I. INTRODUCTION
Li–S batteries are regarded as one of the most promising rechargeable batteries that could meet the demands of the electric vehicles and grid energy storage due to its high theoretical specific energy and volumetric energy density of approximately 2600 W h/kg and 2800 W h/L, Reference Bruce, Freunberger, Hardwick and Tarascon1–Reference Yang, Zheng and Cui4 respectively. Additionally, sulfur as the electroactive material is not only nontoxic, cost-effective, and highly abundant on Earth, but also it possesses a safer operating voltage range (1.5–2.5 V versus Li/Li+). Reference Chen and Shaw5,Reference Gu, Hencz and Zhang6 All the advantages make Li–S cells extraordinarily attractive toward researchers all over the world.
However, the early research on Li–S batteries progress slowly because it suffers several severe issues. First of all, the low electric and Li+-ionic conductivity of elemental sulfur and discharged end-product (Li2S and Li2S2), which will result in low utilization of active materials and poor coulombic efficiency. Reference Liang, Sun, Li and Cheng7,Reference Gu, Wang, Lai, Qiu, Li, Hou, Martens, Mahmood and Zhang8 Secondly and most importantly, the discharged intermediates-polysulphides easily dissolve in the organic electrolyte. Reference Gu, Wang, Lai, Qiu, Li, Hou, Martens, Mahmood and Zhang8 These polysulfide anions (S x 2−) can diffuse to the anode, causing tremendous loss of active materials, extremely low coulombic efficiency, and rapid capacity fading. Reference Gu, Hencz and Zhang6 Thirdly, the huge volume expansion/shrinkage of the sulfur electrode during charge/discharge process, leads to the electrode collapse and short cycle life. Reference Song, Cairns and Zhang3,Reference Gu, Hencz and Zhang6
Therefore multiple and combined strategies, such as developing new electrolyte and binder, Reference Liu, Galpaya, Yan, Sun, Lin, Yan, Liang and Zhang9,Reference Suo, Hu, Li, Armand and Chen10 protecting the anode, Reference Jing, Kong, Liu, Li and Gao11 modifying the separator Reference Li, Jing, Su, Lai, Chen, Yuan, Li and Xiang12 and the sulfur cathode Reference Gu, Tong, Wen, Liu, Lai and Zhang13,Reference Lee, Hwang, Lee, Lee and Choi14 are used to solve the above problems. Among which, using the nanostructured materials to modify the sulfur cathode are most popular. Important progress was made by Nazar and coworkers, Reference Ji, Lee and Nazar15 who showed that by fabricating cathodes where sulfur had been encapsulated into nanostructured mesoporous carbon, high reversible capacities and good rates can be obtained due to the enhanced conductivity and physical confinement of the polysulphides via the mesopores. Since then, various nanostructured conductive carbon, such as carbon fibers, Reference Gu, Lai, Liu, Yang, Hou and Zhang16 graphene, Reference Zhao, Zhang, Huang, Tian, Nie, Peng and Wei17 carbon nanotubes (CNT), Reference Gu, Tong, Wen, Liu, Lai and Zhang13 carbon spheres, Reference Zhang, Wu, Yuan, Guo and Lou18 porous carbon, Reference Gu, Wang, Lai, Qiu, Li, Hou, Martens, Mahmood and Zhang8 etc., have been used to improve the sulfur utilization and extend the sulfur cathode cycling performances. However, the conjugate nonpolar carbon planes have limited sites to strongly anchor polar molecules (e.g., lithium polysulphides and (di)sulphides). Reference Liu, Huang, Zhang and Mai19 To add anchoring sites and enhance anchoring ability of the carbon, tunable polar sites to chemically confine the polysulphides was introduced. Nanostructured carbon doped with heteroatoms (N, Reference Gu, Tong, Rehman, Liu, Hou and Zhang20 S, Reference Xing, Xi, Li, Su, Lai, Zhao and Kumar21 P, Reference Gu, Tong, Lai, Qiu, Huang, Yang, Wen, Liu, Hou and Zhang22 B Reference Yang, Yin, Ye, Jiang, Zhang and Guo23 ), and carbon modified with functional groups (amino-functionized, Reference Wang, Dong, Li, Zhao, Wu, Hao, Liu, Qiu and Lou24 carboxyl-functioned, Reference Wang, Dong, Wang, Zhang and Jin25 sulfonated, Reference Zhou, Lin, Huang and Yu26 fluorinated, Reference Vizintin, Patel, Genorio and Dominko27 hydroxylated Reference Zu and Manthiram28 ) as well as combined with conductive polymers, Reference Yin, Wang, Lin, Yang and Nuli29–Reference Li, Zhang, Zheng, Seh, Yao and Cui31 have all been widely investigated for obtaining high-performance Li–S batteries. However the tap densities of these conductive carbon are commonly very low, Reference Babu and Reddy Arava32 which is not beneficial to the practical applications of S cathodes.
