Introduction
Birth weight, serving as an important indicator of foetal growth,has been influenced by foetal and maternal factors and affected adult later-life health(Reference Horikoshi, Beaumont and Day1–Reference Whincup, Kaye and Owen3). Small for gestational age (SGA) and low birth weight (LBW) are higher risk factors of morbidity and mortality of infants(Reference Katz, Lee and Kozuki4–Reference McIntire, Bloom and Casey6). Maternal nutrition before and during pregnancy plays a critical role in pregnancy outcome(Reference Zeng, Cheng and Dang7–Reference West, Shamim and Mehra9); the recommendations had been given in many countries, including China, for women of child-bearing age to take folic acid (FA)(Reference De Wals, Tairou and Van Allen10–Reference Tamura and Picciano12). Since 2009, free FA supplementation at a dose of 0·4 mg/d during the periconceptional period has been given to the women of child-bearing age in China under a policy issued by the Ministry of Health to ensure good maternal and child health. The policy was implemented through the three-level network of health care in China. The health administration department in the rural areas or the districts of urban areas is in charge of purchase and management of FA supplements. In rural areas, the township hospital distributes the supplements to the village clinics where village doctors are responsible for its free distribution to the women in need. And the women in urban areas might go to the community health service centre for a free supplement(13). However, to date, the effects of FA on other pregnancy outcomes, such as birth weight, SGA and LBW, remain inconclusive except neural tube defects(Reference Berry, Li and Erickson14,Reference Czeizel and Dudas15) and congenital diseases(Reference Shaw, Lammer and Wasserman16,Reference Liu, Joseph and Luo17) . Besides, previous studies mainly focused on the effects of FA supplementation on birth weight in singleton births(Reference Van Dijk, Van Eijsden and Stronks18–Reference Papadopoulou, Stratakis and Roumeliotaki24); no studies had compared the effects of FA supplementation on birth weight indicators in the same samples of singleton and twin births in the round. The present study aimed to explore whether the associations of FA supplementation with birth weight, SGA and LBW are consistent in singletons and twins, as well as the potential effect modifications concerning plurality in a large population-based cross-sectional survey in China, involving 28 174 pregnant women with their infants, during 2010–2013.
Methods
Study design and participants
A population-based, cross-sectional epidemiological survey aiming to investigate the risk factors of birth outcomes was conducted between August and December 2013 in Shaanxi Province of northwest China. Infants born during 2010–2013 and their mothers were recruited using a stratified multistage random sampling method, which has been described elsewhere(Reference Yang, Cheng and Pei25,Reference Yang, Dang and Cheng26) . Briefly, according to the proportion of rural and urban residents and the fertility level of the population of the whole province of Shaanxi, firstly twenty counties and ten districts were sampled randomly. And then six villages each from six townships were selected randomly in each sampled county; six communities each from three streets were selected randomly in each sampled district. Finally, thirty and sixty participants were selected randomly in each sampled village and community, respectively. 30 027 of 32 400 pregnant women completed the questionnaire in the survey (response rate 92·7 %). In our study, 1853 were excluded for the following reasons: miscarriage (n 704); terminations (n 42); stillbirth (n 15); triplet births (n 2); FA supplementation was unknown (n 606); missing data for birth weight (n 484). The final study sample included 28 174 women with their infants – 27 818 single live births and 356 twin live births. Figure 1 displays the flow diagram with exclusion criteria in this study.
