INTRODUCTION
Helminths (animal parasitic nematodes or roundworms) have debilitated human beings over the course of evolution, whether infecting our livestock, our pets, or us directly. Soil-transmitted helminths (STHs; Table 1) establish chronic infections within the gastrointestinal (GI) tract and are by far the most prevalent. The World Health Organization (WHO) currently estimates that STHs infect 24% of the human population, and infection causes extensive morbidity (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006). Almost 900 million children are chronically infected, causing stunted growth and delayed cognitive and intellectual development. Millions of pregnant women are also infected, leading to adverse birth outcomes. Although highly concentrated in developing countries of sub-Saharan African, Southeast Asia and tropical regions of Central and South America, those who travel to and from endemic regions are also at risk of getting infected (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006). Furthermore, when also considering the worldwide prevalence and severity of STHs in livestock (Krecek and Waller, Reference Krecek and Waller2006), some of which are known to cause zoonoses (McCarthy and Moore, Reference McCarthy and Moore2000), greatly reducing the prevalence of these parasites from earth is critical for future global human health and economic growth.
Table 1. Biology of soil-transmitted helminth (STH) infections in humans and livestock
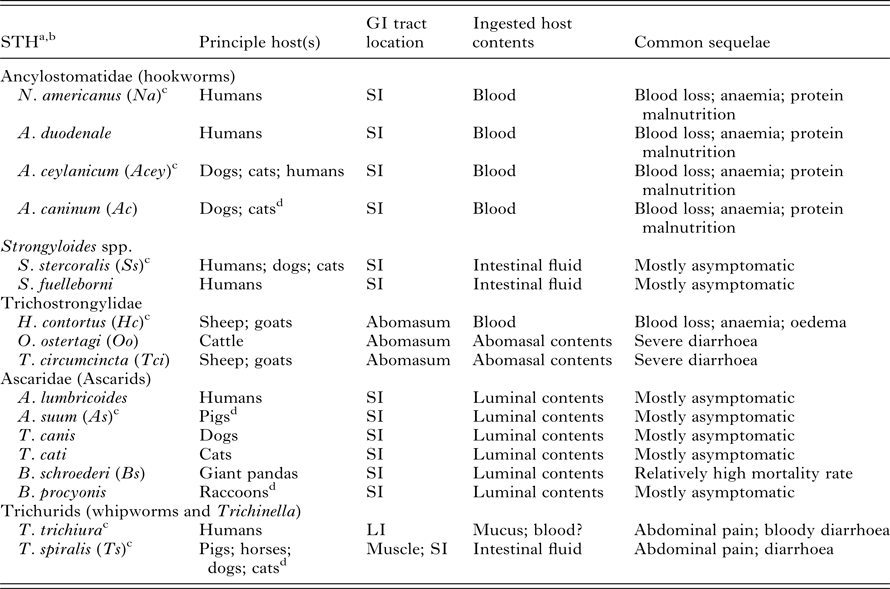
GI, gastrointestinal; SI, small intestine; LI, large intestine.
a Only the STHs that are mentioned in this review and infect humans or livestock (or in the case of B. schroederi endangered wild animal species) are listed.
b Only STH species from which the immunogens in Table 2 are derived from given abbreviations in parentheses.
c Genome has been sequenced.
d Relatively frequent reported transmissions from principle host(s) to humans.
Table 2. Summary of recombinant subunit vaccine studies for STHs
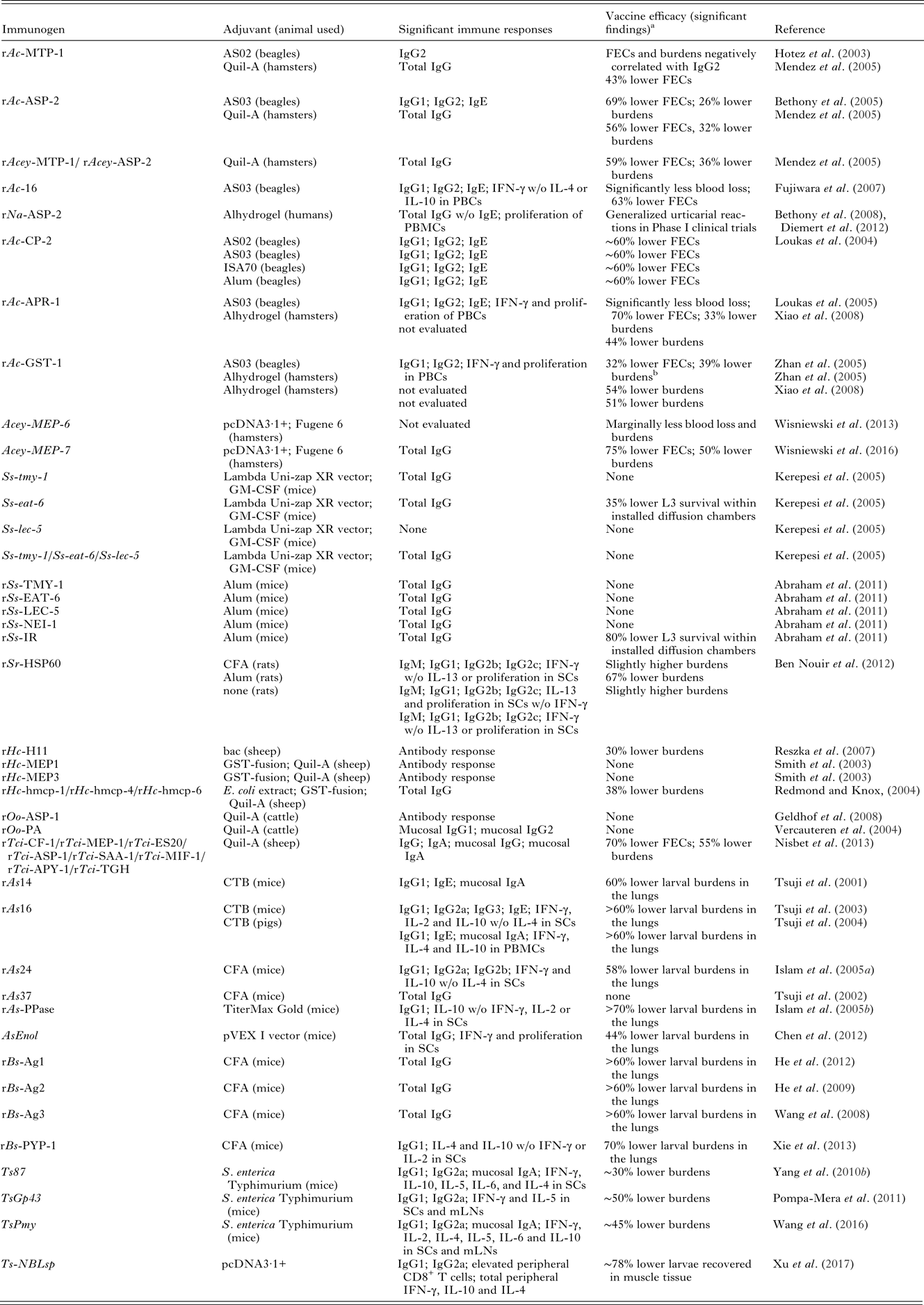
FECs, fecal egg counts; PBCs, peripheral blood cells; PBMCs, peripheral blood mononuclear cells; GM-CSF, granulocyte-macrophage colony-stimulating factor; SCs, spleen cells or spenocytes; bac, baculovirus-insect cell extract; PA, polyprotein allergen; CTB, cholera toxin B subunit; CFA, complete Freund's adjuvant; mLNs, mesenteric lymph nodes; ns, not significant; w/o, without.
a Results are compared with adjuvant alone control.
b These results were not statistically significant.
It is generally accepted that vaccines for STHs would be the most ideal control agents as they would prevent re-infection, which anthelmintic drugs do not. However, there are no licensed vaccines. Currently, chemotherapy is delivered via mass drug administration (MDA), stirring grave concern for the strong selection of drug resistance, especially with limited efficacious anthelmintic drugs (Geerts and Gryseels, Reference Geerts and Gryseels2001). Immunity to STH infections does not develop upon clearance, and represents a vast issue for humans who are rapidly re-infected after chemotherapy (Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006). Thus, vaccines would go a long way towards STH elimination.
An important distinction between STH infections in humans and livestock, ruminants in particular, is that in the latter, protective immunity often develops with age, but in the former, this is not the case. So, although the duration of vaccine-induced protection in livestock may only need to last long enough to protect younger animals, for humans this protection would need to last throughout their lifetime. Thus, developing vaccines for humans will likely be a much greater challenge than for livestock due to the high likelihood that repeated immunizations will be needed throughout life.
What level of efficacy is needed to justify inclusion of an STH vaccine in a control program? Bartsch et al. (Reference Bartsch, Hotez, Hertenstein, Diemert, Zapf, Bottazzi, Bethony, Brown and Lee2016) developed a target product profile (TPP) for a recombinant subunit vaccine against human hookworms and evaluated the vaccines economic and epidemiologic impact on hookworm infection in endemic Brazil. A modelled human hookworm vaccine administered in a single dose to infants (78% coverage rate) with an efficacy of 80% at preventing L3 maturation and providing at least 10 years of protection with a single booster at 15 years of age (78% compliance), and costing $1 per dose, was highly cost-effective and economically dominant compared with no intervention or annual MDA (Bartsch et al. Reference Bartsch, Hotez, Hertenstein, Diemert, Zapf, Bottazzi, Bethony, Brown and Lee2016). Thus, a human STH vaccine with such a TPP should be ideal.
For livestock, a subunit vaccine called Barbervax, developed in Australia, has been commercialized for Haemonchus contortus (barber's pole worm) that consists of a complex combination of native antigens from the worm's gut (described in more detail below). Barbervax is about 70% effective at reducing worm burdens and about 90% effective at reducing the number of eggs in the feces, which greatly reduces egg numbers throughout the pasture, and thus, the number of infections later on. Barbervax is much more cost-effective and efficacious compared with no intervention and MDA alone (especially considering rampant anthelmintic drug resistance in H. contortus) even though it has to be given repeatedly during the summer months (when infection is highest) because a protective memory response is not induced in vaccinated lambs during challenge infection. Clearly any STH vaccine for livestock with such a high efficacy would meet the requirements of an established TPP. As Barbervax is not a recombinant subunit vaccine, it requires numerous sheep to make enough worm guts to produce the vaccine. It is hard to tell if recombinant subunit vaccines for livestock should have similar TPPs and this will have to be addressed in the future.
There have been many attempts to develop vaccines for STHs of humans and livestock (Hewitson and Maizels, Reference Hewitson and Maizels2014). These parasites are relatively large, multicellular, cuticularized animals (i.e. unique from other infectious agents). They have relatively large genomes and produce many thousands of offspring per day, contributing to their rapid evolution (Zarowiecki and Berriman, Reference Zarowiecki and Berriman2015). It is thus clear that the biology of these parasites provides a formidable barrier for vaccine development. Also, although ‘holistic’ vaccines [radiation-attenuated larvae, crude worm extracts and excretory/secretory (ES) products] have provided high levels of protection against many STHs tested, with a few STHs of livestock being some exceptions (Hewitson and Maizels, Reference Hewitson and Maizels2014), because these parasites cannot be cultured on a large enough scale for mass vaccine administration (unlike bacteria and viruses) due to high costs, low stability and shelf-life, among other reasons, recombinant subunit vaccines are necessary. Hence, in this review we do not evaluate the previous vaccine studies that used ‘holistic’ vaccines as this has been extensively reviewed before (Hewitson and Maizels, Reference Hewitson and Maizels2014). Rather, we provide an update and evaluation of the previous studies that used recombinant subunit vaccines, the only realistic type of vaccine possible for controlling STHs.
Below, we first provide a general overview of the immune response(s) that are most relevant for this review. We then summarize and interpret the previous studies that used recombinant subunit vaccines for the major STHs of both humans and livestock (data presented in Table 2), grouped into separate sections based on their phylogenetic relationships (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015). In this review, we use the phylogeny of Nematoda that groups the phylum into five distinct clades (clades I, II, III, IV and V), of which the parasitic species relevant for human and veterinary medicine belong to clades I, III and V (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015). Included in each section is a brief description of each STH's basic biology to compare and contrast with the other STHs. We then provide an evaluation of all of these studies as a whole, and discuss potential avenues that may help to develop more efficacious, recombinant subunit vaccines for these critical parasites.
TH1 and TH2 immune responses
Two key arms of the adaptive immune system are the type 1 and type 2 T helper (TH) cell responses (TH1 and TH2) (Romagnani, Reference Romagnani2000). The TH1 response promotes cell-mediated immunity, while TH2 promotes humoral immunity. Whether a TH1 or TH2 response is induced generally depends on whether the pathogen is intracellular (such as viruses, and some bacteria and protozoa), or extracellular, such as helminths, and also toxins. However, it must be noted that many cracks have been discovered in the foundation for the TH1/TH2 paradigm, in particular that the abundance of effector cytokines, chemokines, co-stimulatory molecules, signalling pathways and transcription factors involved in each response is much greater and more complex than originally thought (Zhu et al. Reference Zhu, Yamane and Paul2010), and there appear to be variations of these responses for particular pathogen types. But for the purpose of this review, we will only mention how TH1 and TH2 responses are mounted, briefly summarizing that covered in Kaiko et al. (Reference Kaiko, Horvat, Beagley and Hansbro2008).
We begin with the TH1 response. Host cells (non-professional antigen presenting cells or APCs) infected with intracellular pathogens present antigens via Class I Major Histocompatibility Complex (MHC) molecules. Cognate naïve CD8+ cytotoxic T (TC) cells then recognize the antigen-bound Class I MHC via the antigen-specific T cell receptor (TCR), resulting in the activation and differentiation of TC cells into antigen-specific effectors that destroy the infected cell, or into memory TC cells for responding to future cognate antigen. Professional APCs such as macrophages or dendritic cells also recognize and endocytose the pathogen or its antigens and present them on Class II MHC molecules. The antigen-loaded professional APCs then travel to nearby lymphoid tissues where they are recognized by cognate naïve CD4+ TH (TH0) cells via antigen-specific TCR. In combination with the cytokine interleukin (IL)-12 secreted by the professional APCs and necessary co-stimulatory molecules (‘signal two’ and ‘signal three’), TH0 cells differentiate into TH1 effector cells that proliferate and produce massive quantities of pro-inflammatory cytokine interferon gamma (IFN-γ), as well as IL-2 and tumour necrosis factor (TNF), triggering the influx of inflammatory cells and the complement system into the site of infection, or into TH1 memory cells. TH1 effector cells also provide help to differentiating TC cells and induce B cell-secretion of opsonizing antibodies or immunoglobulins (Ig), in general IgG2, which along with the complement system enhances phagocytosis, and thus, pathogen clearance (Kaiko et al. Reference Kaiko, Horvat, Beagley and Hansbro2008).