Recently, to increase the tap density of electrodes as well as to keep long cycle life-span, nanostructured polar metal compounds, such as metal oxides, Reference Tao, Wang, Liu, Wang, Yao, Zheng, Seh, Cai, Li, Zhou, Zu and Cui33,Reference Seh, Li, Cha, Zheng, Yang, McDowell, Hsu and Cui34 metal hydroxides, Reference Gu, Tong, Wen, Liu, Lai and Zhang13 metal sulphides, Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35,Reference Zhou, Tian, Jin, Tao, Liu, Zhang, Seh, Zhuo, Liu, Sun, Zhao, Zu, Wu, Zhang and Cui36 metal carbides, Reference Liang, Garsuch and Nazar37 metal nitrides, Reference Cui, Zu, Zhou, Manthiram and Goodenough38 and metal organic frameworks (MOFs), Reference Zheng, Tian, Wu, Gu, Xu, Wang, Gao, Engelhard, Zhang, Liu and Xiao39 have been used as the host materials toward (poly)sulphides. A large amount of work nowadays reports that these metal compounds have much stronger adsorption ability to polysulphides compared to carbon, doped carbon, and conductive polymers. Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35,Reference Ma, Wei, Zhuang, Hendrickson, Hennig and Archer40–Reference Zhang, Shi, Ding, Zhang and Yu43 What’s more, compared to the carbon materials, the metal compounds’ exposed surfaces and morphologies are easier to control by chemical and physical methods. Reference Liu, Huang, Zhang and Mai19 Thus a variety of nanostructured metal compounds were designed, such as hollow, Reference Seh, Li, Cha, Zheng, Yang, McDowell, Hsu and Cui34 porous, Reference Ma, Wei, Zhuang, Hendrickson, Hennig and Archer40 layered, Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35 laminar-structured, Reference Pang, Kundu and Nazar42 and so on, to effectively hold the polysulphides in Li–S batteries. However, compared to many reviews on the carbon and polymers materials used in Li–S batteries, the review on metal compounds for Li–S batteries are rare.
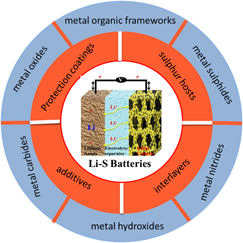
Herein, recent advances in the use of these metal compounds in Li–S batteries are reviewed. We systematically conclude metal oxides, metal hydroxides, metal sulphides, metal carbides, metal nitrides, and MOFs as sulfur host, additives, interlayers, anode/cathode/separator coatings in Li–S batteries (Scheme 1). Also we focus on how these metal compounds interact with polysulphides from both physical and chemical aspects. Meanwhile the disadvantages and bottle-neck of these metal compounds for Li–S batteries have also been proposed. Lastly we prospect feasible solving strategies and future development.

SCHEME 1. Various metal compounds acted as multiply roles in Li–S batteries.
II. NANOSTRUCTURED METAL OXIDES APPLICATION IN LI–S BATTERIES
A. Ti x O y
TiO2, due to its natural abundance, cost-effectiveness, and polar surface, has become the most widespread and popular metal oxide applied in Li–S batteries. Reference Liu, Huang, Zhang and Mai19 It was first used as an additive in the mesoporous carbon–sulphur cathode of Li–S batteries by Nazar’s group in 2012. Reference Evers, Yim and Nazar44 They investigate the role of surface adsorption versus pore absorption by using three kinds of TiO2 with similar surface areas but different pore sizes (mesoporous α-TiO2 with pore size of 5.2 nm, mesoporous β-TiO2 with pore size of 9 nm, and nonporous γ-TiO2, respectively) as the additives. The electrochemical results reveal that the soluble lithium polysulphides are preferentially absorbed within the pores of the nanoporous titania while the surface adsorption also plays a more minor role. Recently, Belharouak and his co-workers also investigated the effects of nano-sized TiO2 particles as the additive on the electrochemical properties. Reference Xu, Li, Lu, Amine and Belharouak45 The cyclic voltammetry measurements at different scan rate indicate that the addition of TiO2 helped in reducing the polarization of the sulfur electrodes. Wang and his co-workers designed a hydrogen reduction TiO2 hollow sphere (H–TiO2), Reference Yang, Wang, Lu, Wang, Zhong, Wang and Jiang46 where the TiO2 micron spheres are composed of TiO2 nano-plates. With such H–TiO2 additive, the sulfur cathode could deliver a high reversible capacity of 928.1 mA h/g after 50 charge–discharge cycles at a current density of 200 mA/g. They attributed the improvement to H–TiO2 spheres’ polar surfaces serving as the surface-bound intermediates for strong polysulphides binding.