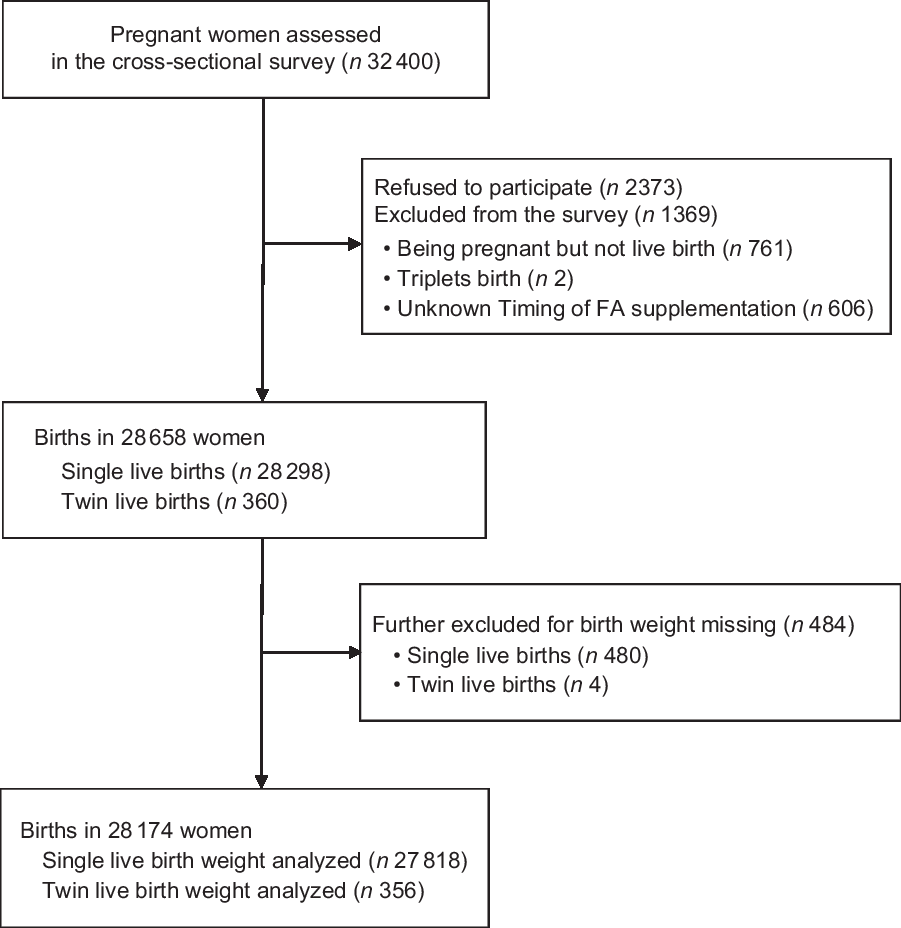
Fig. 1 Study flow diagram with exclusion criteria. FA, folic acid
Ascertainment of folic acid supplementation
In China, the policy of free FA supplementation at a dose of 0·4 mg/d during the periconceptional period has been carried out since 2009. The women who plan to be pregnant might get FA supplements freely in village clinics or community health service centres. In this study, FA supplementation was recorded via a retrospective in-person interview. Women were asked to report the brand and duration of FA supplementation use. In this study, women who took FA pills of 0·4 mg/d at any time from preconception (12 weeks before pregnancy) until the end of the first trimester (1–12 weeks during pregnancy) were classified as FA users(Reference Berry, Li and Erickson14). FA supplementation was divided into four patterns according to the period of use: (1) preconceptional use (12 weeks before pregnancy); (2) postconceptional use (1–12 weeks during pregnancy); (3) periconceptional use (12 weeks before pregnancy and 1–12 weeks during pregnancy); (4) no use (no supplementation during the above periods). We also collected FA supplementation in the second trimester (13–27 weeks during pregnancy) and third trimester (≥28 weeks during pregnancy). This information was used for sensitivity analysis.
Ascertainment of birth outcome
Birth weight and gestational age (GA) were recorded from a review of the Medical Certificate of Birth. Birth weight was measured with precision to the nearest 10 g. GA was calculated in weeks based on the first day of the last menstrual period (LMP)(Reference Li, Li and Ye22,Reference Li, Ye and Zhang27,Reference Conde-Agudelo, Belizan and Norton28) . Information about LMP was obtained from the Medical Certificate of Birth. SGA was defined as the infant whose birth weight was <10th centile based on the GA–gender-specific Chinese reference for foetal growth(Reference Zhu, Zhang and Zhang29). LBW was defined as birth weight <2500 g. Plurality was defined according to the number at births (singletons or twins)(Reference Schieve, Meikle and Ferre30).
Assessment of covariates
Questionnaires administered via retrospective in-person interview were used to collect participants’ characteristics. Covariates considered were infant gender (female or male), residence (rural or urban), maternal age (<25, 25–29, 30–34, ≥35), maternal education (non-educated, primary, secondary, high school, college or above), household wealth index (poor, middle, rich), antenatal care (ANC) visits (<7 or ≥7), gravidity (1, 2, ≥3), parity (1, ≥2), GA (continuous), alcohol drinking (yes or no), passive smoking (yes or no), tea drinking (yes or no), pregnancy-induced hypertension (PIH; yes or no), cold (yes or no), iron supplementation during pregnancy (yes or no), birth order (twin A, twin B). The household wealth index was built by principal component analysis based on four variables representing the family economic level (monthly income, monthly expenditure, housing condition, vehicle), which was classified according to tertiles of poor, middle and rich(Reference Filmer and Pritchett31). Maternal active smoking was also one of the important predictors of reduced birth weight(Reference Larsen, Haavaldsen and Bjelland32), which should be considered as a confounder. However, the percentage of active women smokers was extremely low in China(Reference Liu, Zhang and Yang33), especially among women of child-bearing age (0·3 % for women with singletons and none for women with twins in our study). Therefore, the covariate of active smoking was not included in the present analysis.
