i.e. orthorhombic, Pbcn, a = a0, b = b0, c = c0 and Z = 4) with a disordered distribution of the cations occupied in a single M1 site. The chemical formula of ixiolite-group minerals, as an aristotype structure, is currently defined as (Ta,Mn,Nb)O2 in which Mn is the main charge-balancing component, but also samples with Fe > Mn content in the M1 site [e.g. ixiolite-(Fe2+), see Table 1]. The wolframite-group minerals with the wolframite-type structure (M1M2O4, monoclinic, P2/c, a = a0, b = b0, c = c0, β ≈ 91° and Z = 2) is actually a derivative form of the ixiolite-type structure having a sequence of two kinds of structurally the same, but chemically different, octahedral layers of parallel zig-zag chains. In the wolframite-type structure, as cations with larger radius prefer to keep in the octahedral M1 site and the smaller ones occupy the M2 octahedron, species belonging to the wolframite group are double oxides with the general formula M12+M26+O4 (M1 = Mg, Mn, Fe and Zn; M2 = W) or M13+M25+O4 (M1 = Sc and Fe; M2 = Nb and Ta), except for riesite (Ti4+Ti4+O4) that shows a slightly distorted variant of the wolframite structure (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). The samarskite-group minerals include three valid species [samarskite-(Y), ekebergite and shakhdaraite-(Y)] in monoclinic (P2/c, a = 2a0, b = b0, c = c0, β ≈ 93° and Z = 2), cation-ordered double niobates and tantalates with the general formula AM1M22O8 (A = Y and Th; M1 = Fe2+, Fe3+ and Sc3+; M2 = Nb and Ta). Compared to the other columbite-supergroup minerals, species belonging to the samarskite-group minerals contain a relatively large cation at the A site with 6 + 2-fold coordination owing to the slight irregularity of the hcp. This large cation insertion transforms parallel zig-zag chains into a rigid layer of edge-sharing AO8 polyhedra with the preservation of the cation distribution between the ‘octahedral’ voids of hcp (Lima-de-Faria, Reference Lima-de-Faria2012). Three insufficiently studied metamict minerals including ‘samarskite-(Yb)’, ‘ishikawaite’ and ‘calciosamarskite’ have been tentatively assigned to the samarskite group by Chukanov et al. (Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a) based on their stoichiometry and studies on the powder X-ray diffraction patterns of annealed samples. The columbite-group minerals consist of ten species including double oxides with the general formula M12+M25+2O6 (orthorhombic, Pbcn, a = 3a0, b = b0, c = c0 and Z = 4; M1 = Mg, Ca, Mn and Fe; M2 = Nb and Ta). In the crystal structure of columbite-group minerals, M1O6 octahedra share edges to form infinite zig-zag chains along the c axis with similar chains at the M2O6 octahedra resulting in alternating [100] ‘layers’ with a single ‘layer’ occurring of chains of M1O6 octahedra and double ‘layers’ including chains of M2O6 octahedra (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). The wodginite-group minerals comprise monoclinic species (C2/c; a = 2a0, b = 2b0, c = c0, β ≈ 91° and Z = 4) with the general formula M1M2M32O8 where the dominant cations at the M sites are: M1 = Mn2+, Fe2+ and Li; M2 = Ti, Sn4+ and Ta; M3 = Ta (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). According to Ercit et al. (Reference Ercit, Hawthorne and Černý1992), the structure of wodginite-group minerals are characterised by a different degree of ordering of cations among the M sites with alternating (100) ‘layers’ including chains of edge-sharing MO6 octahedra running along the c axis. Consequently, in the wodginite-group minerals, the ‘layers’ of the first type include chains of M3O6 octahedra, whereas the ‘layers’ of the second type contain chains of alternating M1O6 and M2O6 octahedra (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a).
In the current columbite-supergroup minerals nomenclature scheme, lithiotantite (LiTa3O8) which is chemically and topologically identical to lithiowodginite (LiTa3O8) has been regarded as the ungrouped species. Similarly, ‘qitianlingite’ [Fe2+2Nb2W6+O10], ‘yttrocolumbite-(Y)’ [YNbO4 (?)], ‘yttrotantalite-(Y)’ [YTaO4 (?)] and ‘yttrocrasite-(Y)’ [YTi2O5(OH) (?)] are not currently included in the columbite supergroup due to lack of reliable data on their chemical composition and crystal structure.