Nanostructured TiO2 with different morphologies as the sulfur host are also widely studied. Reference Seh, Li, Cha, Zheng, Yang, McDowell, Hsu and Cui34,Reference Liang, Zheng, Li, Seh, Yao, Yan, Kong and Cui47–Reference Li, Ding, Xu, Hou, Zhang and Yuan54 Cui’s group designed a sulphur–TiO2 yolk–shell nanostructure [as shown in Figs. 1(a)–1(c)], Reference Seh, Li, Cha, Zheng, Yang, McDowell, Hsu and Cui34 where the internal void space accommodates the volume expansion of sulfur and the TiO2 shell minimizes polysulfide dissolution. Accordingly, an initial specific capacity of 1030 mA h/g at 0.5 C (1 C = 1675 mA/g) and Coulombic efficiency of 98.4% over 1000 cycles were achieved. They also synthesized an inverse opal structure TiO2 to achieve both sulfur physical encapsulation and polysulphides binding. Reference Liang, Zheng, Li, Seh, Yao, Yan, Kong and Cui47 The inverse opal structure TiO2 after hydrogen reduction illustrated high conductivity. Furthermore, the relatively enclosed three dimensional (3D) structure [as shown in Figs. 1(d)–1(f)] provided an ideal architecture for sulfur and polysulphides confinement, which contributes to the 3D TiO2–sulphur cathode high initial capacity of 1100 mA h/g and reversible capacity of 890 mA h/g after 200 cycles at 0.5 C. Such good capacity retention for the reduced TiO2–S sample is due to (i) a significantly increased electrical conductivity achieved after hydrogen reduction; (ii) rapid electron and lithium-ion transport resulted from the 3D framework and the thin TiO2 shell; (iii) the generated oxygen vacancies promoting the interaction between the TiO2 and the sulfur, which renders the rational integration of physical confinement and chemical absorption of polysulphides in a working cell. In addition, nanotube TiO2/S composite, Reference Xie, Han, Wei, Yu, Zhang, Wang, Lu and Wei50 nano fibers TiO2–S composites Reference Ma, Jin, Wang, Hou, Zhong, Wang and Xin51 and mesoporous hollow TiO2 sphere–S composites, Reference Li, Ding, Xu, Hou, Zhang and Yuan54 porous TiO2–S composites, Reference Li, Guo, Deng and Huang48,Reference Ding, Shen, Xu, Nie and Zhang55 have also been designed and fabricated a novel cathode for Li–S batteries.
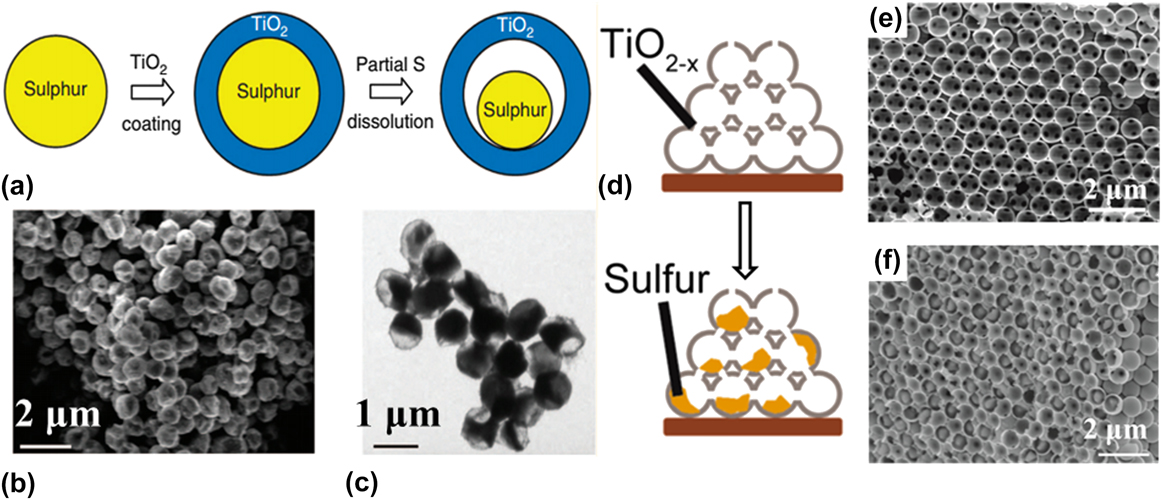
FIG. 1. (a) Synthesis and characterization of sulphur–TiO2 yolk–shell nanostructures. (b) SEM image and (c) TEM image of as-synthesized sulphur–TiO2 yolk–shell nanostructures. Reproduced with permission from Ref. Reference Seh, Li, Cha, Zheng, Yang, McDowell, Hsu and Cui34, Copyright 2013, Nature Publishing Group. (d) Schematic of the synthetic process that involves encapsulating sulfur nanoparticles with reduced TiO2 to form 3D sulphur–TiO2−x core–shell Nanostructures, (e) cross-sectional SEM image of the 3D ordered reduced TiO2 structure, (f) cross-sectional SEM image of the composite structure showing sulfur particles well encapsulated by the reduced TiO2 nanospheres. Reproduced with permission from Ref. Reference Liang, Zheng, Li, Seh, Yao, Yan, Kong and Cui47, Copyright 2014, American Chemical Society.