Statistical analysis
The characteristics of participants are reported as mean (sd) for the quantitative variable, and number (percentage) for the categorical variable. Student’s t test for continuous variables and χ2 test for categorical variables were used in univariate analyses. Based on the data distribution and type (normal distribution with identity-link function, and binomial distribution with logit-link function), we fitted generalised linear models (GLM) and generalised estimating equation (GEE) models (Reference Pollack, Lantz and Frohna34–Reference Zeger and Liang36) to estimate adjusted regression coefficients, or OR and 95 % confidence intervals (CI) for each birth outcome. The consideration of potential confounders was based on previous literature reports(Reference Zeng, Cheng and Dang7,Reference Zheng, Guan and Zhao20,Reference Pastor-Valero, Navarrete-Munoz and Rebagliato23,Reference Zeng, Yan and Cheng37–Reference Acharya, Singh and Kadel40) . Stratified analysis by plurality was built to estimate the association between FA supplementation and birth weight, the risk of SGA and LBW, respectively. In addition, effect modification was examined by adding an interaction term of FA supplementation with plurality in dataset 1 (singleton and twin A) and dataset 2 (singleton and twin B) of the total population, respectively. Furthermore, the association of timing of FA supplementation with birth weight, risk of SGA and LBW was explored. Finally, a sensitivity analysis was conducted to evaluate the robustness of the results by excluding women who sporadically continued to consume FA in the second trimester or third trimester or both (207 cases). And a subgroup analysis was conducted by infant’s sex.
Statistical analyses were conducted with SAS software (version 9.4; SAS Institute Inc.). A two-tailed P value <0·05 was regarded as statistically significant.
Results
Status of folic acid supplementation
The status of FA supplementation is presented in Table 1. Among all the participants, the prevalence of FA supplementation was 63·9 % in singletons and 66·3 % in the twin sample. Women with FA supplementation accounted for 4·3 % for preconception, 46·9 % for postconception and 12·7 % for periconception, respectively, among the singleton sample; and these figures were 4·5, 45·8, 16·0 %, respectively, among the twin sample, but there was no statistical significance between singleton and twin samples.
Table 1 Maternal and infant characteristics by plurality in northwest China during 2010–2013
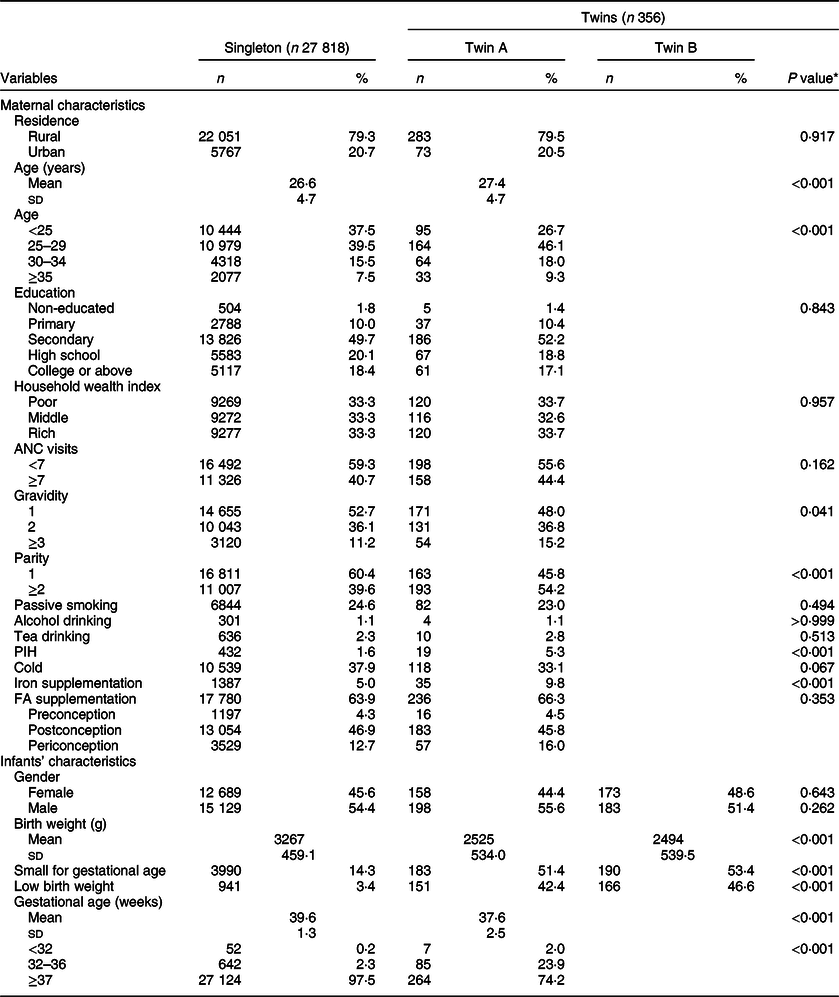
ANC, antenatal care; PIH, pregnancy-induced hypertension, FA, folic acid.
* Comparisons used Student’s t test for quantitative variables and χ2 test for categorical variables.
Demographic characteristics of participants
A total of 28 174 women aged 15–49 years were included in the survey, covering 27 818 single live births and 356 twin live births. The mean maternal age of the twin sample was 27·4 (sd 4·7) years, and 26·6 (sd 4·7) years for the singleton sample. There was no statistically significant difference in infant’s gender (twin A v. singleton, and twin B v. singleton, P = 0·643, 0·262, respectively). Other detailed demographic characteristics of the two samples are presented in Table 1.