Program description
WinClbclas is a user-friendly, compiled program package (≈14 Mb) developed for personal computers running on the Microsoft® Windows operating system. The program first calculates the cation values (in apfu) from analyses made on columbite-supergroup minerals (wet-chemical or electron-microprobe techniques) and then uses these to classify the mineral into the 36 IMA-approved species that belong to five groups including ixiolite, wolframite, samarskite, columbite and wodginite, as well as those of eight species that are currently questionable and insufficiently studied (see Table 1). A list of the calculation steps in the Calculation Screen and in the output of a Microsoft Excel file developed by the program is given in Table 2. Upon successful installation of WinClbclas, the start-up screen, with various pull-down menus and equivalent shortcuts, appears on the screen (Fig. 1a). The program allows the user to input wet-chemical or electron-microprobe wolframite-, samarskite-, columbite-, wodginite- as well as ixiolite-group analytical data, both together or as a separate form, by clicking the New icon on the tool bar, by selecting the New File from the pull-down menu of File option, or pressing the Ctrl + N keys (Fig. 1b). In the New File, Data Entry Screen and Calculation Screen; these parameters are highlighted by the soft green (i.e. data entry for wolframite, samarskite, columbite and wodginite) and pink colours (i.e. data entry for ixiolite). Up to 47 chemical analytes (in wt.%) are used by WinClbclas for calculation and classification of the columbite-supergroup minerals using in the following orders:
Sample No [wolframite, samarskite, columbite and wodginite group], SiO2, TiO2, ZrO2, UO2, ThO2, HfO2, SnO2, Al2O3, Cr2O3, V2O3, Sb2O3, Fe2O3, As2O3, Bi2O3, Y2O3, Sc2O3, La2O3, Ce2O3, Pr2O3, Nd2O3, Sm2O3, Eu2O3, Gd2O3, Tb2O3, Dy2O3, Ho2O3, Er2O3, Tm2O3, Yb2O3, Lu2O3, Nb2O5, Ta2O5, P2O5, WO3, FeO, MnO, PbO, ZnO, MgO, CaO, SrO, BaO, Na2O, K2O, Li2O, F and H2O (in wt.%).
Sample No [ixiolite group], SiO2, TiO2, ZrO2, UO2, ThO2, HfO2, SnO2, Al2O3, Cr2O3, V2O3, Sb2O3, Fe2O3, As2O3, Bi2O3, Y2O3, Sc2O3, TotalREE2O3, Nb2O5, Ta2O5, P2O5, WO3, FeO, MnO, PbO, ZnO, MgO, CaO, SrO, BaO, Na2O, K2O, Li2O, F and H2O (in wt.%).
Data from analysis of a columbite-supergroup mineral can also be input into a blank Excel file following the above order, saving it with the extension of ‘.xls’ or ‘.xlsx’, after which it can then be loaded into the Data Entry Screen of the program by clicking the Open Excel File option from the pull-down menu of File. By selecting the Edit Excel File option from the pull-down menu of File, data can be inserted into a blank Excel file (i.e. MyColumbite), saved using a different file name (with the extension of ‘.xls’ or ‘.xlsx’), and then loaded into the Data Entry Screen of the program by clicking the Open Excel File option from the pull-down menu of File. Additional information about the data entry or similar topics can be accessed by pressing the F1 function key to display the WinClbclas.chm file on the screen. The current version of WinClbclas includes a total of 26 binary and ternary classification and compositional plots. Data on any of these plots can be displayed using the Grapher program by selecting the diagram type from the pull-down menu of Graph in the Calculation Screen of the program (Fig. 1c).

Figure 1. (a) A screenshot of the WinClbclas Start-up window with various pull-down menus and equivalent shortcuts. (b) A screenshot of the WinClbclas Data Entry window with a total of 47 analytes (wt.%). (c) A screenshot of the WinClbclas Calculation Screen with plot options from the pull-down menu of the Graph.
