Introduction
Helminth parasites are one of the most successful groups of organisms on earth and are thought to infect over one third of the human population (Hotez et al., Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008). Many of these infections are endemic in low- and middle-income countries where they typically affect people living in poverty and are considered to be neglected tropical diseases by the World Health Organisation (Hotez et al., Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008). Amongst these are the foodborne trematodes (FBTs) which comprise a diverse group, broadly classified according to their final resting place within their mammalian hosts. Species that traditionally have attracted most attention include the liver flukes, Fasciola hepatica, Fasciola gigantica, Clonorchis sinensis and Opisthorchis viverrini and the lung fluke Paragonimus westermani although others, as discussed later, are emerging as significant human infections and are likely to be worth further research focus.
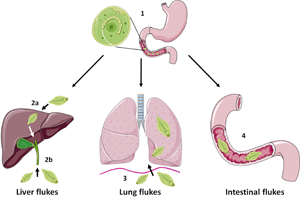
FBTs display complex life cycles which involve asexual reproduction in a freshwater snail host and release of free-swimming cercariae. These can settle on freshwater vegetation, as metacercariae (in the case of Fasciolids) or enter a second intermediate host (typically freshwater fish or crustaceans) as occurs for C. sinesis, O. viverrini and P. westermani (Robinson and Dalton, Reference Robinson and Dalton2009). Humans become infected following ingestion of the metacercariae (e.g. on contaminated vegetables such as watercress or in raw/undercooked fish and crabs), which excyst and release newly excysted juvenile (NEJ) flukes into the small intestine (Fig. 1). At this point the life cycle, and onward migration of the developing flukes, varies considerably depending on the species: F. hepatica and F. gigantica traverse the gut lining and migrate, via the liver parenchyma, to the bile ducts where they mature to adult flukes and produce eggs; O. viverrini and C. sinensis enter the bile duct by direct migration up the ampulla of Vater; P. westermani enters the peritoneal cavity and migrate through the diaphragm before entering the lungs; while intestinal FBTs (e.g. Echinostoma, Gastrodiscoides) parasitize the small intestine or caecum of their definitive hosts. For detailed life cycles of some of the major FBT species, readers are referred to the Centers for Disease Control and Prevention website (https://www.cdc.gov/parasites/). Unlike other infectious diseases, such as malaria which cause considerable mortality, the human impact of FBTs lies with the severe morbidity that is associated with infection. Indeed, the most recent Global Burden of Disease study (Vos et al., 2020) estimates that 780 000 disability-adjusted life years (DALYs) are lost globally due to foodborne trematodiases. However, this is likely an underestimation due to polyparasitism of individuals, which is common in endemic regions (Wai et al., Reference Wai, Han and Oo2017). The major clinical signs of FBT infection arise from mechanical tissue damage or immunopathology as the flukes migrate and feed within the host. However, some serious complications can arise, most notably cancer of the bile duct (cholangiocarcinoma, CCA), which is linked to O. viverrini and C. sinensis infection in Southeast Asia and China (Choi et al., Reference Choi, Han, Hong and Lee2004; IARC, 2012; Sripa et al., Reference Sripa, Tangkawattana and Brindley2018).