Compared with the mean birth weight of the singleton sample (3267 g; sd 459·1), the mean birth weights of twin A (2525 g; sd 534·0) and twin B (2494 g; sd 539·5) were lower (P < 0·001). The prevalence rates of SGA in twin A and twin B were higher (51·4 and 53·4 v. 14·3 %; P < 0·001) than that of the singleton sample. The prevalence rates of LBW in twin A and twin B were higher (42·4 and 46·6 v. 3·4 %; P < 0·001) than that of the singleton sample. The twin sample’s GA was shorter than that of the singleton sample (37·6 (sd 2·5) v. 39·6 (sd 1·3) weeks; P < 0·001).
Associations between folic acid supplementation and birth weight, small for gestational age, low birth weight by plurality
Figure 2 displays the differences in birth weight, prevalence of SGA and LBW, and GA according to the status of FA supplementation in the singleton and twin samples (twin A, twin B). Accordingly, compared to non-users, infants’ birth weight for women with FA supplementation was higher (3283 v. 3240 g, P < 0·001; 2567 v. 2444 g, P = 0·040) in singleton and twin A sample, respectively, but there was no statistically significant difference (2530 v. 2426 g, P = 0·085) in twin B sample. The prevalence of SGA for women with FA supplementation was lower (singleton: 13·0 v. 16·8 %, P < 0·001; twin A: 44·1 v. 65·8 %, P < 0·001; twin B: 47·0 v. 65·8 %, P = 0·001) compared to non-users (Fig. 2(b)). Simultaneously, compared with non-users, the prevalence of LBW for women with FA supplementation was lower (singleton: 2·9 v. 4·2 %, P < 0·001; twin A: 37·7 v. 51·7 %, P = 0·012), but there was no statistically significant difference (44·9 v. 50·0 %, P = 0·363) in twin B (Fig. 2(c)). Compared with non-users, GA for women with FA supplementation was not significantly different in the singleton sample (39·7 v. 39·6 weeks, P = 0·306), but was lower in the twin sample (37·4 v. 38·1 weeks, P = 0·007) (Fig. 2(d)).
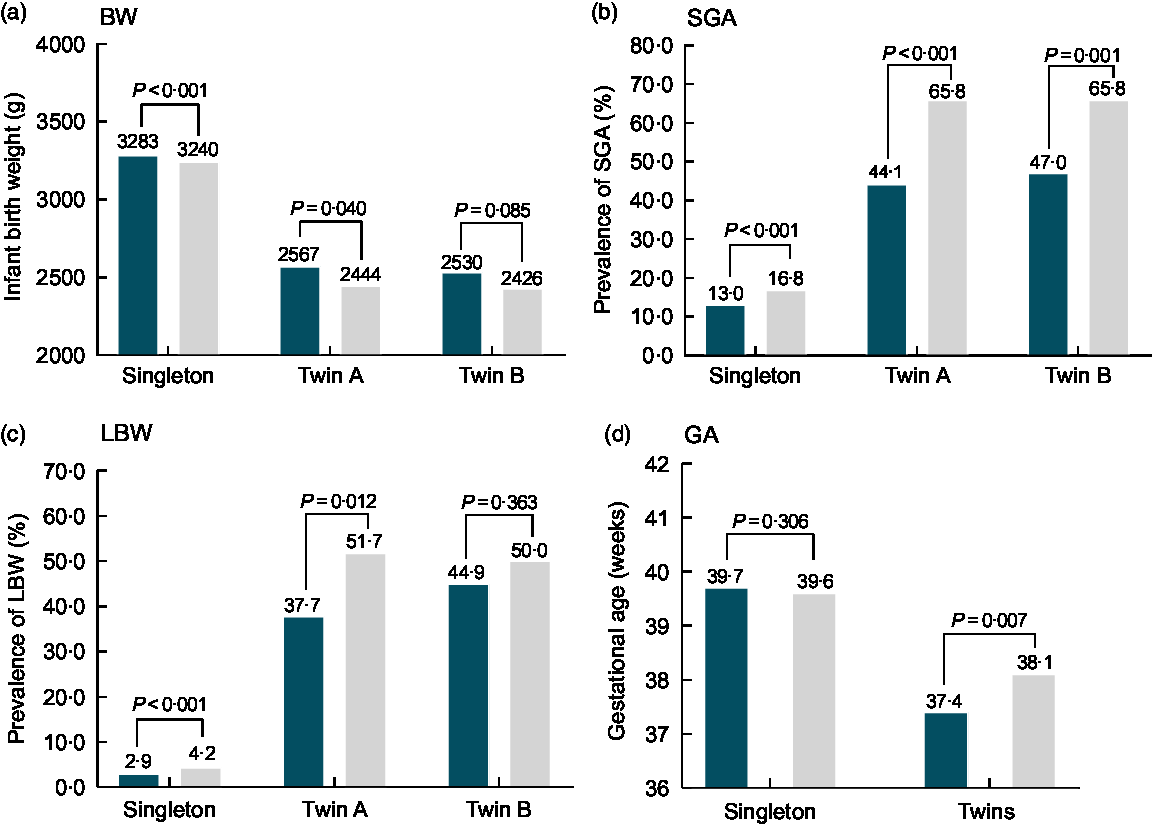
Fig. 2 Differences in BW, SGA, LBW and GA by plurality according to the status of FA supplementation in northwest China during 2010–2013. BW, birth weight; FA, folic acid; SGA, small for gestational age; LBW, low birth weight. ![]() , FA;
, FA; ![]() , non-FA.