Table 2. Description of column numbers in the Calculation Screen window of the WinClbclas program and an output Excel file.
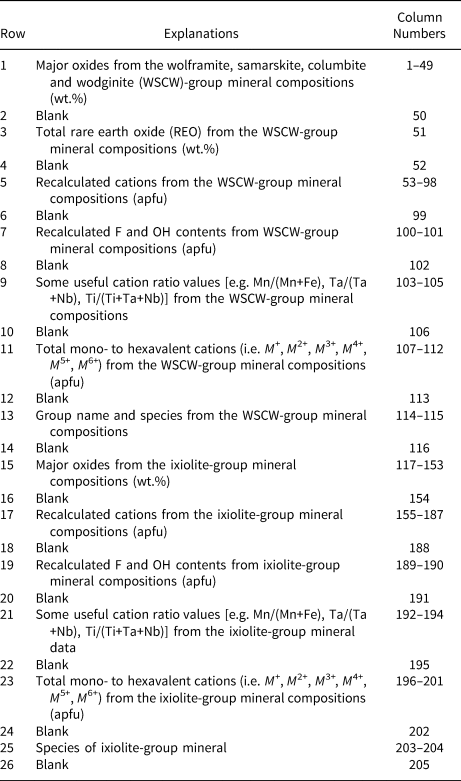
Notes: (apfu) = Atoms per formula unit; M + = Total monovalent cations, M 2+ = Total divalent cations, M 3+ = Total trivalent cations, M 4+ = Total tetravalent cations, M 5+ = Total pentavalent cations, M 6+ = Total hexavalent cations.
Worked examples
Using the selected data set from literature (see references in Tables 3, 4, and 5), examples showing how WinClbclas can be used in the determination of chemical formulae and columbite-supergroup minerals classification are presented. The previously typed or loaded analyses are processed by clicking the Calculate icon (i.e. Σ) in the Data Entry Section of the program, after which all input and estimation parameters are displayed in columns 1–204 (see Table 2) of the Calculation Screen (i.e. 1–116 for wolframite, samarskite, columbite and wodginite groups highlighted by the soft green colour; 117–204 for the ixiolite group, highlighted by the soft pink colour). Pressing the Ctrl + F keys or clicking the Open File to Calculate option from the Calculate menu also executes processing of a selected data file with the extension of ‘.csg’ that refers to the columbite supergroup. By clicking the Send results to Excel file icon in the Calculation Screen, all calculations can be stored in an Excel file (Output.xlsx) and then displayed by clicking the Open and edit Excel file icon.
Table 3. Chemical compositions of selected ixiolite-group minerals with calculations and classifications by WinClbclas.
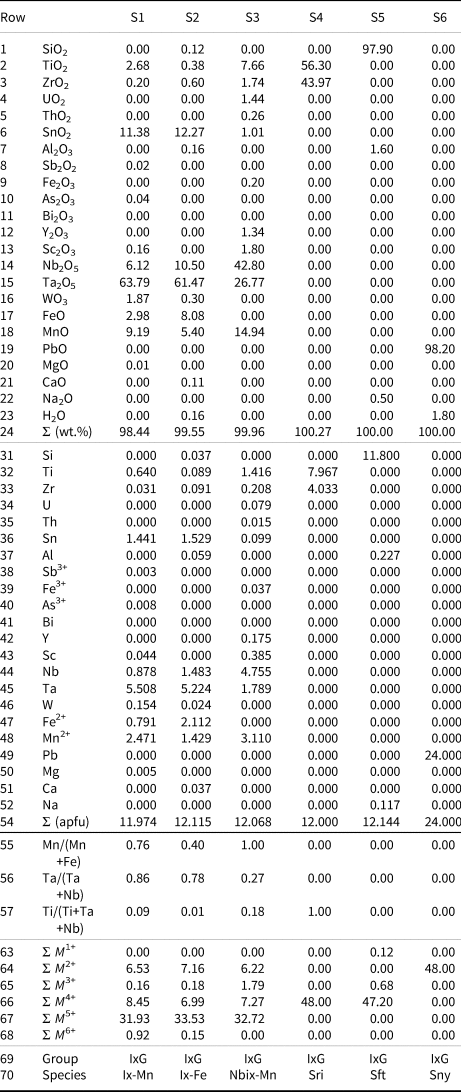
Notes: (apfu) = Atoms per formula unit. Sample sources: S1, S2, S4, S5 and S6 = Handbook of Mineralogy (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2001–2005); S3 = Chukanov et al. (Reference Chukanov, Pekov, Zubkova, Yapaskurt, Shelukhina, Britvin and Pushcharovsky2023b). The formulae were recalculated to content of ions on the basis of 24 O and 12 cations (apfu); M 1+ = Total monovalent cations, M 2+ =Total divalent cations, M 3+ = Total trivalent cations, M 4+ = Total tetravalent cations, M 5+ = Total pentavalent cations, M 6+ = T otal hexavalent cations; IxG = ixiolite group; Ix-Mn = ixiolite-(Mn2+), Ix-Fe = ixiolite-(Fe2+), Nbix-Mn = nioboixiolite-(Mn2+), Sri = srilankite, Sft = seifertite and Sny = scrutinyite.