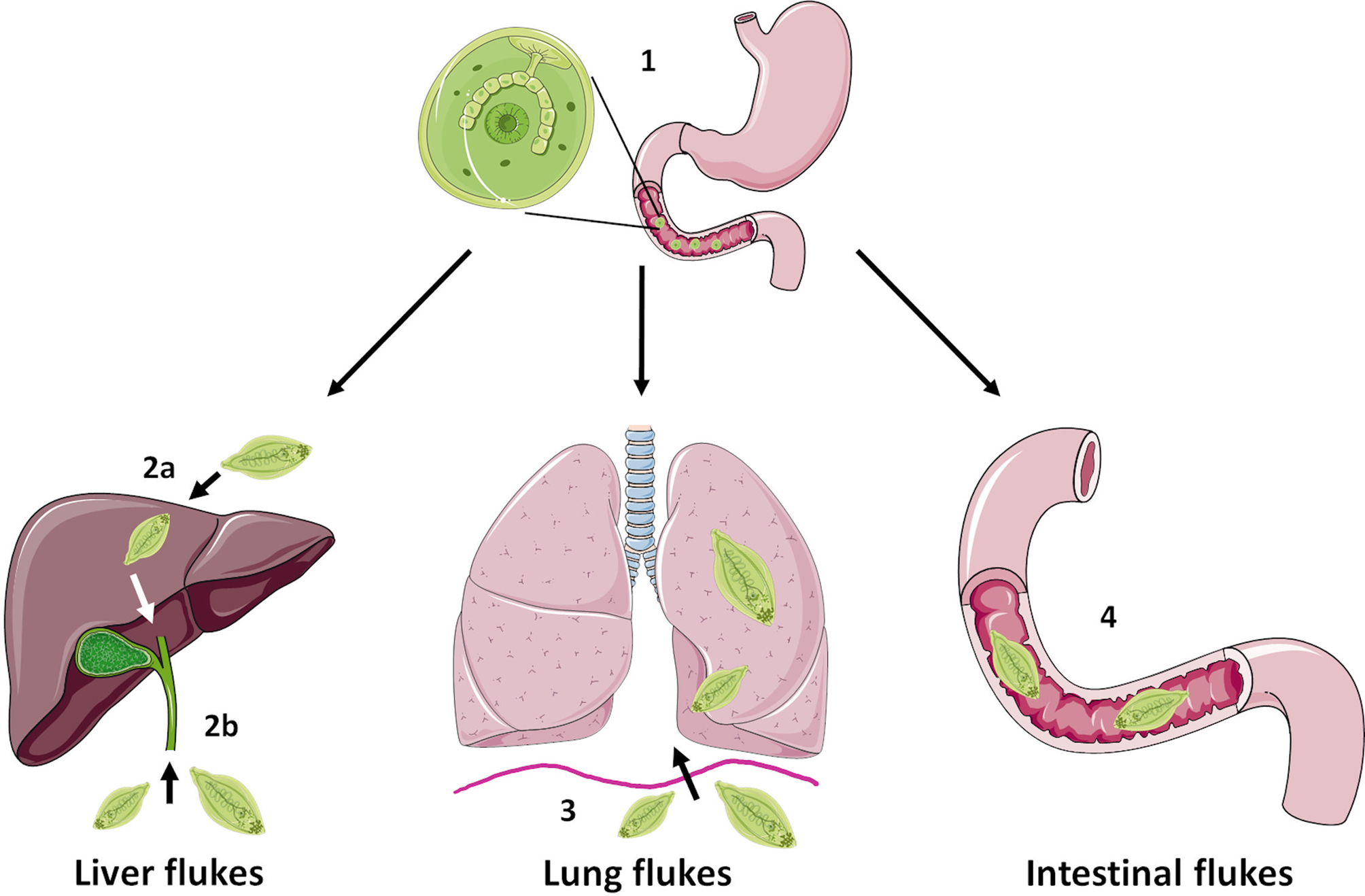
Fig. 1. Schematic representing simplified intra-mammalian life-cycle stages of the major groups of FBTs. Humans become infected with FBTs by ingestion of the infective metacercariae that encyst on vegetation or enter a second intermediate host such as freshwater fish or crustaceans. Initially, the metacercariae excyst and release newly excysted juvenile flukes in the small intestine in a step common to most species (1). However, the next stages of the life cycle vary considerably between species. The liver flukes F. hepatica and F. gigantica traverse the gut wall and migrate through the liver parenchyma before entering the bile ducts where they mature (2a) whilst O. viverrini and C. sinensis enter the bile duct directly by migration up the ampulla of Vater (2b). The lung fluke P. westermani enters the abdominal cavity and penetrates the diaphragm before entering the lungs (3). Intestinal flukes such as E. caproni and G. hominis do not migrate beyond the digestive tract and reside in the small intestine or caecum, respectively (4). Figure created using Les Laboratories Servier, https://smart.servier.com.
Despite significant prevalence and a global burden of disease that rivals some better-known conditions (such as tuberculosis and malaria), the FBTs remain understudied, and underfunded, compared with other infectious diseases. Thus, the aim of this special issue of Parasitology was to collate current knowledge of the major FBT species as well as highlight some emerging infections that have been largely overlooked. With a focus on epidemiology, control and host–parasite interactions, we hope that this collection of articles, from leading researchers in the field, will stimulate further interest and research on these fascinating parasites.