When professional APCs present antigens from extracellular pathogens such as helminths, or toxins/allergens, to TH0 cells in lymphoid tissues, the professional APCs secrete IL-2 and IL-4 that, along with the necessary co-stimulators, drives differentiation of TH0 cells into TH2 effectors or into TH2 memory cells. TH2 effector cells secrete three key cytokines that drive classic features of the TH2 response, IL-4, IL-5 and IL-13. They can also secrete IL-10, which tends to dampen immune responses. IL-4: (1) drives antibody class switching from IgM to IgG1 and IgE; (2) drives affinity maturation by somatic hypermutation in B cells; (3) stimulates B cell proliferation and differentiation into plasma cells; and (4) drives differentiation of TH2 cells, thus providing feedback regulation. IL-5 attracts and activates eosinophils, and also drives the secretion of IgA (Lebman and Coffman, Reference Lebman and Coffman1988), the most abundant mucosal antibody responsible for intestinal homoeostasis (Mantis et al. Reference Mantis, Rol and Corthesy2011). IL-13 mediates the release of granules from basophils and mast cells and can also drive class switching to IgE. In TH2-mediated allergy, IgE is key, with the Fcε region of the heavy chain recognized with high affinity by the FcεRI receptor that is constitutively expressed on basophils and mast cells. This receptor is expressed at much lower levels on professional APCs and is inducible on eosinophils. Basophils, mast cells and eosinophils are granulocytes that collectively release proinflammatory mediators and cytotoxins that result in a robust, hypersensitivity reaction associated with allergy (Kaiko et al. Reference Kaiko, Horvat, Beagley and Hansbro2008; Stone et al. Reference Stone, Prussin and Metcalfe2010).
TH2-type responses are clearly associated with reduced intensities of infection by STHs (Anthony et al. Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007; Nair and De'Broski, Reference Nair and De'Broski2016). These responses involve an interconnected, complex network of IgE-driven mastocytosis and basophilia, IL-5-driven eosinophilia, and IL-4/IL-13-driven alternative activation and recruitment of macrophages and parasite expulsion. Expulsion occurs via intestinal epithielial cell (IEC) secretion of RELM-β and Muc2/Muc5ac (‘mucus’), IEC turnover (‘epithelial escalation’) and/or increased IEC permeability and smooth muscle contraction (‘weep and sweep’ response) (Anthony et al. Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007; Nair and De'Broski, Reference Nair and De'Broski2016). However, subunit vaccines do not necessarily need to induce the natural immune response(s) in order to provide protection. Subunit vaccines for STHs have focused primarily on the idea that the protein antigens targeted by the vaccines are important for infection, rather than simply being immunogenic in order to direct the immune system to the pathogen to attack it. Immunization with most STH antigens triggers IgG responses (IgG1 and IgG2 are by far the most abundant serum antibodies), although titre is highly variable. The common theme is that high IgG titre blocks antigen function, and if the antigen is essential, protection may be achieved. As discussed below, the most promising vaccine candidates discovered from previous studies are consistent with this common theme.
RECOMBINANT SUBUNIT VACCINES FOR STRONGYLIDS
The order Strongylida (Nematoda clade V) contains many important parasitic species, including devastating parasites of humans, livestock, dogs and cats, as well as species that infect birds, reptiles and amphibians (Durettedesset et al. Reference Durettedesset, Beveridge and Spratt1994). Strongylida was once grouped into four suborders: Strongylina, Ancylostomatina, Trichostrongylina and Metastrongylina, of which the former three suborders contained the most significant species for human and veterinary medicine (Durettedesset et al. Reference Durettedesset, Beveridge and Spratt1994). But it is now clear that these suborders are not monophyletic and that the most significant species within the former three suborders are actually in their own monophyletic group (Chilton et al. Reference Chilton, Huby-Chilton, Gasser and Beveridge2006). Thus, we do not use the suborders in this review, but only refer to the most relevant families, which are currently stable. The most extensive studies with recombinant subunit vaccines for strongylids have been on hookworms, with studies on Strongyloides spp. and a few strongylid parasites of livestock also prevalent in the literature. As many of the important strongylid species within these families have relatively recent common ancestries with very similar modes of infection (Durettedesset et al. Reference Durettedesset, Beveridge and Spratt1994; Chilton et al. Reference Chilton, Huby-Chilton, Gasser and Beveridge2006; Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015), a single, pan-strongylid vaccine with efficacy against a majority of the species seems within reason, hence why we have grouped them into their own section.
Hookworms
Hookworms (family Ancylostomatidae) are a serious issue for human health, as well as for dogs and cats, and of all the STHs, these parasites cause the most severe disease due to their voracious blood feeding (Table 1). The human hookworms (Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum) infect almost 500 million people (Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). Necator americanus and A. duodenale infect predominantly humans while A. ceylanicum infects humans, dogs, cats and some rodents. Third stage larvae (L3) penetrate the skin and enter the blood stream, arriving in the lungs for L3 maturation (N. americanus and Ancylostoma spp.). L3 then migrate up the trachea into the pharynx and are swallowed. Ancylostoma spp., but not Necator, are also able to directly enter orally. After passing through the stomach, L3 burrow into the epithelium of the small intestine, most often in the duodenum, and rupture capillaries to feed on blood. Blood feeding by the attached hookworms causes iron deficiency anaemia in humans, and is of major concern for children and pregnant women. Adult hookworms mate within the lumen of the small intestine, and each female can produce over 10 000 eggs per day. These hematophagous parasites can live for years in the same host (Hotez et al. Reference Hotez, Brooker, Bethony, Bottazzi, Loukas and Xiao2004).
Pre-clinical vaccine studies for hookworms have either used the dog hookworm Ancylostoma caninum-beagle or A. ceylanicum-golden hamster (Mesocricetus auratus) pathosystems, or a laboratory strain of N. americanus adapted to golden hamsters. Antigens from both infectious L3 and adult hookworms have been subjected to pre-clinical vaccine studies. These past attempts focused on recombinant subunit vaccines using Escherichia coli, Pichia pastoris or baculovirus-insect cell expression systems.
Secreted L3 antigens
Beagles that received four intramuscular (i.m.) injections at 140 µg each of a recombinant, E. coli-expressed Astacin-like metalloprotease (rAc-MTP-1), natively secreted by L3, and formulated with GlaxoSmithKline's AS02 adjuvant (TH1-biased) in general had lower A. caninum worm burdens and fecal egg counts (FECs) compared with AS02 alone [Table 2; the level of protection was correlated with serum IgG2 endpoint titre (i.e. the inverse of the greatest serum dilution at which there is an above background response)] (Hotez et al. Reference Hotez, Ashcom, Zhan, Bethony, Loukas, Hawdon, Wang, Jin, Jones, Dobardzic, Dobardzic, Bolden, Essiet, Brandt, Russell, Zook, Howard and Chacon2003). This initial study provided proof of concept that recombinant subunit vaccines have efficacy against hookworms.
Ancylostoma secreted protein (Ac-ASP-2) is natively expressed in the oesophageal gland cells, basal lamina and cuticle, and secreted by L3. Baculovirus-insect cell-expressed rAc-ASP-2 formulated with GlaxoSmithKline's AS03 adjuvant (unresolved TH response) and injected i.m. four times into beagles at 100 µg doses had average serum IgG1 and IgG2 endpoint titre of >10 000 and average serum IgE endpoint titre of 1000 (Table 2). rAc-ASP-2/AS03 immunization resulted in an average 26 and 69% lower A. caninum worm burdens and FECs, respectively, compared with AS03 alone (Table 2), although the latter measure had extensive variation. Interestingly, in the same study, intensity of N. americanus infection in individuals from endemic Brazil was negatively correlated with IgE titre specific for Na-ASP-2, revealing this L3 antigen as naturally immunogenic and, possibly, naturally protective.
In another study, P. pastoris-expressed rAcey-MTP-1 and rAcey-ASP-2 were injected three times i.m. into golden hamsters at 25 µg doses as both monovalent and bivalent vaccines formulated with Quil-A adjuvant (mixed TH1/TH2 response). Immunization resulted in impressive average total serum IgG endpoint titre (no IgG subclass-specific secondary antibodies for hamsters) between 50 000 and 200 000, including from both the monovalent and bivalent vaccines (Table 2), suggesting that immunological interference was not a problem. Similar to that found in the A. caninum-beagle pathosystem, both monovalent vaccines resulted in lower A. ceylanicum FECs (rAcey-ASP-2/Quil-A = 56% lower; rAcey-MTP-1/Quil-A = 43% lower) and worm burdens (rAcey-ASP-2/Quil-A = 32% lower; rAcey-MTP-1/Quil-A = 28% lower) compared with Quil-A alone, although the worm burden for rAcey-MTP-1/Quil-A were not significantly less (Table 2). Surprisingly, the bivalent vaccine did not provide significant, additive protection over the monovalents with an average 59 and 36% lower FECs and worm burdens, respectively, compared with Quil-A alone (Table 2). On the other hand, clinicopathological parameters (reduced haemoglobin and body weight) were significantly improved in the bivalent compared with the monovalent vaccines (Table 2) (Mendez et al. Reference Mendez, Zhan, Goud, Ghosh, Dobardzic, Wu, Liu, Deumic, Dobardzic, Liu, Bethony and Hotez2005). Nonetheless, lack of a significant additive effect on FECs and worm burdens suggests that ASP-2 and MTP-1 may have functional redundancies during tissue migration.
Ac-16 is homologous to larval surface antigens of Nematoda clade III helminths that had been previously shown to protect against experimental infections (see section on ascarids below). Ac-16 was expressed in E. coli, formulated with AS03 and injected three times i.m. into beagles at 100 µg doses. Immunization of beagles with rAc-16/AS03 resulted in peak serum IgG1, IgG2 and IgE endpoint titre of around 5000, 100 000 and 1000, respectively, significant rAc-16-stimulated proliferation of peripheral blood cells and production of IFN-γ, but not IL-4 or IL-10 (Table 2). rAc-16/AS03 also resulted in an average 63% lower A. caninum FECs and significantly less blood loss compared with AS03 alone, but worm burdens were similar (Table 2) (Fujiwara et al. Reference Fujiwara, Zhan, Mendez, Loukas, Bueno, Wang, Plieskatt, Oksov, Lustigman, Bottazzi, Hotez and Bethony2007). Nonetheless, this study clearly demonstrated the vaccine efficacy of rAc-16/AS03 for reducing hookworm fecundity and hookworm-associated blood loss, in association with IgG and IgE responses, and a TH1-biased cytokine profile (but without measuring IL-5 and IL-13, contribution from at least some TH2 cytokines cannot be ruled out). Ac-16 was also localized to the L3 cuticle with rAc-16/AS03 serum from beagles (Fujiwara et al. Reference Fujiwara, Zhan, Mendez, Loukas, Bueno, Wang, Plieskatt, Oksov, Lustigman, Bottazzi, Hotez and Bethony2007).
With the promising pre-clinical results for ASP-2 (significantly reduced worm burdens, FECs and clinical pathology), and negative correlation between infection intensity and Na-ASP-2-specific serum IgE responses in individuals from endemic Brazil, P. pastoris-expressed rNa-ASP-2 formulated on Alhydrogel (aluminum hydroxide gel; a widely used TH2 adjuvant) (Goud et al. Reference Goud, Bottazzi, Zhan, Mendez, Deumic, Plieskatt, Liu, Wang, Bueno, Fujiwara, Samuel, Ahn, Solanki, Asojo, Wang, Bethony, Loukas, Roy and Hotez2005) advanced to clinical trials. Three i.m. injections in healthy and unexposed adults of either 10, 50 or 100 µg doses were well tolerated and induced total serum IgG endpoint titre of around 10 000, while serum IgE endpoint titre were below 100, and significant rNa-ASP-2-stimulated proliferative responses in peripheral blood mononuclear cells (PBMCs) (Table 2) (Bethony et al. Reference Bethony, Simon, Diemert, Parenti, Desrosiers, Schuck, Fujiwara, Santiago and Hotez2008). However, rNa-ASP-2/Alhydrogel in only a single injection of 10 µg failed in Phase I as individuals previously infected with or exposed to N. americanus resulted in generalized urticarial reactions due to pre-existing Na-ASP-2-specific serum IgE (Table 2) (Diemert et al. Reference Diemert, Pinto, Freire, Jariwala, Santiago, Hamilton, Periago, Loukas, Tribolet, Mulvenna, Correa-Oliveira, Hotez and Bethony2012). Although rNa-ASP-2 is still being optimized to reduce its allergic potential, namely by removing the IgE epitopes and fusion with Fcγ1, thus promoting its ligation with the FcγRIIb inhibitory receptor (Zhan et al. Reference Zhan, Santiago, Keegan, Gillespie, Xue, Bethony, de Oliveira, Jiang, Diemert, Xiao, Jones, Feng, Hotez and Bottazzi2012), these findings have halted vaccine studies focused on L3 antigens.
Adult intestinal antigens
Results with ASP-2 demonstrated an important problem for targeting antigens that are naturally immunogenic during hookworm infection (at least from L3); there may be an allergic reaction following immunization of individuals that had been previously infected or exposed. Thus, next attempts focused on the so-called ‘hidden antigens’ (i.e. STH proteins that are not exposed to the host immune system during infection but that may be accessed by host immune effectors induced by vaccination) present in the hookworm intestinal lumen and thus accessible to host antibodies in the ingested blood (Hotez et al. Reference Hotez, Bethony, Diemert, Pearson and Loukas2010). Hookworms have a semi-ordered proteolytic cascade in their intestinal lumen for digesting haemoglobin during blood meals (Williamson et al. Reference Williamson, Lecchi, Turk, Choe, Hotez, McKerrow, Cantley, Sajid, Craik and Loukas2004; Ranjit et al. Reference Ranjit, Zhan, Hamilton, Stenzel, Lowther, Pearson, Gorman, Hotez and Loukas2009) and all of the vaccine studies pertaining to adult intestinal antigens have focused on this process (Hotez et al. Reference Hotez, Bethony, Diemert, Pearson and Loukas2010).