Table 4. Compositions of selected wolframite- and samarskite-group minerals with calculations and classifications by WinClbclas.
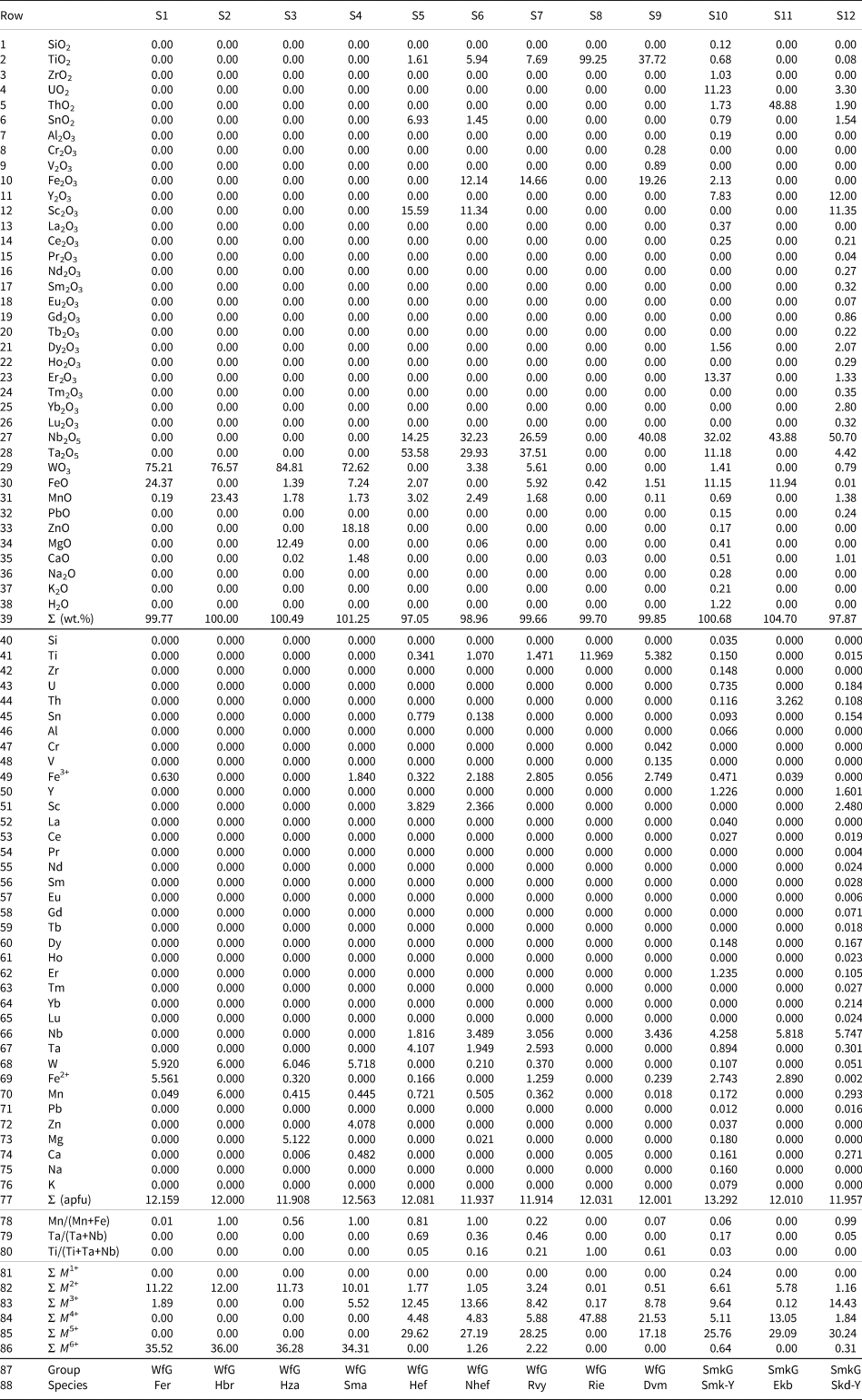
Note: (apfu) =Atoms per formula unit. Sample sources: S1, S2, S3, S4, S5, S7, S8 and S10 = Handbook of Mineralogy (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2001–2005); S6 = Lykova et al. (Reference Lykova, Rowe, Poirier, Mcdonald and Giester2021); S9 = Udoratina et al. (Reference Udoratina, Panikorovskii, Chukanov, Voronin, Luteov, Agakhanov and Isaenko2024); S11 = https://www.mineralienatlas.de; S12 = Pautov et al. (Reference Pautov, Mirakov, Sokolova, Day, Hawthorne, Schodibekov, Karpenko, Makhmadsharif and Faiziev2022). The formulae were recalculated to content of ions on the basis of 24 O and 12 cations (apfu); M 1+ = Total monovalent cations, M 2+ =Total divalent cations, M 3+ = Total trivalent cations, M 4+ =Total tetravalent cations, M 5+ = Total pentavalent cations, M 6+ =Total hexavalent cations; WfG = wolframite group; SmkG = samarskite group; Fer = ferberite, Hbr = hübnerite, Hza = huanzalaite, Sma = sanmartinite, Hef = heftetjernite, Nhef = nioboheftetjernite, Rvy = rossovskyite, Rie = riesite, Dvm = dmitryvarlamovite, Smk-Y = samarskite-(Y), Ekb = ekebergite and Skd-Y = shakhdaraite-(Y).
Table 5. Compositions of selected columbite- and wodginite-group minerals with calculations and classifications by WinClbclas.