, non-FA.
Stratified analysis was performed by plurality after an adjustment for major confounding factors (Fig. 3). Compared with non-users, infants’ birth weight for women with FA supplementation was higher in the singleton sample (17·3 g, 95 % CI 6·1, 28·4; P = 0·002), prominently higher in the twin sample (166·3 g, 95 % CI 69·1, 263·5, P = 0·001; twin A: 179·1 g, 95 % CI 71·7, 286·6, P = 0·001; twin B: 153·3 g, 95 % CI 43·5, 263·1, P = 0·006). FA supplementation was associated with a reduced risk of SGA in both singleton and twin samples (singleton: OR 0·85, 95 % CI 0·80, 0·92, P < 0·001; twins: OR 0·45, 95 % CI 0·30, 0·68, P < 0·001; twin A: OR 0·43, 95 % CI 0·27, 0·70, P = 0·001; twin B: OR 0·47, 95 % CI 0·29, 0·76, P = 0·002) and a reduced risk of LBW (singleton: OR 0·82, 95 % CI 0·71, 0·95, P = 0·008; twins: OR 0·50, 95 % CI 0·33, 0·75, P = 0·001; twin A: OR 0·38, 95 % CI 0·22, 0·64, P < 0·001), but there was no significant reduction of LBW in twin B (OR 0·62, 95 % CI 0·38, 1·03, P = 0·067).
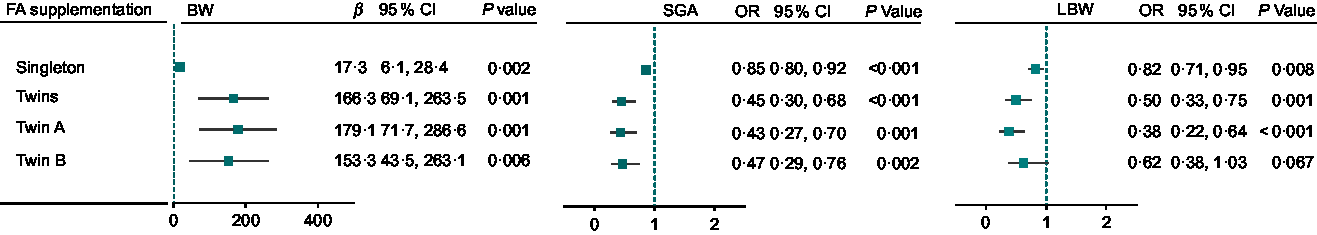
Fig. 3 Birth weight associated with FA supplementation in singletons and twins in northwest China during 2010–2013. For BW, β was adjusted for all covariates of infant’s gender, residence, maternal age, maternal education, household wealth index, antenatal care visits, gravidity, parity, passive smoking, alcohol drinking, tea drinking, pregnancy-induced hypertension, cold, iron supplementation, FA supplementation, gestational age in generalised linear models (GLM) with normal distribution and identity-link function among singletons, twin A, twin B; and β was adjusted for all covariates above plus birth order in generalised estimating equation (GEE) models with normal distribution and identity-link function in twins. For SGA, OR was adjusted for all covariates above except for GA in GLM with binomial distribution and logit-link function among singletons, twin A, twin B, and OR was adjusted for all covariates above except for GA, plus birth order in GEE with binomial distribution and logit-link function in twins. For LBW, OR was adjusted for all covariates above in GLM with binomial distribution and logit-link function among singletons, twin A, twin B, and OR was adjusted for all covariates above plus birth order in GEE with binomial distribution and logit-link function in twins. BW, birth weight; FA, folic acid; SGA, small for gestational age; LBW, low birth weight
Interaction effect analysis of folic acid supplementation with plurality on birth weight, small for gestational age, low birth weight
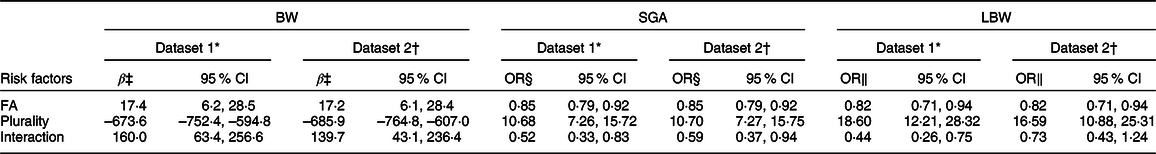
To identify whether the associations were caused by plurality rather than sampling error, the effect modification was examined by adding an interaction term of FA supplementation with plurality in dataset 1 (singleton and twin A) and dataset 2 (singleton and twin B) of the total population, respectively. Table 2 shows that the main effect terms of FA supplementation (dataset 1: 17·4 g, 95 % CI 6·2, 28·5, P = 0·002; dataset 2: 17·2 g, 95 % CI 6·1, 28·4, P = 0·002) and the interaction terms of FA supplementation by plurality (dataset 1: 160·0 g, 95 % CI 63·4, 256·6, P = 0·001; dataset 2: 139·7 g, 95 % CI 43·1, 236·4, P = 0·005) were positively correlated with birth weight. The results indicate that birth weight was approximately 17 g higher in the FA supplementation group compared to non-users for singletons, and was 177·4 g (160·0 + 17·4) and 156·9 g (139·7 + 17·2) higher in twin A and twin B.