Foodborne trematodes: the usual suspects and new kids on the block
The true global prevalence of FBTs remains elusive. Few global studies have been conducted on this and the last systematic review and meta-analysis conducted (Furst et al., Reference Furst, Keiser and Utzinger2012), concluded that although around 56.2 million people were infected with FBTs for the year 2005, this figure is likely to be a significant underestimate due to missing or incomplete data for these species. This study also reported that C. sinensis, F. gigantica, F. hepatica, O. viverrini, O. felineus and Paragonimus spp. infections (largely the ‘usual suspects’ listed above) were responsible for over 85% of the global burden of disease attributable to foodborne trematodiases (Furst et al., Reference Furst, Keiser and Utzinger2012). Nevertheless, a number of other trematode species, seemingly infecting humans in significant numbers, are also emerging.
In the first article of the special issue, Chai and Jung broadly review current understanding of a range of trematodes, including lesser-studied species such as Metagonimus yokogawai, Heterophyes nocens, Haplorchis taichui, Echinostoma revolutum, Isthmiophora hortensis, Echinochasmus japonicus, Artyfechinostomum malayanum, Fasciolopsis buski and Gymnophalloides seoi (Chai and Jung, Reference Chai and Jung2022). The review offers a unique historical perspective of each species and includes detailed coverage of their biology, epidemiology, diagnosis and control. Additionally, the review provides cultural insight into traditional food preparation practices in endemic regions (mostly Southeast Asia), which is an important risk factor for foodborne trematodiases. Accordingly, the article represents a valuable point of reference for students and researchers alike.
In the next review article, Blair deals specifically with lung flukes of the genus Paragonimus (Blair, Reference Blair2022). Here the lung flukes are described as enigmatic parasites that attract less attention than some other neglected tropical diseases. Following an excellent background to the subject, 5 key research questions are posed that are central to a better understanding of Paragonimus epidemiology and evolution. Covering topics such as unravelling lung fluke taxonomy, clarification of life history traits, the value of genomics and the influence of climate change on fluke incidence and distribution, these questions will help bring some research priorities for Paragonimus species into sharp focus.
Next comes a review of the literature pertaining to Clonorchis sinensis infection throughout Korea by Yoo et al. (Reference Yoo, Sohn and Na2022). Despite declining rates of human infection in this country, the authors detail how 5 major river basins retain high metacercarial levels in freshwater fish intermediate hosts and sustained CCA cases within the human population in these endemic areas. Whilst achieving success in various national parasite control initiatives since 1971, the authors stress that continued coordinated efforts, implementing ‘One Health’ concepts are required to further reduce infections and the incidence of C. sinensis-associated CCA in Korea.
Pakharukova and Mordinov review the state-of-the-art knowledge on Opisthorchis felineus, one of the most important neglected helminths affecting Eurasia (Pakharukova and Mordvinov, Reference Pakharukova and Mordvinov2022). These authors highlight the impact that active human intervention into nature and global climate change can have on the spread of this parasite and the resumption of disease outbreaks. Furthermore, due to the similarities in the biology of O. felineus and the closely related liver flukes O. viverrini and C. sinensis, the authors discuss the carcinogenic potential of this species.
The next article covers 2 further neglected trematode species. Toledo et al. review current understanding of the intestinal fluke infections echinostomiasis and gastrodicoidiasis (Toledo et al., Reference Toledo, Alvarez-Izquierdo, Esteban and Munoz-Antoli2022). These foodborne trematodiases, caused by members of the Echinostomatidae and G. hominis respectively, are distributed mainly throughout impoverished areas of Asia. However, as the authors indicate, there are growing numbers in other areas at risk of infection as a result of changing eating habits in developed countries and an increasingly mobile human population. At present, diagnosis of these infections relies on detection of trematode eggs in fecal samples, but due to similarity in appearance, it is often difficult to distinguish between trematode species, particularly within the Echinostomes (Esteban et al., Reference Esteban, Muñoz-Antoli, Toledo and Ash2019). With the spread in geographical range of such intestinal fluke infections, more specific diagnostic tools that will allow rapid diagnosis, and appropriate treatment, will be necessary.