Note: (apfu) = Atoms per formula unit. Sample sources: S1, S2 = Wenger et al. (Reference Wenger, Armbruster and Geiger1991); S3 = https://www.mineralienatlas.de; S4 = Dias and Chavez (Reference Dias and Chavez2015); S5 = Zwaan et al. (Reference Zwaan, Falster and Simmons2016); S6 = Simandl et al. (Reference Simandl, Burt, Trueman and Paradis2018); S7 = Handbook of Mineralogy (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2001–2005); S8 = Aurisicchio et al. (Reference Aurisicchio, Orlandi, Pasero and Perchiazzi1993); S9 = Udoratina et al. (Reference Udoratina, Panikorovskii, Chukanov, Voronin, Luteov, Agakhanov and Isaenko2024); S10 = Handbook of Mineralogy (Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2001–2005); S11 = https://www.mineralienatlas.de; S12 = Galliski et al. (Reference Galliski, Černý, Marquez-Zavalía and Chapman1999); S13 = Hanson et al. (Reference Hanson, Falster, Simmons, Sprague, Vignola, Rotiroti, Andó and Hatert2018); S14 = Galliski et al. (Reference Galliski, Márquez-Zavalía, Černý, Lira, Colombo, Roberts and Bernhardt2016). The formulae were recalculated to content of ions on the basis of 24 O and 12 cations (apfu); M 1+ = Total monovalent cations, M 2+ = Total divalent cations, M 3+ = Total trivalent cations, M 4+ = Total tetravalent cations, M 5+ = Total pentavalent cations, M 6+ = Total hexavalent cations; ClbG = columbite group; WdgG = wodginite group; Clb-Fe = columbite-(Fe), Clb-Mn = columbite-(Mn), Clb-Mg = columbite-(Mg), Ttl-Fe = tantalite-(Fe), Ttl-Mn = tantalite-(Mn), Fsm = fersmite, Eux-Y = euxenite-(Y), Uplc = uranopolycrase, Wdg = wodginite, Fwdg = ferrowodginite, Twdg = titanowodginite, Ftwdg = ferrotitanowodginite, Ttwdg = tantalowodginite and Ahl = Achalaite.