Table 2 Interaction effect of FA supplementation with plurality on BW, SGA, LBW in the total population of northwest China during 2010–2013
BW, birth weight; FA, folic acid; SGA, small for gestational age; LBW, low birth weight.
* Singleton and twin A samples included in analysis.
† Singleton and twin B samples included in analysis.
‡ β represents an estimate of generalised linear models (GLM) with normal distribution and identity-link function, including all covariates of infant’s gender, residence, maternal age, maternal education, household wealth index, antenatal care visits, gravidity, parity, passive smoking, alcohol drinking, tea drinking, pregnancy-induced hypertension, cold, iron supplementation, FA supplementation, gestational age.
§ OR represents results of GLM with binomial distribution and logit-link function, including covariates above except for GA.
‖ OR represents results of GLM with binomial distribution and logit-link function, including all covariates above.
As regards SGA, compared with non-users, the main effect terms of FA supplementation (dataset 1 and 2: OR 0·85, 95 % CI 0·79, 0·92,P < 0·001) and the interaction terms of FA supplementation by plurality (dataset 1: OR 0·52, 95 % CI 0·33, 0·83, P = 0·007; dataset 2: OR 0·59, 95 % CI 0·37, 0·94, P = 0·027) were inversely associated with SGA, indicating that FA supplementation was associated with reduced risks of SGA (singleton: OR 0·85; twin A: OR 0·44 (0·85 × 0·52); twin B: OR 0·50 (0·85 × 0·59)).
For LBW, compared with non-users, the main effect terms of FA supplementation (dataset 1 and 2: OR 0·82, 95 % CI 0·71, 0·94, P = 0·006) and the interaction terms in dataset 1(OR 0·44, 95 % CI 0·26, 0·75, P = 0·003) were inversely associated with LBW, but there was no significant association for the interaction term in dataset 2 (OR 0·73, 95 % CI 0·43, 1·24, P = 0·248), indicating that FA supplementation was associated with a reduced risk of LBW for singletons (OR 0·82) and twin A (OR 0·36 (0·82 × 0·44)), but there was no statistically significant correlation in twin B (OR 0·60 (0·82 × 0·73)).
Timing of folic acid supplementation and birth weight by plurality
Figure 4 shows the association between timing of FA supplementation and birth weight, the risk of SGA and LBW in singletons and twins, respectively. Preconceptional, postconceptional and periconceptional FA supplementation was associated with increased birth weight and reduced SGA in both singletons and twins. Postconceptional and periconceptional FA supplementation was associated with a significant reduction of LBW in singletons and twins, but there was no significant reduction of LBW in preconceptional FA use in singletons and twins.
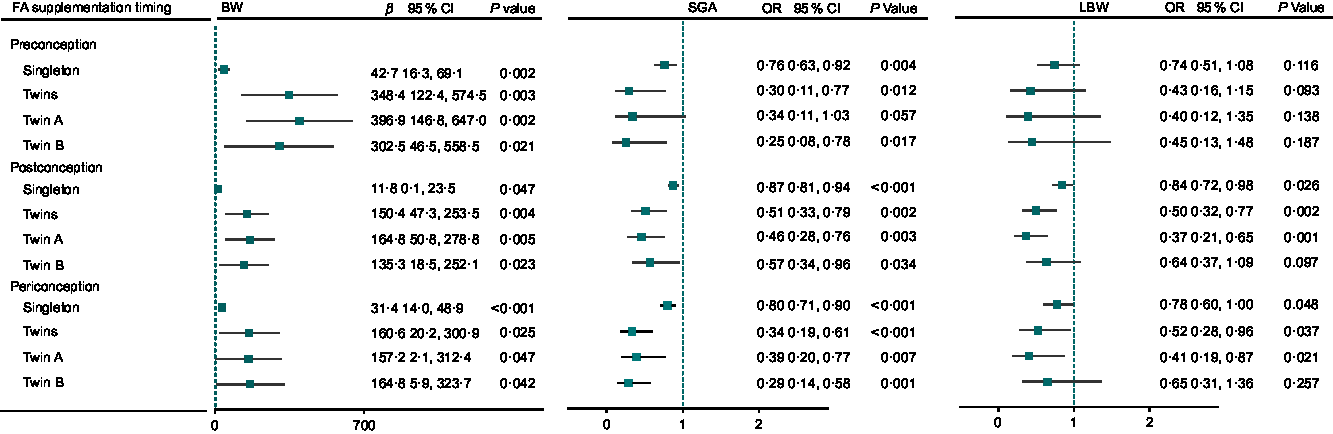
Fig. 4 Birth weight associated with timing of FA supplementation in singletons and twins in northwest China during 2010–2013. For BW, β was adjusted for covariates of infant’s gender, residence, maternal age, maternal education, household wealth index, antenatal care visits, gravidity, parity, passive smoking, alcohol drinking, tea drinking, pregnancy-induced hypertension, cold, iron supplementation, FA supplementation, gestational age (GA) in generalised linear models (GLM) with normal distribution and identity-link function among singletons, twin A, twin B, and β was adjusted for all covariates above plus birth order in generalised estimating equation (GEE) models with normal distribution and identity-link function in twins. For SGA, OR was adjusted for all covariates above except for GA in GLM with binomial distribution and logit-link function among singletons, twin A, twin B, and OR was adjusted for all covariates above except for GA, plus birth order in GEE with binomial distribution and logit-link function in twins. For LBW, OR was adjusted for all covariates above in GLM with binomial distribution and logit-link function among singletons, twin A, twin B, and OR was adjusted for all covariates above plus birth order in GEE with binomial distribution and logit-link function in twins.
Sensitivity analysis