Due to the insulating nature of both TiO2 and sulfur, combining conductive carbon with TiO2–S composites to further improve the electrochemical performances is becoming increasingly favorable by the researchers. Reference Li, Ding, Xu, Hou, Zhang and Yuan54–Reference Wang, Li, Li, Chen, Liu and Guo68 Zhang’s group developed a titanium dioxide anchored on hollow carbon nanofiber hybrid nanostructure (HCNF@TiO2–S). Reference Zhang, Li, Jiang, Zhang, Lai and Li66 The HCNF@TiO2–S composite exhibited much better electrochemical performance than the HCNF–S composite, which delivered an initial discharge capacity of 1040 mA h/g and maintained 650 mA h/g after 200 cycles at a 0.5 C rate. Tilahun and his co-workers designed hybrid nanostructured microporous carbon-mesoporous carbon doped titanium dioxide/sulfur composite (MC-meso C-doped TiO2/S). Reference Zegeye, Kuo, Wotango, Pan, Chen, Haregewoin, Cheng, Su and Hwang57 The incorporation of microporous carbon can effectively increase the electrical conductivity of the material by decreasing the resistance of sulfur which results in enhanced active material utilization. Simultaneously, mesoporous C-doped TiO2 nanotubes prevents the dissolution of polysulfide, and also improves the strength of the entire electrode, thereby enhancing the electrochemical performance. Yu et al., designed a TiO2–nitrogen doped graphene/sulfur (TiO2–NG/S) hybrid structures by atomic layer deposition (ALD) method. Reference Yu, Ma, Song, Wang, Tian, Wang, Qiu and Wang58 The nitrogen doped graphene was used as a conductive matrix and the TiO2 coating on the NG/S electrodes surface was used to inhibit lithium polysulphides shuttle. The performances of the electrodes with different cycled-TiO2 coating have been investigated. Particularly, the NG/S electrode with 20 TiO2 cycles coating illustrated a high initial specific capacity of 1070 mA h/g and a reversible capacity of 918 mA h/g even after 500 cycles at 1 C, showing excellent potential as a cathode material for Li–S batteries.
In addition to using TiO2 as the additive and host for the sulfur cathode, it is also used as an interlayer and a separator coating to enhance electrochemical performances. Reference Liang, Wu, Qin, Liu, Li, He, Kim, Li and Kang69–Reference Yao, Yan, Li, Zheng, Kong, Seh, Narasimhan, Liang and Cui73 A TiO2 nanowire-embedded graphene (TiO2 NW/G) hybrid membrane was prepared by Manthiram’s group. Reference Zhou, Zhao, Zu and Manthiram74 In this hybrid membrane, the graphene with high conductivity was used as the current collector, while the TiO2 NWs were used as a polysulphides shuttling inhibitor as well as the catalyst to accelerate the polysulfide reduction and oxidation. As a result, the Li2S6 catholyte with such a hybrid membrane interlayer showed a high specific capacity of 1327 mA h/g at 0.2 C rate, a Coulombic efficiency approaching 100% and a reversible capacity of 1053 mA h/g over 200 cycles. Recently, Fanqun Li and his co-workers developed a carbonized bacterial cellulose/titania (CBC/TiO2) modified separator. Reference Li, Wang, Wang, Yang, Zhang, Liu and Lai75 This TiO2 modified separator could restrain the shuttle effect of Li–S cells with strong physical and chemical adsorption of polysulphides.
What’s more, Ti4O7, another titanium oxide, has also been investigated due to its positive function in Li–S batteries. Reference Wei, Rodriguez, Best, Hollenkamp, Chen and Caruso76–Reference Pang, Kundu, Cuisinier and Nazar78 Nazar’s group firstly reported that Ti4O7 has a high affinity for lithium polysulphides due to the contained polar O–Ti–O units. Reference Pang, Kundu, Cuisinier and Nazar78 The presence of strong metal oxide-polysulfide interactions has been proved by X-ray photoelectron spectroscopy (XPS), X-ray absorption near-edge structure (XANES) and lithium polysulphides adsorption studies. Following on Cui’s group reported that, compared with the TiO2–S, the Ti4O7–S cathodes exhibited a higher reversible capacity and improved cycling performance. Reference Tao, Wang, Ying, Cai, Zheng, Gan, Huang, Xia, Liang, Zhang and Cui77 The superiorities of Ti4O7–S cathodes could be attributed to the strong adsorption of sulfur species on the low-coordinated Ti sites of Ti4O7, which was revealed by density functional theory (DFT).
As one of the most cost-effective, safe and controllable nanomaterials, TiO2 is a primary candidate for researchers’ investigation. Polysulphides are likely to be chemically bonded at oxygen defect sites and surface defect of TiO2. Reference Liu, Huang, Zhang and Mai19 However, due to the insulation of TiO2, a conductive agent, such as CNT, graphene, etc., should be introduced rationally to generate a synergistic effect to produce high energy density and long life span Li–S batteries. Meanwhile Ti4O7, due to its low-coordinated Ti, is a promising polar host for the complex multi-electron Li–S conversion reaction.