The validity of program output has been tested with representative columbite-supergroup minerals data selected from the literature (see references in Tables 3, 4, and 5). The program calculates Fe3+ and Fe2+ (apfu) from electron-microprobe-derived FeO (wt.%) content using the stoichiometric constraints according to the Droop (Reference Droop1987) method (see sample number S1 in Table 4). WinClbclas calculates the compositional formula for a given columbite-supergroup mineral analytical data on the basis of 24 O and 12 cations (apfu). Alternatively, by clicking the one of options, for example, Columbite group minerals [M1M2(2)O6] on the basis of 6 O and 3 cations (apfu) from the pull-down menu of Content of Ions in the Start-up Screen the program calculates columbite-group minerals according to the selected criteria. Similarly, by clicking one of the options [e.g. Normalize wodginite group minerals [M1M2M3(2)O8] to total cations = 4.00 (apfu)] from the pull-down menu of Normalization in the Start-up Screen the program normalises the total cation content according to the selected criteria. The program provides the users with some useful ratios, such as Mn/(Mn+Fe), Ta/(Ta+Nb) and Ti/(Ti+Ta+Nb) in the Calculation Screen (see rows 78–80 in Table 4). Classification of a given analysis into its proper group is carried out on the basis of the dominant cation at the site(s). WinClbclas lists total monovalent (i.e. M +) to hexavalent (i.e. M 6+) ions, resulting in the same valence state as a single constituent, together with the cations on the type of valence state (i.e. M +) in the Calculation Screen and an Excel output file (e.g. see rows 53–58 in Table 5).
In a case where a chemical composition corresponds to a hitherto unknown species within the columbite supergroup (i.e. a new species), WinClbclas warns the user with a ‘Not classified’ statement in column number 115 of the Calculation Screen. For example, a columbite-supergroup mineral with the following analytical data (see Table 2 in Alekseev, Reference Alekseev2023; wt.%): TiO2 1.68, SnO2 4.35, Nb2O5 17.98, Ta2O5 38.97, WO3 18.39, FeO 8.85, MnO 8.33, Li2O 0.21, total 98.76 is defined as ‘wolframowodginite’ that yields the empirical formula (Mn2.0659Fe2+1.251Li0.247)Σ3.563(Fe3+0.916Sn0.508Ti0.37)Σ1.794(Ta3.102Nb2.379W1.395)Σ6.876O8. As can be seen from the empirical formula, calculated content of ions on the basis of 24 O and 12 cations (apfu), means the dominant ions at the M1, M2 and M3 sites correspond to Mn, Fe3+ and Ta, respectively. As no species corresponding to this composition exists in the current classification scheme (i.e. M1 = Mn, M2 = Fe3+ and M3 = Ta), the program designates it as ‘Not classified’ rather than applying the name of one of known species given in Table 1.
WinClbclas provides options to display various binary and ternary classification and compositional diagrams in the Calculation Screen by using the Grapher program. Some of these plots with selected columbite-supergroup mineral data from the literature are given in Fig. 2. The columbite-group minerals with widespread niobium and tantalum phases in geochemically highly evolved rocks such as leucogranites and granitic pegmatites show compositional variations, especially in Nb–Ta and Fe–Mn pairs. During primary magmatic crystallisation, these pairs, which are sensitive indicators of magmatic to subsolidus evolution of the parental rock, usually show a progressive increase in terms of the Ta/(Ta+Nb) and Mn/(Mn+Fe) ratios with a characteristic evolutionary trend in the columbite–tantalite quadrilateral diagram (Černý and Ercit Reference Černý and Ercit1985; Černý et al., Reference Černý, Goad, Hawthorne and Chapman1986; Tindle and Breaks, Reference Tindle and Breaks2000; Novák et al., Reference Novák, Chládek, Uher and Gadas2018). In a classical columbite–tantalite quadrilateral plot (see Fig. 2a), there exists an empirically derived tantalite–tapiolite miscibility gap restricted by the two, upward and downward, concave curves. The program classifies a sample that plots between these curves as ‘miscibility gap’ instead of naming it tantalite-(Fe) or tantalite-(Mn). Similarly, a sample that plots above the upwards concave curve is classified as tapiolite-(Fe) by the WinClbclas program.