Dealing with outbreaks of disease
The ideal control for pathogens is prophylactic use of vaccines. This offers a cost-effective and safe approach to pathogen control, and in the case of veterinary products, does not leave chemical residues in food. Despite stunning successes in the development of vaccines against viral and bacterial infections, vaccines against most helminth species have yet to be developed commercially (for recent reviews see Claerebout and Geldhof, Reference Claerebout and Geldhof2020; McManus, Reference McManus2020; Perera and Ndao, Reference Perera and Ndao2021). The reasons for this are varied but one major obstacle in the way is the ability of helminths to modulate the host immune system such that vaccines cannot elicit sufficient responses for protection (Dalton et al., Reference Dalton, Robinson, Mulcahy, O'Neill and Donnelly2013). Whilst the search for vaccines continues, we must rely on the use of drugs for the treatment of helminth infections in humans and animals. The next 3 articles in this special issue deal with disease outbreaks and various approaches to trematode control.
In their review article, Coogle et al. highlight the emergence of the lung fluke Paragonimus kellicotti in North America (Coogle et al., Reference Coogle, Sosland and Bahr2021). Despite only 21 cases having been reported to date in North America, the authors emphasize the need for improved diagnosis and awareness of this disease since it is easily overlooked by physicians due to mild symptoms that are usually common to other diseases. Furthermore, domestic and wild animals can act as definitive hosts (and, thus, reservoirs) of P. kellicotti, and the authors suggest that epidemiological studies and alerts for rises in veterinary cases in areas at most risk would help determine the true incidence of this neglected disease.
Animals can act as reservoirs for FBTs, so epidemiological studies aimed at determining the zoonotic and zooanthroponotic transmission of parasites are of great importance. Since cats are well-known hosts of the Thai liver fluke (Sripa et al., Reference Sripa, Suwannatrai, Sayasone, Do, Khieu and Yang2021), Sota et al. analyse the circulation of O. viverrini between domestic cats and humans in an endemic area of Thailand (Sota et al., Reference Sota, Suttiprapa, Tangkawattana, Sripa, Blair and Sripa2022). A population genetics analysis performed in >100 cat and ~1,500 human samples demonstrated the presence of at least 3 different O. viverrini populations in cats and humans. Furthermore, despite most human and cat O. viverrini sequences clustering in separate clades, several sequences grouped together, suggesting that, as expected, O. viverrini can be zoonotically (or vice versa) transmitted.
Despite the new approaches in transmission control and efforts in developing a protective vaccine, anthelmintic drugs are still the cornerstone for treatment and FBT control. However, there are increasing reports of resistance against most flukicidal drugs, including triclabendazole, nitroxynil, closantel and albendazole (Fairweather et al., Reference Fairweather, Brennan, Hanna, Robinson and Skuce2020). Assessing the potency of the most common drugs in hyperendemic zones will provide important information about drugs of choice against human and veterinary fascioliasis. In their research article, Hasan et al. compare the in vitro flukicidal effects of 3 of the most common drugs against F. gigantica (triclabendazole, oxyclozanide and nitroxynil) using a motility assay and scanning electron microscopy to assess morphological changes to the tegument (Hasan et al., Reference Hasan, Roy, Biswas, Rahman, Anisuzzaman, Alam and Talukder2022). All 3 drugs were found to reduce fluke motility and to induce considerable disruption of the tegumental syncytium in a dose-dependent manner. Taken together, the data showed that nitroxynil was the most potent flukicide against these flukes in vitro and that this drug is a valuable alternative to triclabendazole, the ‘drug of choice’ for Fasciola species infections.
Molecular-level understanding of FBT–host interactions
The ability of FBTs to modulate the host's immune response is crucial for their establishment and survival within their host. Furthermore, their complex life cycles, which usually includes an intra-organic migration stage, adds a layer of complexity to these parasite–host interactions. Despite the difficulty in obtaining samples and maintaining life cycles in the laboratory, there have been many advances in the last decade characterizing the tegumental and secreted components that modulate the host's immune response (Cwiklinski et al., Reference Cwiklinski, de la Torre-Escudero, Trelis, Bernal, Dufresne, Brennan, O'Neill, Tort, Paterson, Marcilla, Dalton and Robinson2015; Suttiprapa et al., Reference Suttiprapa, Sotillo, Smout, Suyapoh, Chaiyadet, Tripathi, Laha and Loukas2018; Ryan et al., Reference Ryan, Shiels, Taggart, Dalton and Weldon2020; Fontenla et al., Reference Fontenla, Langleib, de la Torre-Escudero, Dominguez, Robinson and Tort2021). The next 4 articles provide comprehensive reviews and new data on the animal models and molecules that are advancing the characterization of FBTs at a molecular level.