A recombinant, P. pastoris-expressed, Cathepsin B cysteine protease (rAc-CP-2) that natively localizes in the hookworm intestinal lumen (Loukas et al. Reference Loukas, Bethony, Williamson, Goud, Mendez, Zhan, Hawdon, Bottazzi, Brindley and Hotez2004) and that degrades fragments of haemoglobin in vitro (Williamson et al. Reference Williamson, Lecchi, Turk, Choe, Hotez, McKerrow, Cantley, Sajid, Craik and Loukas2004; Ranjit et al. Reference Ranjit, Zhan, Hamilton, Stenzel, Lowther, Pearson, Gorman, Hotez and Loukas2009), was formulated with multiple adjuvants [AS02, AS03, ISA70 and aluminium phosphate (alum)] and injected i.m. three times into beagles at 100 µg doses (Loukas et al. Reference Loukas, Bethony, Williamson, Goud, Mendez, Zhan, Hawdon, Bottazzi, Brindley and Hotez2004). Although rAc-CP-2/adjuvant immunizations resulted in average serum IgG1 and IgG2 endpoint titre of >10 000 and average serum IgE endpoint titre of around 1500 regardless of adjuvant, A. caninum worm burdens were the same as adjuvant controls, while FECs were an average 60% lower (Table 2). The lowest FECs and highest IgG titre were observed with the AS03 adjuvant, although the differences from the other adjuvants were not significant. Also, pooled IgGs from rAc-CP-2/AS03-immunized beagles reduced the in vitro rAc-CP-2 protease activity by an average of 73% and recognized native Ac-CP-2 within the adult hookworm intestinal lumen (Loukas et al. Reference Loukas, Bethony, Williamson, Goud, Mendez, Zhan, Hawdon, Bottazzi, Brindley and Hotez2004), suggesting that blocking/neutralizing IgG contributed to the reduced fecundity.
Another adult antigen, Cathepsin D aspartic protease (Ac-APR-1), which is also localized within the intestinal lumen but degrades whole haemoglobin (Williamson et al. Reference Williamson, Lecchi, Turk, Choe, Hotez, McKerrow, Cantley, Sajid, Craik and Loukas2004; Ranjit et al. Reference Ranjit, Zhan, Hamilton, Stenzel, Lowther, Pearson, Gorman, Hotez and Loukas2009), was recombinantly expressed in P. pastoris. rAc-APR-1 was formulated with AS03 and injected i.m. three times in 100 µg doses into beagles. rAc-APR-1/AS03 immunization resulted in average serum IgG1 and IgG2 endpoint titre of >10 000 and average serum IgE endpoint titre of around 1500 (Table 2). There was significant IFN-γ production and proliferative responses in rAc-APR-1-stimulated whole blood cells (Table 2). rAc-APR-1/AS03 immunization resulted in 70 and 33% lower median A. caninum FECs and worm burdens, respectively, and anaemia was significantly less compared with AS03 alone (Table 2). Similar to rAc-CP-2/AS03, serum IgG from beagles immunized with rAc-APR-1/AS03 reduced the in vitro protease activity of rAc-APR-1 by an average of 71%, and recognized native Ac-APR-1 within the adult hookworm intestinal lumen (Loukas et al. Reference Loukas, Bethony, Mendez, Fujiwara, Goud, Ranjit, Zhan, Jones, Bottazzi and Hotez2005).
The final protective adult hookworm antigen is a glutathione S-transferase (GST-1) that is localized within the intestine, muscular tissue and hypodermis and is also present in adult excretory/secretory (ES) products. GST-1 is believed to scavenge and detoxify haeme liberated from degraded haemoglobin within the hookworm intestine (Zhan et al. Reference Zhan, Liu, Perally, Xue, Fujiwara, Brophy, Xiao, Liu, Feng, Williamson, Wang, Bueno, Mendez, Goud, Bethony, Hawdon, Loukas, Jones and Hotez2005). Based on GST-1's localization in the hypodermis and presence in ES products, the protein may have other functions as well. Pichia pastoris-expressed rAc-GST-1/AS03 i.m. injections into beagles (three times in 100 µg doses), as with rAc-CP-2/AS03 and rAc-APR-1/AS03, resulted in average serum IgG1 and IgG2 titre of >10 000, lower average serum IgE endpoint titre of <500, and significant proliferative responses and IFN-γ production in rAc-GST-1-stimulated whole blood cells (Table 2). rAc-GST-1/AS03 immunization resulted in an average 32 and 39% lower A. caninum FECs and worm burdens, respectively, compared with AS03 alone (Table 2). These reductions were not significant, which was attributed to too much variation between beagles. However, in the same study, rAc-GST-1 formulated on Alhydrogel was subcutaneously (s.c.) injected three times at 25 µg doses into golden hamsters and challenged with the adapted laboratory N. americanus strain, and worm burdens were a significant 54% lower than Alhydrogel alone (Table 2) (Zhan et al. Reference Zhan, Liu, Perally, Xue, Fujiwara, Brophy, Xiao, Liu, Feng, Williamson, Wang, Bueno, Mendez, Goud, Bethony, Hawdon, Loukas, Jones and Hotez2005). Although immune correlates of this protection were not evaluated, this result provided proof of concept for cross-protection against hookworms from different genera using the same immunogen, in addition to revealing GST-1 as a promising vaccine target.
In another study using the N. americanus-golden hamster pathosystem, rAc-GST-1/Alhydrogel under the same immunization regimen as Zhan et al. (Reference Zhan, Liu, Perally, Xue, Fujiwara, Brophy, Xiao, Liu, Feng, Williamson, Wang, Bueno, Mendez, Goud, Bethony, Hawdon, Loukas, Jones and Hotez2005) again had an average of 51% lower N. americanus worm burdens, and in the same study, rAc-APR-1/Alhydrogel had 44% lower N. americanus worm burdens compared with Alhydrogel alone (Table 2) (Xiao et al. Reference Xiao, Zhan, Xue, Goud, Loukas, Liu, Williamson, Liu, Deumic and Hotez2008). Thus, both A. caninum immunogens were capable of cross protecting against N. americanus in golden hamsters, although immune correlates of protection were not evaluated.
The cross-protections against N. americanus in golden hamsters with rAc-GST-1/Alhydrogel and rAc-APR-1/Alhydrogel are the greatest reported to date compared with any other recombinant subunit vaccines against hookworms. A recombinant bivalent subunit vaccine containing rNa-GST-1 expressed in P. pastoris and a catalytically inactive, but still immunogenic (Pearson et al. Reference Pearson, Bethony, Pickering, de Oliveira, Jariwala, Santiago, Miles, Zhan, Jiang, Ranjit, Mulvenna, Tribolet, Plieskatt, Smith, Bottazzi, Jones, Keegan, Hotez and Loukas2009), rNa-APR-1 expressed in tobacco plants (used as an alternative to P. pastoris since expression was low) and formulated on Alhydrogel is currently in Phase I clinical trials (Hotez et al. Reference Hotez, Beaumier, Gillespie, Strych, Hayward and Bottazzi2016). Clinical testing is also evaluating if including synthetic Toll-like receptor agonists glucopyranosyl lipid A (GLA) or CpG oligodeoxynucleotide will help achieve acceptable immunogenicity (Hotez et al. Reference Hotez, Beaumier, Gillespie, Strych, Hayward and Bottazzi2016). Surprisingly, to the best of our knowledge there are no published results on the level of protection provided by the bivalent vaccine. Some important questions remaining to be addressed are whether there is an additive or maybe synergistic effect when combining rAPR-1 and rGST-1, or rather, no additive effect, similar to that observed for the rAcey-ASP-2/rAcey-MTP-1 bivalent (Mendez et al. Reference Mendez, Zhan, Goud, Ghosh, Dobardzic, Wu, Liu, Deumic, Dobardzic, Liu, Bethony and Hotez2005). Given that both APR-1 and GST-1 are assumed to function in haemoglobinolysis (Hotez et al. Reference Hotez, Bethony, Diemert, Pearson and Loukas2010), it seems reasonable to suspect redundancy in blocking/neutralizing both antigens. For example, blocking/neutralizing APR-1 would in theory prevent liberation of heme since haemoglobin would no longer be broken down, thereby making GST-1 redundant. Moreover, adult hookworms can survive in vitro in serum alone, so it is likely that haemoglobin is not the only source of protein in their diet within the intestine. Nevertheless, this vaccine is admittedly not expected to prevent hookworms from establishing infection within the small intestine (Hotez et al. Reference Hotez, Bethony, Diemert, Pearson and Loukas2010; Hotez et al. Reference Hotez, Beaumier, Gillespie, Strych, Hayward and Bottazzi2016), but the evidence does suggest that it will reduce clinicopathological parameters below threshold levels. Thus, although there are still many unanswered questions for this vaccine, there is cautious optimism for significant impact.
DNA vaccines
More recently, a pair of studies used a DNA vaccine approach against A. ceylanicum in golden hamsters (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello and Wedrychowicz2013, Reference Wisniewski, Jaros, Baska, Cappello, Dlugosz and Wedrychowicz2016). With a DNA vaccine, the antigen's cDNA is cloned into a plasmid vector and then the plasmid is injected into the host with the expectation that it will enter host cell nuclei for transcription and will subsequently be translated in the host cell cytoplasm. In the first study, A. ceylanicum metalloprotease 6 (Acey-MEP-6) cDNA (a likely adult intestinal antigen) was cloned into the plasmid vector pcDNA3·1+, mixed with Fugene 6 Transfection Reagent (presumably to enhance uptake into host cells), and administered intranasally (i.n.) both once and three times in 50 µg doses into golden hamsters. Single dose pcDNA3·1+/Acey-MEP-6/Fugene 6, but not triple dose, resulted in significantly lower A. ceylanicum worm burdens and significantly higher haematocrit compared with golden hamsters receiving no vaccine, but not compared with golden hamsters receiving either single or triple doses of pcDNA3·1+/Fugene 6 (empty vector/transfection reagent) control (Table 2) and immune responses were not evaluated (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello and Wedrychowicz2013). Thus, although pcDNA3·1+/Acey-MEP-6/Fugene 6 appeared to have some vaccine efficacy, the clear impact that the empty vector/transfection reagent control had on A. ceylanicum challenge infection and lack of evaluated immune correlates of protection makes it difficult to interpret these results.
In a follow up study with an Acey-MEP-7 cDNA (paralog of Acey-MEP-6), single dose pcDNA3·1+/Acey-MEP-7/Fugene 6 (amount of plasmid was not reported) resulted in significantly lower A. ceylanicum worm burdens and FECs, and significantly higher post-challenge hematocrit compared with pcDNA3·1+/Fugene 6 control in golden hamsters (Table 2) (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello, Dlugosz and Wedrychowicz2016). Also, pcDNA3·1+/Acey-MEP-7/Fugene 6 resulted in significantly greater rAcey-MEP-7-specific total serum IgG responses (measured as optical density at 450 nm; OD450) compared with pcDNA3·1+/Fugene 6 control, although the difference was not great (average OD450 of ~0·7 compared with ~0·4) (Table 2) (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello, Dlugosz and Wedrychowicz2016). Although this study clearly indicates significant vaccine efficacy of pcDNA3·1+/Acey-MEP-7/Fugene 6, the issue with the empty vector/transfection reagent control also having an effect (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello and Wedrychowicz2013) has not been fully resolved, and immune correlates are weak. Thus, efficacy of DNA vaccines for hookworms remains unclear.
Strongyloides spp.
Species within the Strongyloides genus (family Strongyloididae) have been reported to infect humans, non-human primates, dogs, cats and some rodents (Table 1). At least two species, Strongloides stercoralis and Strongyloides fuelleborni, commonly infect humans, with a total of approximately 100 million people infected (Puthiyakunnon et al. Reference Puthiyakunnon, Boddu, Li, Zhou, Wang, Li and Chen2014). Although symptoms are often subclinical, strongyloidiasis can be deadly in immunosuppressed people with hyperinfection syndrome. These strongyloid parasites alternate between free-living and parasitic cycles. Free-living larvae passed in the stool can either moult to infectious L3 or can continue a free-living cycle and ultimately moult to egg-laying adult worms outside of a host for a single generation (an advantage in the absence of a suitable host). Similar to hookworms, in the presence of a suitable host, L3 penetrate the skin and enter the bloodstream where they undergo maturation in the lungs, followed by migration up the trachea and into the pharynx where they are swallowed, finally developing into adult worms within tunnels of the small intestine. Eggs hatch within the small intestine, and larvae are either excreted with the feces outside of the host, or can develop to infectious L3 within the small intestine and immediately reinfect the intestinal mucosa (autoinfection). Chronic asymptomatic infections can be maintained in human hosts for decades (Puthiyakunnon et al. Reference Puthiyakunnon, Boddu, Li, Zhou, Wang, Li and Chen2014).
DNA vaccines
The first study that tested recombinant subunit vaccines against Strongyloides spp. used a DNA vaccine approach against experimental S. stercoralis L3 infection in BALB/cJ mice (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005). Strongloides stercoralis deoxycholate-soluble L3 antigens tropomyosin (Ss-TMY-1), Na+-K+ ATPase (Ss-EAT-6) and galectin (Ss-LEC-5) were shown to be recognized by serum IgG isolated from human plasma obtained from exposed Haitian donors, and this serum was shown to passively transfer protection to BALB/cJ mice (Kerepesi et al. Reference Kerepesi, Nolan, Schad, Lustigman, Herbert, Keiser, Nutman, Krolewiecki and Abraham2004). Six intradermal (i.d.) injections of 20 µg doses were performed in the ears of mice with Lambda Uni-zap XR vector individually containing cDNA inserts for all three S. stercoralis antigens mixed with the plasmid VC1701 containing murine ganulocyte-macrophage colony-stimulating factor (GM-CSF). Only plasmid Ss-tmy-1/GM-CSF and plasmid Ss-eat-6/GM-CSF resulted in significant, synthetic peptide-specific total serum IgG responses compared with control plasmid GM-CSF alone, albeit with very weak responses (OD450 of ~0·25 and ~0·6, respectively, compared to ~0·15 in control using 1 : 100 serum dilutions) (Table 2). Immunized mice were challenged s.c. with S. stercoralis L3 in diffusion chambers (i.e. devices with openings that are small enough to keep the worms inside but large enough to allow immune cells to enter) for 4 days, and only plasmid Ss-eat-6/GM-CSF resulted in significantly lower L3 survival (35%) within the diffusion chambers compared with plasmid GM-CSF control (Table 2). However, mice immunized with a combination of all three DNA vaccines did not result in significantly lower L3 survival compared with plasmid GM-CSF control (Table 2). Although triply vaccinated mice had significant total serum IgG responses against Ss-EAT-6 synthetic peptides, these responses were lower than plasmid Ss-eat-6/GM-CSF alone. Passive transfer of serum from vaccinated into naïve mice for all DNA vaccine groups did not result in significantly lower S. stercoralis L3 survival compared with plasmid GM-CSF control serum, not even for plasmid Ss-eat-6/GM-CSF, although the latter did result in the lowest survival (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005).