Figure 2. Selected plots of the columbite-supergroup minerals classification and compositional diagrams from the pull-down menu of Graph in the Calculation Screen of the WinClbclas program using the selected mineral analyses from the literature. (a) Compositions of the columbite-group minerals in the columbite–tantalite quadrilateral (from Černý and Ercit, Reference Černý and Ercit1985; Reference Černý, Ercit, Moeller, Černý and Saupé1989; samples from Tindle and Breaks, Reference Tindle and Breaks2000) with the empirically derived tantalite–tapiolite miscibility gap (from Černý et al., Reference Černý, Ercit and Wise1992). (b) Compositional plot of the columbite-group minerals in a ternary Fe–Mn–Mg diagram (filled diamonds from Sosa et al., 2002; filled circles from Baumgartner et al., Reference Baumgartner, Romer, Moritz, Sallet and Chiaradia2006; filled squares from Aurisicchio et al., Reference Aurisicchio, De Vito, Ferrini and Orlandi2002; filled triangles from Mackay and Simandl, Reference Mackay and Simandl2015). (c) Compositional plot of the wodginite-group minerals in a Mn/Total A site versus Ti/Total B site diagram (revised from Tindle et al., Reference Tindle, Breaks and Webb1998; filled diamonds from Tindle and Breaks, Reference Tindle and Breaks2000; filled circles from Alekseev, Reference Alekseev2023). (d) Compositional plot of the wodginite-group minerals in a ternary Ti-Ta/4-Sn diagram (filled diamonds from Tindle and Breaks, Reference Tindle and Breaks2000; filled circles from Hanson et al., Reference Hanson, Falster, Simmons, Sprague, Vignola, Rotiroti, Andó and Hatert2018). (e) Environments and mechanisms of W deposition in W versus H/F ratio diagram (from Michaud and Pichavant, Reference Michaud and Pichavant2019; samples from Monnier et al., Reference Monnier, Salvi, Melleton, Bailly, Béziat, de Parseval, Gouy and Lach2019). (f) Compositional plot of the wolframite-group minerals in a ternary Mg–Fe–Mn diagram (from Miyawaki et al., Reference Miyawaki, Yokoyama, Matsubar, Fruta, Gomi and Murakami2010; filled diamonds from Moore and Howie, 1978; filled circles from Michaud and Pichavant, Reference Michaud and Pichavant2019; filled squares from d'Aquin Tumukunde and Piestrzynski, Reference d'Aquin Tumukunde and Piestrzynski2018; filled triangles from Llorens and Moro, Reference Llorens and Moro2012; filled stars from Novák et al., Reference Novák, Johan, Škoda, Černý, Šrein and Veselovský2008). (g) Compositional plot of the ixiolite-group minerals (IGM) in a Mn/(Mn+Fe) versus Ta/(Ta+Nb) diagram (filled diamonds from René, Reference René2019; filled circles from Bergstøl and Juve, Reference Bergstøl and Juve1988; filled squares from Wise et al., Reference Wise, Černý and Falster1998; filled triangles from Tindle and Breaks, Reference Tindle and Breaks2000). (h) Compositional plot of the samarskite-group minerals in a ternary (REE+Y)–Ca–(U+Th) diagram (from Černý and Ercit, Reference Černý, Ercit, Moeller, Černý and Saupé1989; filled diamond from Pieczka et al., Reference Pieczka, Szuszkiewicz, Szełęg, Ilnickki, Nrjbert and Turinak2014; filled circles from Guastoni et al., Reference Guastoni, Secco, Škoda, Nestola, Schiazza, Novák and Pennacchioni2019; filled triangles from Raslan, Reference Raslan2008).