B. Mn x O y
MnO2 is usually characteristically nonstoichiometric and is deficient in oxygen atoms. Reference Liu, Huang, Zhang and Mai19 As stated above, TiO2 with oxygen defect sites and surface defects is beneficial to trap polysulphides, thus MnO2 is also a good candidate to fabricate a high performance S cathode. Reference Lee, Hwang, Lee, Lee and Choi14,Reference Zhang, Shi, Ding, Zhang and Yu43,Reference Hart, Cuisinier, Liang, Kundu, Garsuch and Nazar52,Reference Ni, Wu, Zhao, Sun, Zhou, Gong and Diao79–Reference Liang, Hart, Pang, Garsuch, Weiss and Nazar81 In 2014, Nazar’s group reported δ-MnO2 nanosheets, which served as the prototype, react with initially formed lithium polysulphides to form surface-bound intermediates. Reference Liang, Hart, Pang, Garsuch, Weiss and Nazar81 Different from TiO2 chemical retention for the polysulphides, MnO2 chemical retention relied on mediating polysulfide redox through insoluble thiosulfate species in a two-step process, where the thiosulfate groups are first created in situ by oxidation of initially formed soluble lithium polysulfide species on the surface of ultra-thin MnO2 nanosheets, and then the surface thiosulfate groups are proposed to anchor newly formed soluble ‘higher’ polysulphides by catenating them to form polythionates and converting them to insoluble ‘lower’ polysulphides. Because of such chemical retention mechanism, the sulfur/manganese dioxide nanosheet composite with 75 wt% sulfur exhibited a reversible capacity of 1300 mA h/g at 0.2 C and a fade rate over 2000 cycles of 0.036%/cycle at 2 C. From then on, nanowire α-MnO2-coated sulfur cathode, Reference Lee, Hwang, Lee, Lee and Choi14 core–shell sulphur-δ-MnO2 cathode, Reference Liang and Nazar80 and core–shell γ-MnO2-coated sulfur cathode, Reference Ni, Wu, Zhao, Sun, Zhou, Gong and Diao79 have been reported in succession.
Similar to TiO2, the conductivity of MnO2 is also not good. Therefore a considerable amount of conductive additives, such as the conductive polymer or conductive carbon, should be introduced to overcome the dead active material and achieve high performances. Reference Zhang, Shi, Ding, Zhang and Yu43,Reference Xu, Qie and Manthiram82–Reference Rehman, Tang, Ali, Huang and Hou85 Yu and her co-workers designed a S/Polypyrrole–MnO2 (S/PPy–MnO2) ternary nanostructure as shown in Fig. 2(a). Reference Zhang, Shi, Ding, Zhang and Yu43 Combining the advantages of conductive polypyrrole and MnO2, the S/Polypyrrole–MnO2 cathode with 70 wt% S content showed a reversible capacity of 550 mA h/g after 500 cycles with an extremely low decay rate of 0.07% per cycle at 1 C-rate as shown in Fig. 2(b). Lou’s group designed a hollow carbon nanofiber-MnO2 nanosheet-S (MnO2@HCF/S) nanostructure that the sulfur particle and MnO2 nanosheets were filled in the hollow carbon nanofiber as shown in Fig. 2(c). With such a unique structure, the MnO2@HCF hybrid host not only facilitated electron and ion transfer during the redox reactions, but also efficiently prevented polysulfide dissolution. As a result, the MnO2@HCF/S electrode, with 71 wt% S content in the composite and an area of sulfur mass loading of 3.5 mg/cm2, maintained a reversible capacity of 662 mA h/g at 0.5 C over 300 cycles as shown in Fig. 2(d).
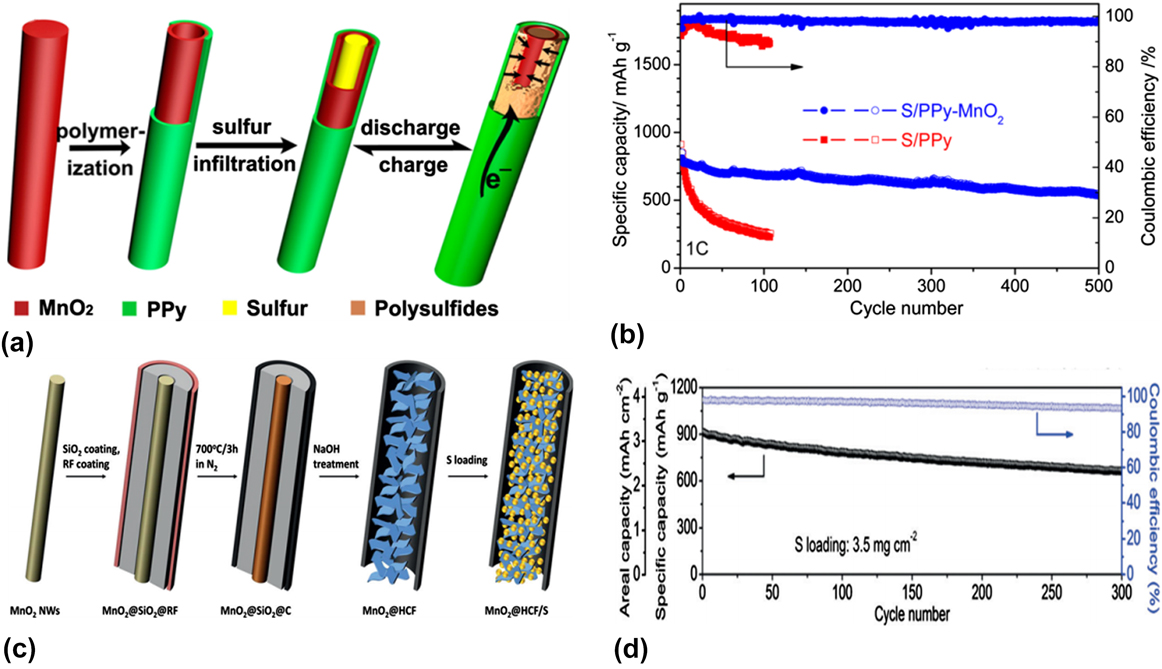
FIG. 2. (a) Illustration of the synthesis of S/PPy-MnO2 ternary composites. (b) Cycling performance of PPy-MnO2 nanotubes encapsulated sulfur electrode compared with pure PPy nanotubes encapsulated sulfur electrode at 1 C. Reproduced with permission from Ref. Reference Zhang, Shi, Ding, Zhang and Yu43, Copyright 2016, American Chemical Society. (c) Synthesis of the MnO2@HCF/S composite. (d) Prolonged cycling performance of MnO2@HCF/S at 0.5 C and the corresponding Coulombic efficiency. Reproduced with permission from Ref. Reference Li, Zhang and Lou84, Copyright 2015, Wiley-VCH.