An additional sensitivity analysis indicated a robust association of FA supplementation with birth weight, SGA and LBW when excluding women who sporadically continued to consume FA in the second trimester or third trimester or both (online Supplemental Table S1), and no statistically significant interaction effect was found between FA supplementation and infant’s sex (online Supplemental Table S2).
Discussion
FA supplementation from preconception (12 weeks before pregnancy) to the first trimester was associated with increased infant birth weight and reduced risk of SGA and LBW at birth in both singletons and twins, and this association might be more prominent in twins. Our findings are in accordance with previous studies showing that FA supplementation is associated with increased birth weight and reduced risk of LBW and SGA(Reference Timmermans, Jaddoe and Hofman19–Reference Li, Li and Ye22). In contrast, a majority of studies principally focused on singleton birth population, which excluded twin births. And few studies had compared twin and singleton births in the same sample. The birth weight of twins could increase 166·3 g if their mothers took FA, whereas this figure was only 17·3 g for singletons, and this association became more prominent when mothers took FA during 1–12 weeks before pregnancy. Further, the risk of SGA and LBW might be reduced by 55 and 50 % for twins if mothers took FA, but only 15 and 18 % for singletons. Therefore, twins might benefit much more from earlier-pregnancy FA supplementation. As we know, twins are the high-risk group of small size at birth and neonatal mortality(Reference Monden and Smits41,Reference Hanson, Munjanja and Binagwaho42) ; they require more attention. Considering the growth and development of the foetus and infant survival, FA supplementation might be beneficial for pregnant women with a family history of twins.
The association of timing of FA supplementation and birth weight has been explored in singleton birth population. The Generation R study in the Netherlands(Reference Timmermans, Jaddoe and Hofman19) suggested that preconceptional FA supplementation is associated with higher birth weight and lower risk of LBW and SGA. A prospective cohort study in Jiaxing indicated(Reference Zheng, Guan and Zhao20) an association between preconceptional FA supplementation and lower risk of SGA, but a large prospective cohort study in Jiangsu and Zhejiang provinces(Reference Li, Li and Ye22) showed that periconceptional or postconceptional FA supplementation is associated with a reduced risk of SGA and LBW. Nevertheless, our study found that FA supplementation is associated with increased birth weight, reduced risk of SGA for women who took FA during preconceptional, postconceptional and periconceptional periods in both singletons and twins. Similarly, postconceptional and periconceptional FA supplementation was associated with a reduced risk of LBW in singletons and twins, but there was no significant reduction of LBW in preconception FA use in both singletons and twins.
Previous studies have suggested that nutrient supplementation during pregnancy increased infant birth weight through prolonging gestational weeks(Reference Zeng, Cheng and Dang7,Reference Kang, Dang and Zeng43) . However, in our study, there was no difference in gestational weeks between the FA group and non-FA group in singletons, but the gestational week of the FA group was a little shorter than that of the non-FA group in twins. This result implies that increased infant birth weight with FA supplementation during pregnancy might not be through prolonging gestational weeks. Moreover, a higher sex ratio (about 119) at birth was observed in our sample, which was close to 118·06 from the Sixth Censuses of China in 2010(Reference Shi44). Moreover, our sample was in line with the characteristics of birth population in China. Meanwhile, a subgroup analysis by infant’s sex suggested that there was no significant interaction effect between FA use and infant’s sex on birth weight, risk of SGA and LBW.