In their research article, Fang et al. compare some of the most widely used animal models to study F. hepatica infections, including rats, mice and rabbits (Fang et al., Reference Fang, Yang, Wang, Chen, Li, Liu, Duan and Li2022). These authors conclude that the Sprague–Dawley rat is a permissive model, surviving for more than a year post-infection, making them a suitable model to study the pathological response. However, mice and rabbits died after several weeks post-infection, showing different severity of hepatic lesions, and thus the authors suggested using mice only for studies focusing on the acute phase of the infection.
FBTs secrete a plethora of molecules and other components (e.g. extracellular vesicles) that have a direct impact on the host's response to infection. One of the most important and abundant proteins secreted by the carcinogenic liver fluke O. viverrini is Ov-M60-like-1, a mucinase-like protein capable of degrading bovine sub-maxillary mucin and, thus, facilitating infection (Ta et al., Reference Ta, Nguyen, Jala, Dontumprai, Plumworasawat, Aighewi, Ong, Shawley, Potriquet, Saichua, van Diepen, Sripa, Hokke and Suttiprapa2020). Herein, Wendo et al. demonstrate, for the first time, the ability of Ov-M60-like-1 to degrade epithelial mucus and localize the expression of this protein to the tegumental syncytium, tegumental cells, vitelline glands and mature eggs, suggesting a key role for this protein in parasite–host interactions as well as having housekeeping functions within the fluke itself (Wendo et al., Reference Wendo, Tangkawattana, Saichua, Ta, Candra, Tangkawattana and Suttiprapa2022).
Similarly, a significant effort is being made on studying the host's response to FBT infections. In this regard, macrophages play an important role in the innate immune response against helminthiases in general, being classically or alternatively activated depending on the fluke life stage (Lechner et al., Reference Lechner, Bohnacker and Esser-von Bieren2021). In the next paper, Quinteros et al. summarize the responses of macrophages to parasite-derived signals with a focus on liver flukes and discuss their importance in host susceptibility to fluke-induced cancer (Quinteros et al., Reference Quinteros, O'Brien and Donnelly2022). These authors also highlight the need to view these cells not only as M1 or M2 types, but as multiple functional variants. Accordingly, future research should focus on characterization of the genotype and phenotype of macrophage populations in the context of helminth infections.
Finally, the impact of FBTs on their hosts cannot be fully understood unless the microbiome is included in the equation and any interactions are analysed as a 3-way communication between the host, the parasite and the host's microbiome (Lechner et al., Reference Lechner, Bohnacker and Esser-von Bieren2021). Not only can helminths impact host immunity by modifying its microbiome (Brosschot and Reynolds, Reference Brosschot and Reynolds2018) but they can also harbour bacteria that can colonize the human gastrointestinal tract and promote pathogenesis (Sripa et al., Reference Sripa, Deenonpoe and Brindley2017). This is the case for Helicobacter pylori, a pathogenic bacterium suggested to be a commensal of O. viverrini that can colonize host cholangiocytes and contribute to disease (Boonyanugomol et al., Reference Boonyanugomol, Chomvarin, Hahnvajanawong, Sripa, Kaparakis-Liaskos and Ferrero2013). In the last paper of this special issue, Thanaphongdecha et al. analyse the factors involved in H. pylori–O. viverrini commensalism with a particular focus on bacterial glycan ligands and cognate fluke receptors (Thanaphongdecha et al., Reference Thanaphongdecha, Chamgramol, Pairojkul, Deenonpoe, Suttiprapa, Brindley and Sripa2022). They demonstrate that l-fucose, a glycan expressed through the tegument and gut epithelium of O. viverrini, is important for H. pylori adhesion to the parasite's organs and subsequent hepatobiliary pathogenesis.
Concluding remarks and future research perspectives
Controlling FBT infections is challenging and will only be successful through joint efforts and combined approaches. Epidemiological studies on well-known (e.g. Fasciolids and Opisthorchids) and other neglected trematode species (e.g. Gastrodiscoides hominis, Paragonimus kellicotti and Echinostoma spp.) are needed to understand the real burden and potential threat of endemic and emerging FBT infections. These studies will also provide important information for the design of appropriate control measures using One Health approaches, chemotherapy or testing potential vaccines. However, in order to develop novel control methods, a deeper understanding of the biology of the flukes at a molecular level is also needed. This Special Issue provides an overview of all these aspects of FBTs, with the aim of stimulating discussion and advancing research and understanding of this important group of parasites.
Author contributions
MWR and JS wrote the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.