Recently, MnO2 and graphene composites used as the interlayer for Li–S batteries have been reported. Reference Sun, Ou, Yue, Yang, Wang, Rooney and Sun86,Reference Guo, Zhao, Wu, Zhang, Xiang, Wang, Liu and Wu87 For example, Zhang et al., synthesized a free-standing MnO2 nanowires and graphene nanoscroll (GNSM) interlayer, where the weight ratio of graphene nanoscroll to MnO2 nanowires is 4:1. The insertion of the hybrid GNSM interlayer between sulfur cathode and separator could not only reduce the overall electrode resistance but also offer strong physical/chemical interactions for efficiently mitigating the shuttling of polysulphides, ensuring continuous reactivation and reutilization of the trapped sulfur active materials.
In addition, manganese monoxide (MnO) modified CNTs as sulfur host for improving the performance of Li–S batteries has been reported latterly. Reference An, Deng, Lei, Wu, Tian, Zheng and Dong88 The CNTs/MnO–S cathode showed a better cycling stability over 100 cycles than CNTs–S cathodes with a same carbon/sulfur weight ratio at around 1:8, which demonstrated MnO is a potential additive for sulfur cathode to address the insufficiencies of carbon hosts.
Similar to TiO2, MnO2 also shows strong chemical adsorption for the polysulphides. However, the mechanism of the MnO2 in the sulfur cathodes was not clearly elucidated, especially for the different polymorphs of MnO2, e.g., α-MnO2, δ-MnO2, γ-MnO2. In other words, the interactions between the polysulphides and MnO2 with different crystal phases should be further explored by theoretical and experimental investigations. Meanwhile other manganese based oxide, such as MnO, Mn2O3 could be a promising sulfur host candidate. However, the working mechanisms also need to be further understood.
C. V x O y
V2O5, has been attracting much attention, as it offers the essential advantages of low cost, abundant sources, and better safety relative to commercial cathodes such as LiCoO2 and LiNiO2. Reference Liu, Liu, Gao, Wu and Xue89 However, recently it has received increasing attention due to its strong interaction with polysulphides. Reference Liu, Li, Qin, Liang, Han, Zhou, He, Li and Kang90,Reference Li, Hicks-Garner, Wang, Liu, Gross, Sherman, Graetz, Vajo and Liu91 As early as 2009 and 2010, there were two works that reported V2O5–S composites as cathode materials for Li–S batteries. Reference Zhang, Wu, Feng, Wang, Zhang, Xia and Dong92,Reference Zhang, Wang, Zhang, Song, Li, Feng, Wu and Du93 V2O5–S composites illustrated better electrochemical performances compared to the pure sulfur cathode, wherein the researchers attributed such improvements to the addition of V2O5 which could decrease the resistance of the composite electrode. Reference Zhang, Wang, Zhang, Song, Li, Feng, Wu and Du93 Following on, Oh et al., Reference Kim, Shin, Kim, Cho and Oh94 found the V2O5/carbon nano-composite as an additive to the Li2S6 polysulfide solution was highly effective at capturing these long-chain polysulphides on its surface. As a result, Liu and his co-workers directly used micrometer-scale V2O5 layer as the interlayer for Li–S batteries. Reference Li, Hicks-Garner, Wang, Liu, Gross, Sherman, Graetz, Vajo and Liu91 A 5 mA h pouch cell with V2O5 interlayer could cycle 300 times over 1 year without noticeable degradation. Additionally a V2O5-decorated carbon nanofiber interlayer for suppressing self-discharge and shuttle effect has also been reported recently. Reference Liu, Li, Qin, Liang, Han, Zhou, He, Li and Kang90
What’s more, Nazar’s group found that by coating a layer of VO x onto CMK-3-S composites, the electrochemical reduction of polysulphides on the cathode surface is inhibited. Reference Lee, Black, Yim, Ji and Nazar95 Manthiram’s group tried to incorporate VO2(B) into sulfur cathodes to improve the performances but finally found VO2(B) was incompatible with the glyme-based electrolytes that are usually used in Li–S cells. Reference Su and Manthiram96
D. SnO x
SnO2, was found to be a better conductive material than most other oxides in porous shell morphologies, Reference Zhang, Wang, Gou and Zeng97 therefore SnO2 shells with micromesopores were synthesized to load the sulfur inside by Zhang. Reference Zhang, Wang, Gou and Zeng97 The core–shell S/SnO2 composites with 66 wt% S content exhibited a high initial capacity of 1176 mA h/g at 0.5 C and retained a capacity of 736.6 mA h/g after 50 cycles. To further enhance the conductivity and thus enhance the performance, double-shell SnO2@C hollow nanospheres were designed to encapsulate sulfur by Cao. Reference Cao, Li, Hou, Mo, Yin and Chen98 The S/SnO2@C composite illustrated a high reversible capacity of 616 mA h/g at 3200 mA/g after 100 cycles.