The exact mechanisms behind the association of FA deficiency with adverse pregnancy outcomes were not fully understood(Reference Van der Molen, Verbruggen and Novakova45). FA is an essential water-soluble B vitamin, acting as a substrate in the biological pathways of cellular processes(Reference Tamura and Picciano12,Reference Bailey and Gregory46) , which might affect foetal growth indirectly by the optimisation of the FA-dependent homocysteine pathway because of its critical role in DNA synthesis and repair, as well as methylation. Higher serum homocysteine accumulation is associated with decreased foetal growth, while reduced FA status is associated with elevated homocysteine. Furthermore, the improvement of birth weight might be due to epigenetic modification for the effect of periconceptional women with FA supplementation(Reference Sinclair, Allegrucci and Singh47).
A systematic review has reported(Reference Muggli and Halliday48) a relationship between periconceptional FA supplements and increased twinning. But a population-based cohort study has suggested(Reference Li, Gindler and Wang49) that FA supplementation during pregnancy is not associated with an increased occurrence of multiple births. Our data also found no significant association (crude OR: 1·26, 95 % CI 0·97, 1·63) between preconceptional FA supplementation and the occurrence of twins. This problem requires more evidence. However, our study confirms a positive association between periconceptional 0·4 mg/d FA supplementation and increased birth weight in singletons and twins, which implies that periconceptional supplementation of 0·4 mg/d would play an active role in improving birth weight. It is noteworthy that high doses of FA supplements (≥1 mg/d) might have a detrimental effect on child’s birth weight and neuropsychological development(Reference Pastor-Valero, Navarrete-Munoz and Rebagliato23,Reference Valera-Gran, de la Hera and Navarrete-Munoz50) . Thus, further researches are required to examine the mechanism behind the association of FA supplementation with foetal development.
Our study has several strengths. First, it was a large population-based, cross-sectional study with a high response rate in northwest China using a stratified multistage random sampling method, which is generalisable to some extent. Second, birth weight and GA were recorded from a review of Medical Certificate of Birth, which might be relatively accurate. Third, a multi-parameter (birth weight, SGA, LBW) and multi-statistical (stratified analysis, interaction effect analysis, sensitivity analysis) approach was employed, and the results were robust and reliable. Besides, the associations of FA supplementation with birth weight, SGA and LBW in twins were explored, which had not been reported previously.
However, some limitations should be addressed. First, since this study was observational research, information on FA supplementation and other covariates was retrospectively self-reported by mothers after delivery. FA supplementation was not determined and supervised by the researcher. Therefore, there might be a misclassification of FA supplementation types due to the retrospective investigation. To minimise recall bias, efforts were made to help participants recall as accurately as possible. For one thing, standard and detailed classification questionnaires were used to control the recall bias. For another thing, before the formal investigation, interviewers were trained rigorously and a pilot study was performed. During the analysis, we just included live births in order to analyse the association of birth weight with FA supplementation. We had also controlled for possible confounders when data-analysing as far as possible. However, the potential for bias in our findings as a result of only including live births might still exist. Accordingly, systematic reviews and meta-analyses of high-quality prospective longitudinal data should be done to assist in clarifying this association. In addition, causal modelling and mediation analysis of direct and indirect effects could be also helpful. Second, the sample size was relatively small in the twin sample, and the power of analysis was limited. The association might be more prominent if the sample size was larger. In addition, data on the availability of maternal or infant folate biomarkers were lacking. Finally, observational studies are subject to unobserved confounding factors even though the observed confounding factors are controlled in the multivariable regression analysis, but residual confounding might still exist. Maternal height and weight gain during pregnancy could be an important predictor of the size of the baby at birth. Unfortunately, maternal height and weight gain was not recorded in this cross-sectional survey, especially because mothers could not effectively recall such information during pregnancy.
Conclusion
The present study suggests an association of periconceptional 0·4 mg/d FA supplementation with increased birth weight and reduced risk of SGA and LBW in both singletons and twins, and this association may be more prominent in twins. Further researches with high-quality prospective longitudinal data are required to explore their associations and interpret underlying mechanisms.
Acknowledgements
Acknowledgements: We are grateful to the many individuals and organisations that have contributed to this work, including all the mothers and their infants who participated in the survey. We specifically acknowledge support of the local government, the local education bureau, and the dedication and hard work of the field team and data manager in the Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center. Financial support: This work was supported by the National Natural Science Foundation of China (grant number 81230016) and Shaanxi Health and Family Planning Commission (grant number sxwsjswzfcght2016-013), National Key R&D Program of China (grant number 2017YFC0907200, 2017YFC0907201). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: B.Z., S.S., S.D. and H.Y. conceived and designed the study; B.Z. and S.S. analysed the data and drafted the article; S.D. and H.Y. revised the article; B.Z., S.S., S.L., B.M., G.S., M.L., M.M. and Q.W. collected and cleared the data. All authors approved the final version of the article to publish. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Xi’an Jiaotong University Health Science Center (number 2012008). Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this article visit https://doi.org/10.1017/S1368980019004580