Protein vaccines
Ss-TMY-1, Ss-EAT-6 and Ss-LEC-5, as well as two highly and naturally immunogenic S. stercoralis antigens used for diagnostics in humans [Ss-NEI-1 and Ss-IR; (Ramanathan et al. Reference Ramanathan, Burbelo, Groot, Iadarola, Neva and Nutman2008)] were tested for expression in E. coli, baculovirus-insect cells, and the yeast Kluyveromyces lactis expression systems (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011). All but rSs-TMY-1 expressed well in the baculovirus-insect cell expression system, while rSs-TMY-1 expressed well in K. lactis. All immunogens were formulated with alum and injected s.c. twice in 25 µg doses into BALB/cByJ mice. All vaccines resulted in total serum IgG responses against cognate immunogen (albeit weak; ODs between only 0·5 and 2·0 at 1:100 serum dilutions, with background in controls around 0·25) (Table 2). Cellular immune responses were not significantly different for any of the vaccines, measured as the number of neutrophils, macrophages and eosinophils infiltrated into diffusion chambers after challenge. Of all the vaccines tested, only immunization with rSs-IR/alum resulted in significantly lower survival of S. stercoralis L3 with an average 80% lower survival in three different trials compared with alum alone (Table 2). Passive transfer of serum from mice immunized with rSs-IR/alum into naïve mice again resulted in an approximately 80% lower L3 survival. Finally, serum from mice immunized with rSs-IR/alum localized native Ss-IR within granules of the glandular oesophagus in immunoelectron microscopy, and on the surface of S. stercoralis L3 in immunoconfocal microscopy (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011).
Consistent with DNA vaccination (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005), neither Ss-TMY-1 nor Ss-LEC-5 showed any evidence of efficacy as recombinant, protein-based, subunit vaccines (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011). Although the lack of significant vaccine efficacy for Ss-EAT-6 (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011) somewhat contradicts its efficacy (albeit low) as a DNA vaccine (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005), it was speculated that rSs-EAT-6 might need a different adjuvant to induce protection. On the other hand, rSs-IR/alum is a potential candidate whose efficacy as a recombinant subunit vaccine for strongyloidiasis (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011) in principle could be further investigated. However, the weak IgG response, the lack of significant immune correlates with rSs-IR/alum-induced protection, and the presence of Ss-IR on the surface of L3 (potentially risking allergic reactions in clinical trials as was found with rNa-ASP-2 detailed above) present important hurdles for this vaccine. Abraham et al. (Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011) speculate that the mechanism of rSs-IR/alum-induced protection may be through complement and/or antibody-dependent cellular cytotoxicity (ADCC) as these mechanisms were demonstrated to be important for vaccine-induced reduction of S. stercoralis L3 survival (Ligas et al. Reference Ligas, Kerepesi, Galioto, Lustigman, Nolan, Schad and Abraham2003; Kerepesi et al. Reference Kerepesi, Nolan, Schad, Lustigman, Herbert, Keiser, Nutman, Krolewiecki and Abraham2004). Although these mechanisms may be important for rSs-IR/alum-induced protection in the S. stercoralis-mouse experimental model with implanted diffusion chambers, it seems plausible that such mechanisms may not be sufficient to prevent the establishment of patent infections within the small intestine, and more thorough studies are needed.
Heat shock protein 60 (HSP60) from the rodent parasite Strongyloides ratti is a naturally immunogenic antigen during infection in C57BL/6 mice that has been shown to be recognized by IgM and to stimulate proliferative responses in spleen and mesenteric lymph node (mLN) cells (Ben Nouir et al. Reference Ben Nouir, Eschbach, Piedavent, Osterloh, Kingsley, Erttmann, Brattig, Liebau, Fleischer and Breloer2012). rSr-HSP60 expressed in E. coli was emulsified with complete Freund's adjuvant (CFA; TH1-biased), formulated with alum, or used without adjuvant, and injected i.p. twice at 100 µg doses. Immunization with rSr-HSP60/CFA, and interestingly, rSr-HSP60 alone induced a skewed TH1 response characterized by significant IgM, IgG1, IgG2b/c, and IFN-γ, without IL-13 or significant proliferative splenocyte responses (Table 2). rSr-HSP60 alone was also found to increase IFN-γ production in DO11·10 mouse splenocytes in vitro stimulated with cognate ovalbumin peptide. Not only did either vaccine fail to protect against challenge infection, but also immunization with rSr-HSP60/CFA or rSr-HSP60 alone actually slightly increased susceptibility. Conversely, although immunization with rSr-HSP60/alum resulted in similar IgM, IgG1 and IgG2b/c responses compared with rSr-HSP60/CFA and rSr-HSP60 alone, significant proliferation and production of IL-13 without IFN-γ in splenocytes was observed after rSr-HSP60 stimulation (Table 2). Immunization with rSr-HSP60/alum resulted in >50% protection against S. ratti challenge infection (Table 2) (Ben Nouir et al. Reference Ben Nouir, Eschbach, Piedavent, Osterloh, Kingsley, Erttmann, Brattig, Liebau, Fleischer and Breloer2012). IgE responses were not evaluated, but given the association between IL-13 and protection, it seems plausible that IgE was involved. These findings clearly indicate that a TH2 adjuvant such as alum can overrule an antigen's intrinsic induction of TH1 and provide significant protection against a STH infection.
Trichostrongyloids (family Trichostrongylidae) of livestock
Haemonchus contortus
Haemonchus contortus is a worldwide trichostrongyloid of small ruminants, mostly sheep and goats, with a life cycle similar to hookworms except that L3 attach to the abomasal mucosa in the ruminant stomach (abomasum) to feed on blood (Zajac, Reference Zajac2006) (Table 1). Targets of subunit vaccines for H. contortus have focused primarily on three different integral membrane protease complexes located within the worm's intestine, H11 aminopeptidase, galactose-containing glycoprotein (H-gal-GP) and Thiol Sepharose-Binding Protein (TSBP). In all cases, vaccine-induced immunity is believed to be due to blocking/neutralizing antibodies. Although high levels of protection have not yet been achieved with any recombinant subunit vaccine against haemonchosis, various technical factors are likely accountable and further studies are needed.
An adult H. contortus protein extract enriched and sub-fractionated for the H11 aminopeptidase complex resulted in an average 71% lower H. contortus FECs and worm burdens in Merino sheep compared with adjuvant control (Munn et al. Reference Munn, Smith, Graham, Greenwood, Tavernor and Coetzee1993). An H11 aminopeptidase component was cloned and expressed in the baculovirus-insect cell expression system (Reszka et al. Reference Reszka, Rijsewijk, Zelnik, Moskwa and Bienkowska-Szewczyk2007). Recombinant H11 aminopeptidase-expressing baculovirus-infected insect cell extracts (bac-rHc-H11) – used instead of purified protein due to anticipated immunostimulatory effects of insect cell extracts – were injected twice i.m. in estimated 300 µg doses into Merino sheep. Serum antibody responses (no mention of classes) were monitored throughout infection and responses were higher than bacWT for bac-rHc-H11 after immunization, although the difference was not great (OD of ~2·25 for bac-rHc-H11 with a background OD of ~1·0 for bacWT; responses were low despite lack of serum dilution). Sheep immunized with bac-rHc-H11 had a significant 30% lower H. contortus worm burdens compared with bacWT control (Table 2) (Reszka et al. Reference Reszka, Rijsewijk, Zelnik, Moskwa and Bienkowska-Szewczyk2007). Although this study indicated significant vaccine efficacy of bac-rHc-H11 against H. contortus challenge in sheep, adjuvanted, purified recombinant protein, as well as other aminopeptidase components of the H11 complex, may provide greater protection, and further investigation is warranted.
H-gal-GP is a detergent-soluble, heavily aspartyl and metalloproteolytic complex containing 12 different proteins, mostly pepsins, metalloproteases and cysteine protease-like enzymes, as well as a cysteine protease inhibitor and thrombosponin, that when adjuvanted with CFA provided immunized Suffolk–Greyface cross lambs 72% lower H. contortus worm burdens and 93% lower FECs compared with CFA alone (Smith et al. Reference Smith, Smith and Murray1994; Longbottom et al. Reference Longbottom, Redmond, Russell, Liddell, Smith and Knox1997; Redmond et al. Reference Redmond, Knox, Newlands and Smith1997; Smith et al. Reference Smith, Pettit, Newlands, Redmond, Skuce, Knox and Smith1999; Skuce et al. Reference Skuce, Newlands, Stewart, Pettit, Smith, Smith and Knox2001). SDS–PAGE separation of H-gal-GP determined that the isolated, individual components adjuvanted with CFA are much less protective alone (Smith and Smith, Reference Smith and Smith1996). Moreover, reduced and urea-denatured H-gal-GP and the most protective metalloprotease components, as well as their recombinant forms in E. coli, all adjuvanted with Quil-A, eliminated significant protection against H. contortus, while native H-gal-GP/Quil-A still resulted in protection similar to the previous studies (Table 2) (Smith et al. Reference Smith, Newlands, Smith, Pettit and Skuce2003). These findings demonstrate that vaccine-induced protection with H-gal-GP, whether adjuvanted with CFA or Quil-A, most likely requires a complex combination of conformational epitopes provided only by the complex, which has been a major challenge for its application as a recombinant subunit vaccine for haemonchosis. The use of random peptide phage-display libraries has been suggested as a potential method to identify peptide sequences in H-gal-GP that potentially mimic the antigenic epitopes (Ellis et al. Reference Ellis, Newlands, Nisbet and Matthews2012).
TSBP is another intestinal, membrane-bound glycoprotein complex exposed to the luminal surface, and is enriched with cysteine proteases (Knox et al. Reference Knox, Smith and Smith1999), drawing parallels with the partially protective A. caninum intestinal cysteine protease reported by Loukas et al. (Reference Loukas, Bethony, Williamson, Goud, Mendez, Zhan, Hawdon, Bottazzi, Brindley and Hotez2004). Suffolk–Greyface cross-lambs injected three times i.m. with TSBP formulated with CFA, IFA, or Quil-A at 200 µg doses had 95, 54 and 82% lower H. contortus FECs, and 52, 43 and 45% lower adult worm burdens, respectively, compared with adjuvant controls (reduction in adult worm burdens for TSBP/IFA was not significant) (Knox et al. Reference Knox, Smith and Smith1999). The TSBP intestinal membrane complex was therefore determined as a highly protective immunogen when formulated with either CFA or Quil-A, but surprisingly, protection was lesser with IFA, a classic TH2 adjuvant.
Immunoscreening of an H. contortus adult, gut-derived cDNA library with TSBP antisera recognized three Cathepsin-B-like cysteine proteases (hmcp-1, 4 and 6), and hmcp-1, 4 and 6 were immunolocalized to the gut microvillar surface and found to be exclusively expressed during blood-feeding (Skuce et al. Reference Skuce, Redmond, Liddell, Stewart, Newlands, Smith and Knox1999). Consistently, TSBP further fractionated for cysteine proteases, formulated with Quil-A, and injected i.m. resulted in similar levels of protection compared with the unfractionated TSBP complex with ~30% lower H. contortus worm burdens in trial 1 and ~46% lower in trial 2, even with only 3 µg doses per injection of the former and 100 µg for the latter, and the non-cysteine protease TSBP fraction failed to protect (Redmond and Knox, Reference Redmond and Knox2004). Also, although the TSBP cysteine protease fraction resulted in slightly higher numbers of abomasal eosinophils and mast cells, as well as serum IgE and IgA, but not serum IgG endpoint titre, compared with unfractionated TSBP and the non-cysteine protease TSBP fraction, only serum IgG titre slightly correlated with protection between individual lambs within the group. For the TSBP cysteine protease fraction, not only did total serum IgG titre slightly negatively correlate with FECs, but also an equal balance between IgG1 and IgG2 subclasses appeared to be most protective, though this conclusion did not hold up in a second trial. Importantly, although recombinant, E. coli-expressed, trivalent hmcp-1, 4 and 6 expressed as GST-fusions in E. coli cell extracts (~80–90% pure proteins), formulated with Quil-A, and injected three times i.m. at 100 µg doses failed to lower H. contortus FECs in immunized lambs compared with Quil-A alone, adult worm burdens were 38% lower and along with the cysteine protease fraction and unfractionated TSBP, female worm size was lower (Table 2). A couple possible explanations for the lack of reduced FECs for recombinant hmcp-1, 4 and 6/Quil-A might be immunization with unpurified E. coli cell extracts and/or expression as GST-fusions, of which the GST tag was shown to account for nearly 80% of the total serum IgG response in immunized lambs (Redmond and Knox, Reference Redmond and Knox2004). Another possible explanation for the observed reductions in worm burdens but not FECs might be due to the fewer number of worms having elevated fecundities (eggs/adult female), which could be a possible compensation. Either way, these findings clearly indicate that the intestinal TSBP cysteine proteases are partially protective hidden antigens against haemonchosis and hmcp-1, 4 and 6 are candidate immunogens for a recombinant subunit vaccine. However, although there appears to be some trend between protection and serum IgG responses, lack of significant immune correlates indicates the need for further studies of the intestinal TSBP cysteine proteases, as well as optimization in different recombinant expression systems, which should also be extended to the H11 aminopeptidase and H-gal-GP complexes.