Recently, Lee’s group firstly reported multi-walled carbon nanotubes (MWCNT) filled with ordered tin-monoxide (SnO) nanoparticles as the sulfur host for high-rate lithium–sulphur batteries. Reference Kim, Kim, Kim, Wen, Gu, Dao, Choi, Byun and Lee99 The resulting MWCNT–SnO/S composite cathodes even exhibited a high capacity of 257.7 mA h/g at an extremely high current rate of 20 C. Such an excellent rate capability could be attributed to the dipole–dipole interaction between the SnO and polysulphides as well as the MWCNT–SnO host accommodating the volume expansion, protecting the sulfur from dissolution, and enhancing the electrical conductivity. Reference Kim, Kim, Kim, Wen, Gu, Dao, Choi, Byun and Lee99
E. Al2O3
Al2O3 is an electrochemically inactive and ceramic material with a low cost and natural abundance. Reference Jing, Kong, Liu, Li and Gao11 It was firstly applied as the additive in the sulfur cathode to improve the electrochemical performances of Li–S batteries. Reference Choi, Jung, Lee, Jeong, Kim, Ahn, Cho and Gu100,Reference Dong, Wang, Zhang and Wu101 Later Al2O3 was applied as the coating layer on both sulfur cathodes Reference Han, Xu, Chen, Chen, Weadock, Wan, Zhu, Liu, Li, Rubloff, Wang and Hu102–Reference Yu, Yuan, Li, Hong and Shi104 and separators, Reference Li, Jing, Su, Lai, Chen, Yuan, Li and Xiang12,Reference Yao, Yan, Li, Zheng, Kong, Seh, Narasimhan, Liang and Cui73,Reference Zhang, Lai, Zhang, Zhang and Li105 which has proved to be effective in enhancing the cycle stability of Li–S batteries. For instance, Shi’s group coated an ultrathin Al2O3 film via ALD on the graphene–sulphur (G–S) composite. Reference Yu, Yuan, Li, Hong and Shi104 The ALD-Al2O3 coated-G–S composite cathode delivered a high specific capacity of 646 mA h/g after 100 cycles at 0.5 C, which was about twice that of the bare G–S composite. Subsequently, Song et al., applied the Al2O3 and graphene to modify the polypropylene (GPA) separator for application in Li–S batteries as shown in Fig. 3. Reference Song, Fang, Wen, Shi, Wang and Li106 The Al2O3 coating could further enhance the thermal stability and safety of the graphene coated polypropylene (GCP) separator.
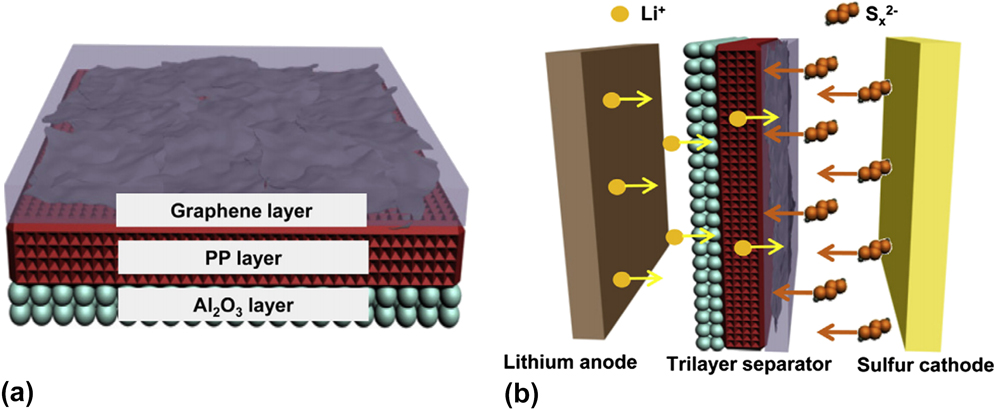
FIG. 3. Schematic illustration of (a) the structure of GPA separator, and (b) Li–S battery with GPA separator. Reproduced with permission from Ref. Reference Song, Fang, Wen, Shi, Wang and Li106, Copyright 2016, Elsevier.
Recently, porous Al2O3 was applied to protect the lithium anode by using a spin-coating method. Reference Jing, Kong, Liu, Li and Gao11 The Al2O3 protective layer can restrict the side reactions between soluble lithium polysulphides and the lithium anode through a combination of physical separation and chemical adsorption by the Al2O3 layer, which can alleviate lithium corrosion due to polysulfide attack.