Differentiation of highly evolved peraluminous crustal magmas may cause a high Mn/Fe ratio in the fluid that controls the deposition of hübnerite. Hence, in understanding the wolfram deposition environments in perigranitic ore-forming systems, Michaud and Pichavant (Reference Michaud and Pichavant2019) proposed the H/F ratio [i.e. hübnerite/ferberite = 100*Mn/(Mn+Fe)] as an indicator of contrasted wolframite deposition mechanisms, as well as environments in perigranitic wolfram ore-forming systems, in three distinctive domains: (1) for a H/F ratio > 60, wolframite precipitates from a Mn-rich magmatic fluid evolving under a fluid-buffered path; (2) for a H/F ratio between 40 and 60, wolframite precipitates from a fluid buffered by granite/fluid interactions; and (3) for a H/F ratio < 40, wolframite precipitates from a fluid carrying a significant non-magmatic signature derived from country rocks. In this context, for example, by clicking option nine belonging to the Wolframite Group from the pull-down menu of Graph in the Calculation Screen (see Fig. 1c), the W versus H/F Ratio plot with selected data file in the Data Entry Screen is displayed on screen through the Grapher software (Fig. 2e). All input and calculated parameters from an Output tab of an Excel file (i.e. Output.xlsx) are transposed automatically by the Transpose tab of the program. This procedure provides the user with the ability to prepare a quick table for presentation as well as publication by using the Copy-Paste options.
Conclusions
WinClbclas is a user-friendly program developed specially for personal computers running on the Windows operating system to estimate and classify columbite-supergroup minerals using data obtained from both electron-microprobe and wet-chemical analyses. The program processes multiple analytical data (up to 200) for each program execution. The current version of WinClbclas classifies a total of 44 species for a given analysis into one of five groups – ixiolite, wolframite, samarskite, columbite and wodginite, as well as other questionable and ungrouped species, using the current IMA-approved nomenclature scheme (Chukanov et al., Reference Chukanov, Pasero, Aksenov, Britvin, Zubkova, Yike and Witzke2023a). The program generates two main windows. The first window (i.e. Start-up/Data Entry Screen), with several pull-down menus and equivalent shortcuts, enables one to edit a given analysis, based on chemistry (wt.%). By clicking the Calculate icon (i.e. Σ) in the Data Entry Screen, all input and estimated parameters by WinClbclas are displayed in the second window (i.e. Calculation Screen). The program reports the output in a tabulated form with a numbered column number from 1 to 204 (1–115 for wolframite, samarskite, columbite and wodginite groups; 117–204 for the ixiolite group) in the Calculation Screen window as well as in an Output Excel file. The results in the Calculation Screen can be exported to a Microsoft® Excel file (i.e. Output.xlsx), by clicking the Send Results to Excel File (Output.xlsx) icon or selecting the Send Results to Excel File (Output.xlsx) option from the pull-down menu of Excel. This file is then opened by Excel by clicking the Open and Edit Excel File (Output.xlsx) icon or selecting the Open Excel File (Output.xlsx) option from the pull-down menu of Excel. WinClbclas is a compiled program that consists of a self-extracting setup file containing all the necessary support files (i.e. ‘.dll’ and ‘.ocx’) for the 32-bit system. By clicking the setup file, the program and its associated files (i.e. support files, help file, data files with the extension of ‘.csg’, ‘.xls’, ‘.xlsx’, and plot files with the extension ‘.grf’) are installed into the personal computer (i.e. the directory of C:\Program Files\WinClbclas or C:\Program Files (x86)\WinClbclas) with Windows XP and subsequent operating systems. An installation of the program into a personal computer with the 64-bit operating system may require the msflexgrd adjustment (see explanations in the Supplementary Material). The self-extracting setup file is ~14 Mb and can be obtained from the Supplementary material (see below) (i.e. the WinClbclas setup.exe file).
Acknowledgements
I am grateful to Marco Pasero for his constructive comments, contributions and suggestions on an earlier draft, which improved the overall quality and clarity of the manuscript. I wish to thank Daniel Atencio and anonymous reviewers for their constructive comments on the earlier version of the manuscript.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2024.35.
Competing interests
The authors declare none.