In a separate study, another thiol-binding fraction of H. contortus adult ES enriched with cysteine and metalloprotease activity was formulated with Alhydrogel and immunized three times s.c. at 15 µg doses into Zwart-Bles lambs (Bakker et al. Reference Bakker, Vervelde, Kanobana, Knox, Cornelissen, de Vries and Yatsuda2004). The thiol-binding fraction of ES induced elevated ES-specific serum IgG, IgA and IgE, but did not significantly elevate mucosal antibody and lymphoproliferative responses. These immunizations resulted in an average 52 and 50% lower H. contortus FECs and adult worm burdens, respectively, compared with Alhydrogel alone, but these results were not significant. Although the lower FECs and adult worm burdens were not statistically significant, fecundity was significantly reduced for the thiol-binding fraction, while not for the unbound fraction or, surprisingly, total ES, injected at 60 and 75 µg doses, respectively, and all formulated with Alhydrogel. It was speculated that the choice of adjuvant, although good for thiol-binding fractions, might not have been good for total ES products and thus led to the poor vaccine efficacy observed (Bakker et al. Reference Bakker, Vervelde, Kanobana, Knox, Cornelissen, de Vries and Yatsuda2004). There are no published data on the vaccine efficacy of recombinant ‘ES-thiol’ proteins against haemonchosis.
To date, the combination of native H11 and H-gal-GP gut membrane complexes are the most protective antigen cocktail against haemonchosis. As mentioned above, the commercialized H. contortus vaccine Barbervax includes these native complexes and is highly effective at reducing worm burdens and egg counts below troublesome levels. A major issue though is that a protective memory response is not induced in vaccinated lambs during challenge infection, so repeated immunizations are required. It is not yet clear why a protective memory response fails to be induced during challenge infection, but it might be due to the complexes containing exclusively hidden antigens.
Ostertagia ostertagi
Ostertagia ostertagi is a major trichostrongyloid of cattle that infects the abomasal mucosa feeding on mucus and mucosal tissue, but unlike H. contortus, is not an obligate blood feeder (Table 1). These differences in feeding habit likely reflect the contrasting data found in a vaccine efficacy study that included the H-gal-GP fraction, already detailed above and O-gal-GP fraction, both formulated with Quil-A, against haemonchosis and ostertagiasis, respectively (Smith et al. Reference Smith, Smith and Pettit2000). While H-gal-GP/Quil-A was again found to be highly protective against haemonchosis at 100 µg doses in Suffolk–Greyface cross-lambs, immunization of MontBéliard calves with O-gal-GP/Quil-A at 200 µg doses failed to provide consistent protection against ostertagiasis. Quite amazingly though, immunization of O-gal-GP/Quil-A into lambs cross-protected against H. contortus with 81–97% and 57–84% lower FECs and worm burdens, respectively, compared with Quil-A alone. Thus, without sufficient blood ingestion by O. ostertagi, host antibodies are most likely incapable of binding to and blocking/neutralizing O-gal-GP within the intestine unlike for H. contortus, indicating that differences in feeding habits can yield very contrasting results with the same vaccine (Smith et al. Reference Smith, Smith and Pettit2000). These data also support the notion that blocking/neutralizing antibodies may be a good strategy for vaccine-induced protection against blood-feeding STHs.
An O. ostertagi adult ES-thiol fraction enriched with cysteine protease activity, but with the most abundant antigens being activation-associated secreted proteins 1 (Oo-ASP-1) and 2 (Oo-ASP-2), as well as (separately) a membrane-bound thiol subfraction similar to TSBP above, were formulated with Quil-A and immunized three times i.m. at 100 µg doses into MontBéliard calves (Geldhof et al. Reference Geldhof, Claerebout, Knox, Vercauteren, Looszova and Vercruysse2002, Reference Geldhof, Vercauteren, Gevaert, Staes, Knox, Vandekerckhove, Vercruysse and Claerebout2003). Interestingly, the membrane-bound thiol subfraction, which, considering its homology to TSBP, is most likely intestinal, failed to protect against O. ostertagi challenge, consistent with the contrasting results with H-gal-GP and O-gal-GP mentioned above. However, immunization with the ES-thiol fraction resulted in an average 60% lower FECs, 18% lower worm burdens (though not significant) and significantly smaller worms compared with Quil-A alone. Protection was correlated with the levels of IgA, IgG1 and IgG2 in the abomasal mucus, and the numbers of infiltrated eosinophils and mast cells within the abomasal mucosa were also slightly correlated with protection (Geldhof et al. Reference Geldhof, Claerebout, Knox, Vercauteren, Looszova and Vercruysse2002). In a follow up study (Geldhof et al. Reference Geldhof, Vercauteren, Vercruysse, Knox, Van den Broeck and Claerebout2004), the same ES-thiol fraction was formulated with either Quil-A or Alhydrogel, and ES-thiol/Quil-A again resulted in similar levels of protection, while ES-thiol/Alhydrogel failed to protect. ES-thiol/Quil-A resulted in elevated IgA, IgG1 and IgG2 levels in the abomasal mucus, and elevated numbers of eosinophils, mast cells and ‘global leucocytes’ in the abomasal mucosa compared with ES-thiol/Alhydrogel. These findings indicated that Quil-A induces stronger mucosal antibody and cellular immune responses to ES-thiol compared with Alhydrogel, which likely accounts for the superior protection (Geldhof et al. Reference Geldhof, Vercauteren, Vercruysse, Knox, Van den Broeck and Claerebout2004). Taken together, the data suggest that for blood-feeding STHs, blocking/neutralizing serum antibody responses may be more important whereas for non-blood feeding STHs, it might be mucosal antibody and cellular immune responses that are more important.
It was later shown that immunization of cattle with three different subfractions of ES-thiol (ASP-enriched, cysteine protease-enriched and the remaining non-cysteine protease-enriched or ‘rest’ subfraction) all resulted in even lower O. ostertagi FECs compared with ES-thiol (Meyvis et al. Reference Meyvis, Geldhof, Gevaert, Timmerman, Vercruysse and Claerebout2007). Thus, proteins within all three ES-thiol subfractions have protective capacity against ostertagiasis. Unfortunately, data for recombinant (r)Oo-ASP-1 alone, a dominant component in ES-thiol subfractionation, are not as promising. rOo-ASP-1 was expressed in baculovirus-insect cell expression system, the cell extract was formulated with Quil-A and injected three times i.m. at 100 µg doses into cattle, and no differences in O. ostertagi FECs or worm burdens were observed (Table 2) (Geldhof et al. Reference Geldhof, Meyvis, Vercruysse and Claerebout2008). Serum from rOo-ASP-1/Quil-A resulted in much weaker general antibody responses to a native Oo-ASP-1/2-enriched fraction compared with serum from cattle immunized with the native Oo-ASP-1/2-enriched fraction (ODs of ~0·3 and ~2·1, respectively) (Table 2). It was proposed that rOo-ASP-1 was incorrectly folded or lacked the protective post-translational modifications, or that rOo-ASP-1 alone may be insufficient for protection possibly requiring the addition of rOo-ASP-2 (Geldhof et al. Reference Geldhof, Meyvis, Vercruysse and Claerebout2008). However, there was no mention of the possibility that purification of rOo-ASP-1 from the crude insect cell extract may improve vaccine efficacy, similar to that mentioned above for bac-rHc-H11.
Native O. ostertagi polyprotein allergen (Oo-PA) formulated with Quil-A resulted in ~60% lower O. ostertagi FECs with protection correlated with mucosal IgG1 and IgG2 responses, while as with rOo-ASP-1, recombinant protein (this time made in E. coli) completely failed to protect (Table 2) (Vercauteren et al. Reference Vercauteren, Geldhof, Vercruysse, Peelaers, Van Den Broeck, Gevaert and Claerebout2004). Thus, although recombinant forms of protective O. ostertagi antigens have failed to protect cattle from challenge infections, optimization of and/or use of alternative expression systems and/or optimized purification are warranted in order to conclusively determine their vaccine efficacy.
Teladorsagia circumcincta
Teladorsagia circumcincta is another abomasal mucus dweller very similar to O. ostertagi, but mostly infects sheep (Table 1) (Zajac, Reference Zajac2006). Eight different antigens either, (1) naturally immunodominant for IgA [cathepsin F-1 (Tci-CF-1); astacin-like metalloproteinase-1 (Tci-MEP-1); 20 kDa protein of unknown function (Tci-ES20); and Tci-ASP-1]; (2) homologous to protective antigens from other strongylids (Tci-SAA-1); or (3) potential immunomodulatory proteins [macrophage migration inhibitory factor-1 (Tci-MIF-1); calcium-dependent apyrase-1 (Tci-APY-1); and transforming growth factor β homologue (Tci-TGH)], were evaluated for efficacy in a single, recombinant multivalent vaccine. Recombinant proteins were either expressed in E. coli (rTci-MEP-1; rTci-ASP-1; rTci-SAA-1; rTci-MIF-1; rTci-APY-1; and rTci-TGH) or P. pastoris (rTci-CF-1 and rTci-ES20), formulated with Quil-A, and injected three times s.c. at 50 µg doses each into Texel cross-bred lambs. Immunization resulted in elevated serum and abomasal mucosal IgG and IgA responses for nearly all recombinant immunogens, and 70% lower T. circumcincta FECs and 55% lower worm burdens compared with Quil-A alone (Table 2) (Nisbet et al. Reference Nisbet, McNeilly, Wildblood, Morrison, Bartley, Bartley, Longhi, McKendrick, Palarea-Albaladejo and Matthews2013). Thus, this study provided proof of concept that recombinant subunit vaccines can work against trichostrongyloids of livestock. However, whether individual components of the recombinant vaccine cocktail can protect against teladorsagiasis, or whether specific combinations are required has not been resolved.
RECOMBINANT SUBUNIT VACCINES FOR ASCARIDS
Ascarids (Nematoda clade III, family Ascarididae) are serious parasites of humans (Ascaris lumbricoides), pigs (Ascaris suum), dogs (Toxocara canis) and cats (Toxocara cati) (Table 1). Species of the Baylisascaris genus are also important parasites of wild animals such as giant pandas (Baylisascaris schroederi) and raccoons (Baylisascaris procyonis), of which the latter can cause lethal zoonoses. A. lumbricoides, the human giant roundworm (can be up to 50 cm in length), infects over 800 million human beings, making it the most common of the human STHs (Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). Embryonated ascarid eggs, which can persist in the soil for over 10 years, are ingested and hatch within the small intestine, where the infectious larvae burrow into the intestinal mucosa entering the bloodstream, and then pass through the liver and heart ultimately breaking free in the pulmonary alveoli where the larvae undergo maturation. Similar to N. americanus, larvae migrate up the bronchial tree into the throat, and are then swallowed eventually reaching the small intestine once again where they develop into adult worms. Adult females can produce over 200 000 eggs per day, and these worms can live within the small intestine for years (Crompton, Reference Crompton2001). Infection by A. lumbricoides (and A. suum) is relatively asymptomatic as this nematode consumes luminal contents rather than host tissue, yet stunts physical and cognitive development in children due to significant malnutrition (Stephenson et al. Reference Stephenson, Latham and Ottesen2000). On the other hand, larval migration can lead to various pulmonary pathologies, and heavy patent infections can cause life threatening gut obstructions, and obstructions to biliary and pancreatic ducts due to occasional upstream migration. Baylisascaris procyonis infection is particularly and highly dangerous if eggs are ingested by humans due to the potential for larvae to migrate to the brain. Also, severe symptoms in young dogs and cats result from infection by T. canis and T. cati, respectively, and if humans are infected by them syndromes known as visceral and ocular larva migrans can develop where larvae migrate to the heart, brain or eye, of which the former can be fatal. Thus, preventing infections by ascarid parasites is critical (Crompton, Reference Crompton2001).
Currently there are no published results for recombinant subunit vaccines against A. lumbricoides, while several studies have been published for A. suum and B. schroederi. Given the recent common ancestries between these ascarid species and A. lumbricoides, such studies are highly relevant to this most prevalent human STH.
Ascaris suum
Intranasal immunization with cholera toxin B (CTB)subunit
Ascaris suum 14 and 16-kDa proteins (As14 and As16), as well as As24 and As37 mentioned below, are conserved in clade III helminths, and were suspected to be highly antigenic as they were determined to be abundantly expressed on the surface of the infective L3 stage (Kasuga-Aoki et al. Reference Kasuga-Aoki, Tsuji, Suzuki, Arakawa, Matsumoto and Isobe2001) and are present in ES products. Both As14 and As16 are homologous to Ac-16 mentioned above (Fujiwara et al. Reference Fujiwara, Zhan, Mendez, Loukas, Bueno, Wang, Plieskatt, Oksov, Lustigman, Bottazzi, Hotez and Bethony2007).
rAs14 was expressed in E. coli, conjugated to CTB subunit, an adjuvant that enhances mucosal and systemic immune responses, and immunized i.n. into BALB/c mice at a primary dose of 50 µg with two boosters of 30 and 10 µg (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Matsumoto, Arakawa, Ishiwata and Isobe2001). Mice immunized with rAs14-CTB had significant mucosal IgA and serum IgG (with IgG1 the highest) and IgE responses, and resulted in 60% lower larval A. suum worm burdens in the lungs compared with both CTB and rAs14 alone (Table 2) (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Matsumoto, Arakawa, Ishiwata and Isobe2001).
rAs16 was also expressed in E. coli and conjugated to CTB, and was immunized three times i.n. into BALB/c mice with a primary dose of 25 µg and two boosters of 15 and 10 µg in one study (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Isobe, Arakawa and Matsumoto2003) and into cross-bred Landrace × Whitelarge × Duroc piglets at 300 µg in another study (Tsuji et al. Reference Tsuji, Miyoshi, Islam, Isobe, Yoshihara, Arakawa, Matsumoto and Yokomizo2004). In the former study, mice immunized with rAs16-CTB had significant serum IgG (with equal contributions from IgG1, IgG2a and IgG3) and IgE responses, but an insignificant mucosal IgA response, and highly elevated splenic rAs16-stimulated IFN-γ and IL-2, as well as significant IL-10, but not IL-4 (Table 2) (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Isobe, Arakawa and Matsumoto2003). Somewhat differently in the latter study, piglets immunized with rAs16-CTB had significant mucosal IgA and serum IgG (with IgG1 the highest) (IgE responses were not evaluated), and was also shown to stimulate elevated IFN-γ, IL-4 and IL-10 from PBMCs (Table 2) (Tsuji et al. Reference Tsuji, Miyoshi, Islam, Isobe, Yoshihara, Arakawa, Matsumoto and Yokomizo2004). IL-4 and IL-10 were more highly elevated than IFN-γ in the piglets (Table 2). Both mice and piglets immunized with rAs16-CTB, similar to rAs14-CTB above, had 60% lower larval worm burdens in the lungs compared with both CTB and rAs16 alone (Table 2) (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Isobe, Arakawa and Matsumoto2003, Reference Tsuji, Miyoshi, Islam, Isobe, Yoshihara, Arakawa, Matsumoto and Yokomizo2004).
Thus, both rAs14-CTB and rAs16-CTB induced protection against A. suum larval colonization of the lung in association with mixed TH1/TH2 mucosal and/or systemic responses, of which such protection was completely dependent upon CTB conjugation. Also, anti-rAs16-CTB mouse and piglet serum localized As16 to the worm hypodermis and intestine (Tsuji et al. Reference Tsuji, Suzuki, Kasuga-Aoki, Isobe, Arakawa and Matsumoto2003, Reference Tsuji, Miyoshi, Islam, Isobe, Yoshihara, Arakawa, Matsumoto and Yokomizo2004) and inhibited moulting of A. suum L3 in vitro (Tsuji et al. Reference Tsuji, Miyoshi, Islam, Isobe, Yoshihara, Arakawa, Matsumoto and Yokomizo2004). It was speculated that the mechanism of rAs16-CTB-induced immunity involves induction of antibodies that block As16, resulting in moulting defects.
Parenteral immunization
rAs24 was expressed in E. coli, formulated with CFA, and injected three times s.c. at 50 µg doses into BALB/c mice (Islam et al. Reference Islam, Miyoshi and Tsuji2005a ). Immunization of mice with rAs24/CFA resulted in highly significant serum IgG1, IgG2a and IgG2b responses, significant rAs24-stimulated splenic IFN-γ and IL-10 (but not IL-4) responses, and a significant 58% less larvae recovered from the lungs compared with CFA alone (Table 2). This study clearly indicated an association between rAs24/CFA-induced protection and a mixed TH1/TH2-type immune response. The finding that anti-rAs24 serum IgG obtained from immunized mice inhibited the L3 to L4 moult by 26% in vitro suggests a role for serum IgG in rAs24/CFA-induced protection. As24 was also strongly localized to the hypodermis, supporting a potential role in the moulting process (Islam et al. Reference Islam, Miyoshi and Tsuji2005a ).
As37, in addition to being abundantly expressed on the surface of A. suum L3, is immunodominant and recognized by serum obtained from previously infected, fenbendazole-treated pigs (Kasuga-Aoki et al. Reference Kasuga-Aoki, Tsuji, Suzuki, Arakawa, Matsumoto and Isobe2001). The As37 amino acid sequence harbours multiple Ig domains and has similarity to proteins of the Ig superfamily, as well as nematode twitchin and mammalian skeletal muscle titin. Native As37 was localized in the muscle and hypodermis, as well as in the ovaries, eggs and lateral and dorsal chords of A. suum adult females, indicating a rather ubiquitous expression pattern. Sera from rabbits, mice and pigs infected with A. suum embryonated eggs reacted with E. coli-expressed rAs37, supporting natural immunogenicity of As37. BALB/c mice were injected with rAs37/CFA three times s.c. (doses not reported), and, although immunized mice had significant serum IgG responses, significant reduction in the number of larvae in the lungs was not observed (Table 2). It was proposed that alternative expression systems and/or routes of immunization need to be tested in order to determine whether or not rAs37 has protective vaccine efficacy (Tsuji et al. Reference Tsuji, Kasuga-Aoki, Isobe, Arakawa and Matsumoto2002).
Ascaris suum inorganic pyrophosphatase (As-PPase) is conserved in ascarids and was shown to play a role in larval development and moulting, but was also shown to be expressed throughout the life cycle, and to localize in the hypodermis and adult reproductive tissues (Islam et al. Reference Islam, Miyoshi, Kasuga-Aoki, Isobe, Arakawa, Matsumoto and Tsuji2003). Knockdown of As-PPase by RNA interference (RNAi) inhibited moulting of L3 to L4 in vitro by 31%, with only around 56% reduction in As-PPase protein levels (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005b ). rAs-PPase was expressed in E. coli, formulated with TiterMax Gold adjuvant, and injected three times s.c. at 50 µg doses into BALB/c mice. Immunization with rAs-PPase/TiterMax resulted in significant serum IgG responses (predominantly IgG1), significant production of splenic IL-10 (but not IFN-γ, IL-2 or IL-4) after rAs-PPase stimulation, and over 70% less A. suum larvae in the lungs compared with TiterMax alone (Table 2). Also, anti-rAs-PPase serum from immunized mice recognized native As-PPase, localized it to the hypodermis of lung-stage larvae, and inhibited L3 to L4 moulting in vitro by up to 57%. Finally, immunoreactive plasmids from the Novotope system mapped IgG-binding epitopes to sites spread across the whole rAs-PPase protein, including the active site (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005b ). Although the latter experiment did determine that epitopes overlap with the active site, the Novotope system is limited to contiguous epitopes, and it was not resolved for whether or not protection is mediated by serum IgG-binding to linear or discontinuous conformational epitopes within the As-PPase active site. Nonetheless, this study revealed rAs-PPase/TiterMax Gold as the recombinant subunit vaccine that provides the greatest protection against invasive L3 tissue migration to date in mice. It was speculated that the mechanism of vaccine-induced immunity involves IgG-binding and blocking/neutralizing native As-PPase in the hypodermis, thus disrupting larval development and moulting, and hence, efficient migration and development within the lung (Islam et al. Reference Islam, Miyoshi, Yamada and Tsuji2005b ). Surprisingly, the protection was clearly associated with IL-10, an anti-inflammatory cytokine (Couper et al. Reference Couper, Blount and Riley2008).
Enolase is suspected to be involved in glycolysis, and knockdown of A. suum enolase (AsEnol) by RNAi indicated an important role in larval development (Chen et al. Reference Chen, Xu, Nisbet, Huang, Lin, Yuan, Song and Zhu2011). AsEnol has been identified in ES products, despite the fact that it is missing an N-terminal signal peptide (Huang et al. Reference Huang, Gasser, Cantacessi, Nisbet, Zhong, Sternberg, Loukas, Mulvenna, Lin, Chen and Zhu2008), suggesting a function in the host. Chen et al. (Reference Chen, Yuan, Xu, Zhou, Zhang, Zhang, Wang, Yan, Lin and Zhu2012) investigated the vaccine efficacy of AsEnol using a DNA vaccine approach in mice. An AsEnol cDNA was cloned into the pVEX I expression plasmid, transfected into Marc-145 cells and expression and immunogenicity of rAsEnol was verified by indirect immunofluorescence assay using pVEX I-AsEnol serum from mice. pVEX I-AsEnol was immunized three times i.m. at 100 µg doses into Kunming mice in the absence of a transfection reagent or adjuvant. Immunization resulted in significant total serum IgG responses against A. suum crude larval extract (AsCE), and significant splenic AsCE-stimulated IFN-γ, IL-2, IL-4 and IL-10 responses (Table 2). IFN-γ was much more highly elevated compared with the other cytokines. Also, immunization with pVEX I-AsEnol resulted in significant proliferative responses in splenocytes stimulated with AsCE (Table 2), indicating an established memory TH cell response. Finally, pVEX I-AsEnol resulted in an average 44% less larvae recovered in either the liver or lung compared with pVEX I alone (Table 2), thus indicating significant efficacy of this DNA vaccine, characterized by a TH1-skewed cytokine profile with minor TH2 contributions (Chen et al. Reference Chen, Yuan, Xu, Zhou, Zhang, Zhang, Wang, Yan, Lin and Zhu2012).
Baylisascaris schroederi
Baylisascaris schroederi antigens (Ag) 1, 2 and 3 are homologous to As14, As16 and As37 mentioned above, respectively. Hence, their efficacies as recombinant subunit vaccines against experimental B. schroederi infection in mice were evaluated in order to develop alternative control measures for this critical STH of the endangered giant panda, of which the prevalence of infection is between 70 and 100% (Wang et al. Reference Wang, He, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2008; He et al. Reference He, Wang, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2009, Reference He, Chen, Wang, Yan, Zhang, Li, Yu, Xie, Wang, Gu, Wang, Peng and Yang2012). rBs-Ag1 (He et al. Reference He, Chen, Wang, Yan, Zhang, Li, Yu, Xie, Wang, Gu, Wang, Peng and Yang2012), rBs-Ag2 (He et al. Reference He, Wang, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2009) and rBs-Ag3 (Wang et al. Reference Wang, He, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2008) were expressed in E. coli, formulated with CFA, and injected three times i.p. at 30 µg doses into BALB/c mice. Mice immunized with all three vaccines had significant serum IgG responses and had a significant 60% less B. schroederi larvae recovered in the lungs compared with CFA alone (Table 2) (Wang et al. Reference Wang, He, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2008; He et al. Reference He, Wang, Yang, Fei, Zhang, Wang, Yang, Lan, Luo and Liu2009, Reference He, Chen, Wang, Yan, Zhang, Li, Yu, Xie, Wang, Gu, Wang, Peng and Yang2012). These studies were in concordance with the vaccine studies for rAs14 and rAs16, but different from the results obtained with rAs37, which may reflect the differences in route of immunization (s.c. vs i.p.) or other technical/biological factors. Regardless, these three recombinant immunogens serve as possible candidates for a vaccine to control baylisascariasis in giant pandas.
The efficacy of the B. schroederi homologue of As-PPase mentioned above (Bs-PYP-1) as a recombinant subunit vaccine against experimental infection in mice was also investigated (Xie et al. Reference Xie, Chen, Yan, Zhang, Li, Yu, Wang, Nong, Zhou, Gu, Wang, Peng and Yang2013). rBs-PYP-1 was expressed in E. coli, formulated with CFA, and injected three times s.c. at 50 µg doses into BALB/c mice in two separate trials. In both trials, immunization with rBs-PYP-1/CFA resulted in significant serum IgG responses (with IgG1 much greater than IgG2a), significant production of rBs-PYP-1-stimulated splenic IL-4 and IL-10 (with IL-10 much greater than IL-4), without production of IFN-γ or IL-2, and a significant 70% less B. schroederi larvae recovered in the lungs (Table 2). Other than the significant production of IL-4, these results were in concordance with the findings for the rAs-PPase homologue mentioned above, again demonstrating a unique role for IL-10 in vaccine-induced immunity to these ascarids. Also similar to As-PPase, native Bs-PYP-1 was localized to the hypodermis and reproductive tissues with anti-rBs-PYP-1 serum, but also localized to the muscle tissues and intestine, and thus, was more ubiquitously expressed (Xie et al. Reference Xie, Chen, Yan, Zhang, Li, Yu, Wang, Nong, Zhou, Gu, Wang, Peng and Yang2013). The inorganic pyrophosphatase conserved in ascarids therefore remains as the most promising vaccine candidate to date for these critical parasites of animals and humans.
It must be mentioned that all of these previous studies of recombinant subunit vaccines in ascarids have focused on target antigens abundantly expressed and/or present on the surface of infective L3. Although there is no direct evidence for pre-existing serum IgE against ascarid L3 antigens resulting in allergic reactions when immunized into previously infected humans or animals, the studies in hookworms present an alarming issue. Studies must be conducted to determine if these candidate immunogens will pass safety testing in humans and veterinary animals, because if not, future developments in ascarids should no longer focus on L3 antigens.
RECOMBINANT SUBUNIT VACCINES FOR TRICHURIDS
Trichurids (Trichuris spp.; whipworms; Nematoda clade I) are critical parasites of livestock, dogs, cats and humans, and infect various rodents. Trichuris trichiura (Table 1) is the only well-known human trichurid, infecting almost 500 million people (Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). Trichuriasis can be characterized by painful passage of the stool and rectal prolapse with heavy infections, and in children, can lead to severe anaemia, stunted growth and cognitive development. Trichurids begin their life cycle when embryonated eggs are ingested by the host. Once the eggs reach the duodenum, they hatch. Larvae either mature in the small intestine, or travel to the cecum of the large intestine for maturation, feeding on tissue fluids, the mucosal epithelium, and possibly blood, but evidence for the latter remains inconclusive. Adult trichurids anteriorly fixed within the intestinal mucosa mate, and each female lays between 3000 and 20 000 unembryonated eggs per day in the cecum and ascending colon that are passed with the feces, embryonating after several weeks and continuing the next cycle once ingested. The lifespan of T. trichiura is about 1 year (Bundy and Cooper, Reference Bundy and Cooper1989).
Although there are no published studies for recombinant subunit vaccines for any trichurid, vaccination of mice with adult Trichuris muris ES products resulted in almost complete protection against T. muris egg challenge, in association with a classical TH2 response (Dixon et al. Reference Dixon, Little and Else2010). Also, a few studies have obtained promising results for the zoonotic parasite Trichinella spiralis (Table 1), which, like trichurids, is also found in clade I Nematoda. Although T. spiralis has a considerably different life cycle than trichurids – transmission to humans occurring from ingestion of larvae-contaminated meat, followed by larval maturation to adults that deposit eggs within the small intestine that hatch, and then the larvae penetrate the intestinal mucosa and subsequently migrate to muscle tissue for maturation into cysts – many antigens are likely to contain high amino acid identities and to potentially have similar functions.
Three related studies in mice determined the vaccine efficacy of the immunodominant and/or immunomodulatory surface and/or ES proteins Ts87, TsGp43 and paramyosin (TsPmy) delivered as DNA vaccines in recombinant, attenuated Salmonella enterica serovar Typhimurium for induction of mucosal immune responses (Yang et al. Reference Yang, Zhang, Yang, Chen, Cui and Zhu2010b ; Pompa-Mera et al. Reference Pompa-Mera, Yepez-Mulia, Ocana-Mondragon, Garcia-Zepeda, Ortega-Pierres and Gonzalez-Bonilla2011; Wang et al. Reference Wang, Wang, Bi, Sun, Yang, Gu, Huang, Zhan and Zhu2016). Vaccines were administered three times either orally or i.n. and elicited significant mucosal IgA and/or serum IgG1/IgG2a as well as elevated TH1 and TH2-associated cytokines (Table 2). Vaccination resulted in significantly less (between 30 and 50%) T. spiralis worm burdens in muscle tissue compared with control (Table 2). Though these recombinant subunit, oral vaccines for T. spiralis are encouraging for trichurids, the efficacy of oral vaccination against T. muris in mice was shown to depend upon host genetics, and less so for parenteral vaccination (Robinson et al. Reference Robinson, Bellaby and Wakelin1995). Nonetheless, TsPmy resulted in similar vaccine efficacy when administered s.c. as an E. coli-expressed protein-based vaccine formulated with CFA (Yang et al. Reference Yang, Gu, Yang, Wei, Wang, Cui, Pan, Li and Zhu2010a ), and thus, appears to be the most encouraging of the three antigen targets for potential application to trichurids.
A very recent study (Xu et al. Reference Xu, Bai, Wang, Shi, van der Giessen, Boireau, Liu and Liu2017) used a naked DNA vaccine approach (i.e. no adjuvant or transfection reagent) to test the vaccine efficacy of a serine protease (Ts-NBLsp) expressed specifically in the newborn larval (NBL) stage, which is the stage when T. spiralis colonizes the striated muscle tissue after migrating from the small intestine. A Ts-NBLsp cDNA was cloned into pcDNA3·1+ and injected twice i.m. at 60 µg doses into the quadriceps of Kunming mice, where expression of the recombinant Ts-NBLsp protein was confirmed by immunofluorescence. Mice immunized with pcDNA3·1(+)-Ts-NBLsp had elevated serum IgG1 and IgG2a responses, with the latter higher than the former, but responses were weak overall (maximum IgG1 OD450 = ~0·3; maximum IgG2a OD450 = ~0·55; background = ~0·1; 1 : 50 serum dilution) (Table 2), consistent with the previous studies on DNA vaccines mentioned above. Interestingly, the ratio of CD4+/CD8+ T cells in peripheral blood was significantly lower in both pcDNA3·1+/Ts-NBLsp and pcDNA3·1+ empty vector control mice compared with PBS control mice (i.e. the population of CD4+ T cells were reduced and/or CD8+ T cells expanded) (Table 2). Consistent with an expansion of CD8+ T cells in peripheral blood, IFN-γ was also significantly elevated in the peripheral blood for both pcDNA3·1+/Ts-NBLsp and pcDNA3·1+ empty vector control mice compared with PBS control mice, and higher (but not significant) in pcDNA3·1+/Ts-NBLsp compared with pcDNA3·1+ (Table 2). On the other hand, IL-10 and IL-4 were significantly elevated in the peripheral blood of only pcDNA3·1+/Ts-NBLsp mice (Table 2). Mice immunized with pcDNA3·1+/Ts-NBLsp had ~78% less T. spiralis larvae recovered in muscle tissue (Table 2), and interestingly, pcDNA3·1+ empty vector control mice also had a significant 30% less T. spiralis larvae, compared with PBS control mice (Xu et al. Reference Xu, Bai, Wang, Shi, van der Giessen, Boireau, Liu and Liu2017). These findings indicated that: (1) the pcDNA3·1+ vector alone induces a non-specific cell-mediated immune response within the muscle tissue that reduces susceptibility to T. spiralis larval colonization, and (2) pcDNA3·1+/Ts-NBLsp is a highly protective DNA vaccine that greatly reduces NBL colonization of the muscle tissue, in association with a mixed TH1/TH2-type immune response. It is also worth mentioning that the apparent elevation in CD8+ T cells, and association with protection, makes sense as TH1 effectors are known to be protective against the T. spiralis NBL stage (but not TH2 effectors), which becomes an intracellular pathogen within the muscle tissue. Finally, although T. spiralis infects its host much differently, it would be worthwhile to explore the NBLsp homologue in trichurids to determine if by targeting this antigen, similarly high levels of protection can be achieved.
DISCUSSION
These previous studies of recombinant subunit vaccines for STHs have reported many possible candidates for further optimization, and some that progressed to clinical trials. However, collectively reviewing all of these studies has revealed many differences in vaccine design and their administration. Examples include the use of protein or DNA vaccines, E. coli or eukaryotic expression systems, TH1 or TH2-biased adjuvants, many different routes of immunization, different amounts of immunogen and the number of injections, among many other inconsistencies. Below, we briefly discuss and interpret the results from these previous studies as a whole in an attempt to help guide future developments.
Protein or DNA vaccines?
An important difference between protein and DNA vaccines, is that the former has been used successfully in humans since the mid-20th century (Plotkin, Reference Plotkin2014), but not a single DNA vaccine has been shown to work in humans. An advantage of protein vaccines is that the immunogen (i.e. an extracellular antigen) is processed and presented on Class II MHC by professional APCs, and along with adjuvant, this can shape intricate TH responses, and clearly the majority of successes with recombinant subunit vaccines for STHs have been protein-based. However, with DNA (and RNA) vaccines the immunogen must be translated within the host cell cytoplasm, and hence, can only be presented by Class I MHC. Thus, the immune response(s) is generally limited to TH1, and particularly to CD8+ TC cells (Ferraro et al. Reference Ferraro, Morrow, Hutnick, Shin, Lucke and Weiner2011). This may explain the weak antibody responses generated against rAcey-MEP-6 and rAcey-MEP-7 in golden hamsters using the pcDNA3·1+/Fugene 6 system against A. ceylanicum (Wisniewski et al. Reference Wisniewski, Jaros, Baska, Cappello and Wedrychowicz2013, Reference Wisniewski, Jaros, Baska, Cappello, Dlugosz and Wedrychowicz2016), against rSs-EAT-6 peptide in mice using the Lambda Uni-zap XR vector/GM-CSF system against S. stercoralis (Kerepesi et al. Reference Kerepesi, Keiser, Nolan, Schad, Abraham and Nutman2005), and against rTs-NBLsp in mice using pcDNA3·1+ alone against T. spiralis. However, DNA vaccines in mice have proven to be highly efficacious against A. suum (Chen et al. Reference Chen, Yuan, Xu, Zhou, Zhang, Zhang, Wang, Yan, Lin and Zhu2012) and T. spiralis (Yang et al. Reference Yang, Gu, Yang, Wei, Wang, Cui, Pan, Li and Zhu2010a ; Pompa-Mera et al. Reference Pompa-Mera, Yepez-Mulia, Ocana-Mondragon, Garcia-Zepeda, Ortega-Pierres and Gonzalez-Bonilla2011; Wang et al. Reference Wang, Wang, Bi, Sun, Yang, Gu, Huang, Zhan and Zhu2016), with significant immunogenicity (albeit relatively weak for pcDNA3·1+/Ts-NBLsp) and protection achieved. Moreover, though DNA vaccines against A. suum and T. spiralis resulted in significant TH1 responses, as would be expected, there were contributions from TH2 cytokines as well. These studies demonstrate that the immune responses to DNA vaccines against STHs are likely to be more complex than that appreciated, and this type of recombinant subunit vaccine may prove to be broadly applicable in the future with more thorough studies. However, it will be important for future studies to address the safety concerns of host gene modification and uncontrollable gene expression before DNA vaccines can be a realistic STH control measure.
Escherichia coli or eukaryotic expression systems for protein-based vaccines?
Escherichia coli would be the most cost effective system for expressing recombinant proteins for STH vaccines, which is important given that the target populations are people living in poverty. However, it is widely known that recombinant expression of eukaryotic proteins in E. coli often results in the proteins being expressed in insoluble inclusion bodies. Inclusion bodies must then be solubilized with harsh detergents, and often reduced, thus causing denaturation with the major challenge of refolding into native confirmation. Also, even in the rare event that the protein does express in E. coli in the soluble fraction, still, folding is often incorrect, and eukaryotic post-translational modifications – common on secreted proteins – are deficient. These deficiencies of E. coli are significant for vaccine development given that adequate immunogenicity often requires discontinuous conformational B cell epitopes (Andersen et al. Reference Andersen, Nielsen and Lund2006). Hence, eukaryotic expression systems such as yeast (P. pastoris or K. lactis) and baculovirus-insect cells are likely to be more suitable for production of recombinant proteins for vaccines against STHs. On the other hand, although the majority of successful recombinant subunit vaccines for the strongylids have been with these eukaryotic expression systems, with most attempts having failed with E. coli (especially in the trichostrongyloids), the major successes in ascarids with E. coli presents a significant incongruence. Could it be that some immunogens (e.g. those mentioned above for ascarids) induce protection through linear T cell epitopes independent from B cells, similar to the natural immune responses (Anthony et al. Reference Anthony, Rutitzky, Urban, Stadecker and Gause2007; Nair and De'Broski, Reference Nair and De'Broski2016), while other immunogens (e.g. many of those mentioned above for stongylids) require non-linear B cell epitopes? It seems likely that success in any expression system will vary on a protein-to-protein basis for any STH, and more thorough studies of the immunobiology of STH infections will help to explain the protein-to-protein variations that have been observed.
What are the best immune correlates of vaccine-induced protection?
B cells
There have been many immune factors found to be associated with vaccine-induced protection against STHs. Serum IgG1, IgG2 and IgE, and mucosal IgA [i.e. secretory (s)IgA], IgG1 and IgG2 are antibodies often found to be elevated in immunized animals. IgG1/IgG2 in both serum and mucosa have been found to be correlated with the level of protection, and in some cases, so has sIgA. In some cases, equal balance of IgG1 and IgG2 were found to correlate with the greatest level of protection. As IgG1 is considered the TH2 subclass, while IgG2 is considered the TH1 subclass, most relate this equal balance to a mixed TH1/TH2 response. However, although TH2-dominated cytokine profiles were nearly always associated with IgG1-dominated serum antibody, many of the previous studies report IFN-γ-dominated cytokine profiles also with highly elevated IgG1, often higher than IgG2. So it is unclear if IgG1 is specific for TH2 in vaccine-induced immune responses to STHs, and cytokines must also be evaluated to determine the type of TH response. Also, in some studies protection was correlated with IgG1 or balanced IgG1/IgG2, while in others, sIgA was more highly correlated. Importantly, although the IgG class has been ascribed the function of ‘blocking/neutralizing’ target antigen function for hookworms, inhibiting protein activity in vitro, passive transfer experiments with purified antibodies have not been performed. Thus, it has not been conclusively determined if antibodies in general are sufficient for vaccine-induced protection, and if any one class is sufficient. However, it was shown that passive transfer of serum specific for rSs-IR resulted in similar levels of protection against Ss challenge infection compared with immunized donor mice (Abraham et al. Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011), thus supporting the role of antibodies in vaccine-induced protection. That said, Abraham et al. (Reference Abraham, Hess, Mejia, Nolan, Lok, Lustigman and Nutman2011) speculated that antibodies were not blocking/neutralizing antigen function per se, but that the protection was via complement and/or ADCC.
T cells
Antigen recall responses in peripheral blood, spleens, or mLNs of immunized animals have found that IFN-γ, IL-4 and IL-13 are frequently elevated indicating that protection is often associated with mixed TH1/TH2 responses, consistent with common elevations of both IgG1 and IgG2. In some cases, IL-10 was also elevated, and in other cases, IL-10 dominated the cytokine pool, thus demonstrating that IL-10s role in vaccine-induced immunity to STHs may be more complicated than typically appreciated (i.e. it may not be just an anti-inflammatory cytokine). But the levels of all these cytokines were highly variable between studies. As with antibodies, it is unclear which, if any, of these cytokines are necessary for vaccine-induced protection. In vivo experiments with cytokine knockout animals or with blocking/neutralizing antibodies will need to be performed to determine if any of these cytokines are necessary for protection, and adoptive transfer experiments should be performed to determine whether T cells are sufficient, or whether antibodies are also required. Furthermore, many more cytokines should be evaluated in future studies to more thoroughly characterize the TH responses associated with protection. Finally, studies should compare the levels of cytokines produced by lymphocytes in response to antigen stimulation in the different lymph tissues. Although most studies have found that lymphocytes from immunized animals proliferate significantly in response to antigen, it would also be worthwhile to compare proliferative responses between different lymph tissues.
Innate immune cells
Studies of the trichostrongyloids showed nicely that the granulocytes eosinophils, basophils and mast cells were elevated locally within the abomasum, and in some cases correlated with the level of protection. Such experiments should be extended to the other STHs to determine whether these innate immune cells, or others, such as neutrophils, macrophages, dendritic cells and even natural killer cells, are (or are not) significantly elevated within the local tissue, which could ultimately help to enhance vaccine-induced protection.
Does adjuvant really matter for parenteral vaccines?
One of the most variable factors among these previous studies was the choice of adjuvant. Given the conserved association of TH2-type responses with immunity to STHs, TH2-biased adjuvants make sense. However, the paradigmatic natural immune responses to STHs are clearly not conserved with the immune responses associated with recombinant subunit vaccines. Loukas et al. (Reference Loukas, Bethony, Williamson, Goud, Mendez, Zhan, Hawdon, Bottazzi, Brindley and Hotez2004) showed that whether TH1 or TH2-biased adjuvant was used with rAc-CP-2 did not significantly change the immunogenicity and protection against A. caninum challenge infection induced in beagles. And multiple studies for hookworms used either TH1 or TH2 adjuvants with success, and the associated immune responses – elevated IgG1/IgG2 – were overall consistent with both classes of adjuvants (although cytokines were never evaluated for TH2 adjuvants, so they cannot be compared with the cytokine changes observed for TH1 adjuvants). Also, studies in ascarids and T. spiralis had much success with CFA, a heavily TH1-biased adjuvant, with elevated, and in some cases dominant, TH2 cytokine profiles. It seems reasonable to question if the immunogens alone can override adjuvant induction of TH1 and/or TH2, but such experiments were not conducted to be certain.
On the other hand, studies in the non-hookworm strongylids found that the choice of adjuvant can determine the outcome of vaccination. Ben Nouir et al. (Reference Ben Nouir, Eschbach, Piedavent, Osterloh, Kingsley, Erttmann, Brattig, Liebau, Fleischer and Breloer2012) showed that rSr-HSP60, which inherently induces TH1, resulted in an IL-13-dominated cytokine profile and partial protection when formulated with alum, but with CFA actually increased susceptibility to S. ratti challenge infection. Conversely, in the trichostrongyloids, multiple native immunogens lacked significant protective effects when formulated with TH2 adjuvants (IFA or Alhydrogel), but with Quil-A (and CFA) resulted in significant protection (Knox et al. Reference Knox, Smith and Smith1999; Geldhof et al. Reference Geldhof, Vercauteren, Vercruysse, Knox, Van den Broeck and Claerebout2004), possibly suggesting that adjuvant induction of TH1 or a mixed TH1/TH2 response is necessary for the mechanism of vaccine-induced protection, at least in these trichostrongyloids.
Clearly we are far from understanding the mechanism(s) of vaccine-induced protection against STHs, and it is likely that each STH will be distinct in one way or another. Thus, the field of STH vaccinology needs to better bridge with immunobiology for us to more rationally design better recombinant subunit vaccines.
Parenteral or oral/intranasal immunization?
Parenteral immunizations have dominated the literature for the development of STH vaccines. However, in considering the target populations that are most afflicted by STHs, oral/i.n. vaccines are likely to be more ideal due to their ease of self-administration, which would likely allow for a much wider dispersal, and for their induction of local, mucosal immune responses. Previous studies of A. suum and T. spiralis had much success with oral or i.n. immunizations, and the success in A. suum with simple conjugation of immunogen to CTB (CTB subunit) is promising for all STHs. However, as mentioned above, Robinson et al. (Reference Robinson, Bellaby and Wakelin1995) showed that BALB/c high and C57BL/10 and B10.BR low-responder mice were all protected against T. muris challenge infection when immunized s.c. with T. muris adult worm extract, with elevated serum IgG1 and IFN-γ and IL-5 cytokine profiles. However, only BALB/c high and C57BL/10 low-responder mice were protected against T. muris when immunized orally, while B10.BR low-responder mice were not protected and had negligible immune responses. Also, the success of oral/gut mucosal vaccines in endemic human populations for other enteric pathogens such as S. enterica serovar Typhi and Vibrio cholera has been demonstrated to depend largely on underlying genetics (Pasetti et al. Reference Pasetti, Simon, Sztein and Levine2011). Thus, although oral/i.n. vaccines may be a hopeful goal for STH vaccines (especially for humans), the lesser dependence on host genetics for parenteral vaccines suggests a greater likelihood of success in clinical trials.
Does immunization regimen matter?
A likely underappreciated factor that is important for vaccine design is the number of injections and amount of immunogen per injection. With reductionist vaccine design (at least in the simplest terms), the number of injections and amount of immunogen (as well as route of administration and form of immunogen) can be optimized in order to achieve a desired immune response (e.g. sufficient affinity maturation) (Jardine et al. Reference Jardine, Sok, Julien, Briney, Sarkar, Liang, Scherer, Dunand, Adachi, Diwanji, Hsueh, Jones, Kalyuzhniy, Kubitz, Spencer, Pauthner, Saye-Francisco, Sesterhenn, Wilson, Galloway, Stanfield, Wilson, Burton and Schief2016). Nearly all of the previous studies of recombinant subunit vaccines for STHs used three equally dosed injections, with small rodents (mice and golden hamsters) generally receiving between 20 and 50 µg doses, and larger animals (beagles, sheep and cattle) receiving from 50 to 300 µg doses. No studies have actually adequately compared different numbers of injections and doses of the same vaccine, but such a study may prove worthwhile, especially considering that minimizing the number of immunizations (and doses) required for protection will be critical for the success of these vaccines in target populations. A few studies did, however, differ from the standard of three injections, with significant protection achieved with just two injections (Reszka et al. Reference Reszka, Rijsewijk, Zelnik, Moskwa and Bienkowska-Szewczyk2007; Ben Nouir et al. Reference Ben Nouir, Eschbach, Piedavent, Osterloh, Kingsley, Erttmann, Brattig, Liebau, Fleischer and Breloer2012), suggesting that this may be the minimum number. Regardless, it is very likely that the dose and frequency of exposure will vary with antigen and host and will probably have to be empirically determined for each case.
Should naturally hidden or exposed antigens be targeted?
The mechanisms of vaccine-induced protection against STHs have not been completely resolved, only extensively postulated. To use hookworms and H. contortus as an example, the idea behind targeting STH proteins that are present within STHs’ intestinal lumens is that these proteins are accessible to antibodies ingested in the hosts’ blood. The antibodies bind to the target proteins, and block/neutralize their activities/functions. These proteins have been called hidden antigens, as mentioned above, because naturally they are physically concealed from the host immune system inside of the blood-feeding STHs’ bodies. The current definition of hidden antigen does not include those antigens that naturally are physically exposed to the host immune system (e.g. proteins in ES products) and immunologically silent for unknown reasons, which could be distinguished from hidden antigens by from now on being called immune-silent ES proteins. Now hidden intestinal proteins in STHs that do not normally ingest blood (see Table 1) may not be as useful to target, as exemplified by comparing the efficacy of the same vaccine against H. contortus and O. ostertagi (Smith et al. Reference Smith, Smith and Pettit2000). However, as most non-blood-feeding STHs normally ingest large amounts of mucus within the GI tract, induction of high titre of sIgA as well as IgG within the mucosa might grant these antibodies access to block/neutralize the hidden intestinal proteins within these worms. But again, since passive transfer experiments of purified antibodies specific for individual immunogens have not been performed, it is uncertain whether blocking/neutralizing antibodies are in fact solely responsible for vaccine-induced protection, even against hookworms and H. contortus.
Targeting hidden intestinal antigens is justified by studies in hookworms. Naturally immunodominant ASP-2 secreted by hookworm infective L3, although partially protective, resulted in allergic reactions when immunized into previously infected individuals due to pre-existing ASP-2-specific IgE, as mentioned above. Thus, targeting antigens that are not naturally immunogenic avoids such risks. But an important question unaddressed for the hidden antigen approach is whether or not recall responses will be sufficiently induced in memory lymphocytes in vaccinated individuals if in fact the native antigens are hidden from the immune system. In the experimental vaccine studies (including with hidden intestinal antigens), animals were challenged soon after they were immunized, and thus, acute immune responses were likely still existent. In order to answer this important question, it would be worthwhile to at least investigate the lifetime of protection for such vaccines that target hidden intestinal antigens in the animal models for experimental vaccination.
On the other hand, what about targeting antigens exposed on the surface of L4 and/or adult worms (while also absent from L3), or that are secreted into the local environment (i.e. proteins in ES products)? In theory, many of these antigens would be both immunologically accessible and more likely to induce long-lasting recall responses in immunized individuals, because they are in fact exposed. And as long as these antigens are not produced by infective L3 (most notably in hookworms), natural immunogenicity in these later stages may not matter. There would also be the option of using the exposed antigens to direct the natural immune mechanisms that exist to expel STHs from the GI tract. Such an approach may also help to better bridge the currently largely independently operating fields of STH vaccinology and immunobiology.
How valid are the laboratory challenge infection models?
All of the preclinical vaccine studies above for human STH infections used various laboratory challenge infection models. Although there is no way of conducting preclinical studies directly on humans, there are likely limitations with these models. These limitations include the use of model hosts, STH species other than the target species, laboratory strain of the target STH species, unnatural infection and other limitations. With the use of model hosts such as golden hamsters, mice rats, and beagles, and laboratory STH species/strains that are genetically different from natural species/strains, it is unclear if any of the vaccines will translate to humans, and such studies are warranted. Also, the use of unnatural infections such as the diffusion chambers for S. stercoralis in mice, or inoculating mice with ascarids, which are not able to establish patent infections in the mouse small intestine, add further limitation for extrapolating to humans. Furthermore, most of the vaccine trails for each STH were conducted by only one or two different labs. More technical, laboratory-to-laboratory variations such as different laboratory STH strains, different viability and dose of inocula, different host species or strains and just simply different reagents, equipment and researchers could cause extensive variation in results. As the field of STH vaccinology continues to grow, the reproducibility of the results obtained for these recombinant subunit vaccines will likely be addressed.
How can we better streamline antigen selection?
If blocking/neutralizing antibodies really are the bases of the protection achieved from the previous studies on recombinant subunit vaccines for STHs, then the antigens must have essential/important functions for infection. With the advent of whole genome sequencing combined with transcriptomics and proteomics, we are now capable of filtering out candidate antigens unlikely to be important for infection and/or to be present in immunologically inaccessible sites within the worms through bioinformatics and computational biology. Although a challenge, we can similarly prioritize for candidate antigens using the same methods, which will greatly increase the probability of testing antigens that have essential/important functions. The genomes have already been published for many of the most critical STHs (Table 1), and thus, much of the data needed for prioritization is already at hand.
Even with genomics-assisted selection of candidate antigens for experimental vaccination, importance/essentiality for infection can only be validated through reverse genetics. RNAi was used successfully to validate important functions of multiple A. suum antigens and subsequent vaccine studies resulted in comparatively high levels of protection (Islam et al. Reference Islam, Miyoshi and Tsuji2005a , Reference Islam, Miyoshi, Yamada and Tsuji b , Chen et al. Reference Chen, Xu, Nisbet, Huang, Lin, Yuan, Song and Zhu2011, Reference Chen, Yuan, Xu, Zhou, Zhang, Zhang, Wang, Yan, Lin and Zhu2012). Although RNAi has had variable success so far in other STHs such as H. contortus (Britton et al. Reference Britton, Samarasinghe and Knox2012), this tool may still be useful for validation studies of particular antigens. An alternative reverse genetics tool for validation studies might be the CRISPR-Cas9 system for generating single gene knockouts. But although heritable loss-of-function mutants have been obtained in C. elegans using CRISPR-Cas9 (Friedland et al. Reference Friedland, Tzur, Esvelt, Colaiacovo, Church and Calarco2013), no results have been published in STHs, so the efficacy of this system for validating important functions of candidate antigens is unknown at this time.
Another possible method to better streamline antigen selection could be to screen STH antigens for recognition by putative immune sera collected from people living in endemic regions that have high antibody titre and low worm burdens. However, if these antigens happen to be recognized by IgE in these humans, it is not likely that they will be useful as vaccine candidates given the findings with hookworm ASP-2 (Diemert et al. Reference Diemert, Pinto, Freire, Jariwala, Santiago, Hamilton, Periago, Loukas, Tribolet, Mulvenna, Correa-Oliveira, Hotez and Bethony2012).
Are pan-anthelmintic vaccines possible?
The idea of a single, multivalent, pan-anthelmintic vaccine to control the three major human STHs (hookworms, A. lumbricoides and T. trichiura) has been proposed (Zhan et al. Reference Zhan, Beaumier, Briggs, Jones, Keegan, Bottazzi and Hotez2014). By including one or two recombinant protein immunogens for each STH, it is believed that all can be immunologically controlled simultaneously (Zhan et al. Reference Zhan, Beaumier, Briggs, Jones, Keegan, Bottazzi and Hotez2014). However, though such a vaccine would be ideal, the different locations that these STHs occupy within the GI tract and/or different feeding habits (Table 1) may present an enormous obstacle for a single vaccine. As mentioned above, adjuvants that worked well against one STH completely failed against another, and targeting hidden intestinal antigens in hookworms may work differently (or not at all) in A. lumbricoides and T. trichiura. And in combination with these three different STHs being phylogenetically distant relatives (i.e. from Nematoda clades V, III and I, respectively), the immunobiological factors involved in vaccine-induced protection against each of them is likely to be considerably different. On the other hand, pan-strongylid, pan-ascarid and pan-trichurid vaccines would seem to be more attainable goals given the appreciation of their phylogenetic relationships, and thus, biological similarities, and would also aim to target STHs of livestock. The latter ‘pan’ approach may also be achievable with mono or at most bivalent vaccines as antigens that are highly conserved within each group of target STHs, antigen(s) likely to have very similar and important functions for all of them, could be targeted.
Conclusions/future directions
Recombinant subunit vaccines not only offer the potential to immunologically control STHs, but also are capable of being manufactured on a large enough scale to vaccinate over one fourth of all human beings and the majority of livestock in developing regions. Studies in hookworms have reported several recombinant immunogens with significant immunogenicity and protection, some of which progressed to clinical trials. The current best candidates in hookworms are those present in the adult intestine with roles in digestion of haemoglobin; those expressed in infective L3 are at risk of inducing allergic reactions in individuals with pre-existing IgE, and thus, should be avoided. Studies of another family of strongylids, the strongyloids, have revealed a number of immunogenic and protective recombinant immunogens in experimental vaccinations. But so far in the trichostrongyloids of livestock, minimal success has been achieved, likely due to the more technical factors of recombinant protein expression and/or purification. There are a number of recombinant immunogens from the ascarids that provided significant protection, some of which have been validated via RNAi to have important roles during infection. However, all of the target ascarid antigens are naturally immunodominant, abundant L3 surface proteins and thus, the studies in hookworms suggest potential issues for clinical application of the candidate immunogens. A few protective immunogens have been reported from T. spiralis that may also be effective against the relatively closely related trichurids.
Although there have been many successes with experimental vaccination using recombinant subunit vaccines, we still are far from understanding the mechanisms of vaccine-induced protection, which will require a sturdy bridge between vaccinology and immunobiology. A better understanding of the immunobiology and mechanism(s) of protection will lead to more potent, broad-spectrum vaccines. Also, more technical factors need to be better explored, such as why certain recombinant expression systems work and others do not for particular immunogens, how many injections and what doses per injection are optimal, which route of immunization is optimal (i.e. parenteral or oral/intranasal) and how long the immunological memory responses remain protective. Furthermore, many of the pre-clinical vaccine studies mentioned in this review focused on laboratory models, and thus, it is difficult to extrapolate to the target animals (and humans) as it is unclear whether the variation in immunological and biological factors will result in different vaccine efficacies. Better understanding these more technical factors and integrating genomics for testing higher priority target antigens will undoubtedly result in durable vaccines to protect our planet from these critical parasites.
ACKNOWLEDGEMENTS
The authors do not have acknowledgments to report.
FINANCIAL SUPPORT
This work was supported by the National Institutes of Health (NIH)’s National Institute of Allergy and Infectious Diseases (NIAID) R01 (grant number 7R01AI056189), NIAID R21 (grant number 1R21AI111173) and the US Department of Agriculture (USDA)’s National Institute of Food and Agriculture (NIFA) (grant number 2015-11323) grants to RVA.