The ALD method is commonly used to deposit a very thin Al2O3 layer on the sulfur cathode or separator. The Al2O3 layer could not only enhance thermal stability of Li–S batteries, but also can decrease the risk of short circuits. Reference Song, Fang, Wen, Shi, Wang and Li106 However, the mechanism of Al2O3-polysulfide chemical bonding still needs to be studied.
F. ZnO
Zinc oxide (ZnO) is a nontoxic n-type semiconductor with a wide band gap of 3.37 eV. Reference Li, Yang, Xu, Xiaoqiang, Yang, Wei, Ren, Shen and Han107 Due to the large exciton binding energy of 60 meV and the high mechanical, and thermal stabilities, ZnO is attractive for high-efficiency short-wavelength optoelectronic nanodevices. Reference Li, Yang, Xu, Xiaoqiang, Yang, Wei, Ren, Shen and Han107 However, recently, ZnO attracts much attention as adsorbent for migrating polysulphides due to strong chemical bonding. Reference Gu, Tong, Wen, Liu, Lai and Zhang13,Reference Zhao, Ye, Peng, Divitini, Kim, Lao, Coxon, Xi, Liu, Ducati, Chen and Kumar108–Reference Liang, Song, Liu and Liu110 Our group used ball-milling method to coat ZnO on the CNT–S cathode to improve the performances. Reference Gu, Tong, Wen, Liu, Lai and Zhang13 The results showed that ZnO coated-CNT–S cathode illustrated much better cycling stability than that of CNT–S composites. While Gaoquan Shi’s group used ALD method to coat ZnO on reduced graphene oxide (RGO)–sulphur composite. Reference Zhao, Ye, Peng, Divitini, Kim, Lao, Coxon, Xi, Liu, Ducati, Chen and Kumar108 With the ZnO coating, the RGO–S composites exhibited high discharge capacity, excellent cycling stability and good rate-performance.
Recently, inspired by the brush-like membrane of cells for nutrient adsorption, a similar structure of interlayer consisting of ZnO nanowires and conductive frameworks as shown in Fig. 4 has been designed for chemical adsorption of polysulphides by Kumar and his co-workers. Reference Zhao, Ye, Peng, Divitini, Kim, Lao, Coxon, Xi, Liu, Ducati, Chen and Kumar108 The S/MWCNTs composite cathode with a ZnO/C interlayer exhibited a reversible capacity of 776 mA h/g after 200 cycles at 1 C with only 0.05% average capacity loss per cycle.
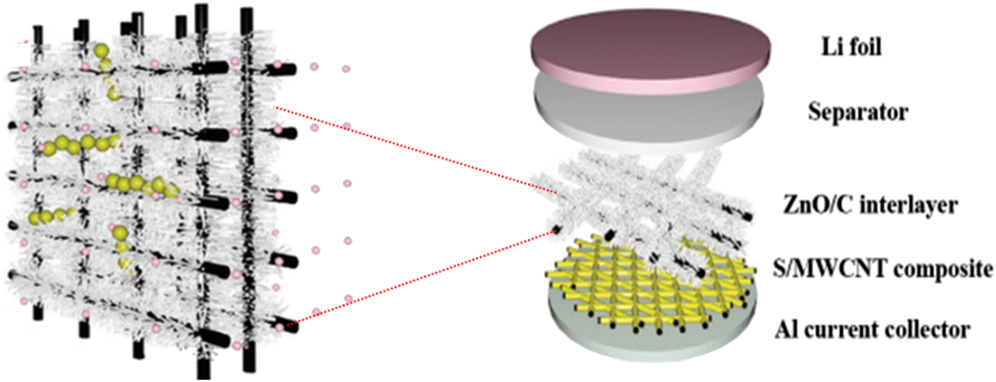
FIG. 4. Schematic of the structural and chemical function of the hybrid ZnO nanowires/carbon nanofibers interlayer in Li–S batteries. Reproduced with permission from Ref. Reference Zhao, Ye, Peng, Divitini, Kim, Lao, Coxon, Xi, Liu, Ducati, Chen and Kumar108, Copyright 2016, Wiley-VCH.
G. Other metal oxide
Intrinsically polar metal oxides, which can interact with polar lithium polysulphides, have been widely used to modify the sulphur-based cathodes, such as representative Mg0.8Cu0.2O, Reference Zhang, Wu, Feng, Wang, Zhang, Xia and Dong92 Mg0.6Ni0.4O, Reference Tang, Yao, Jing, Wu, Hou, Qian, Rao, Shen, Xi and Xiao111–Reference Song, Han, Kim, Kim, Kim, Kang, Ahn, Dou and Lee113 Li4Ti5O12, Reference Zhao, Liu, Lv, He, Wang, Yun, Li, Kang and Yang114 BaTiO3, Reference Xie, You, Yuan, Lu, Zhang, Xu, Ye, Ke, Shen, Zeng, Fan and Wei115 LiFePO4, Reference Kim, Guerfi, Hovington, Trottier, Gagnon, Barray, Vijh, Armand and Zaghib116 NiFe2O4, Reference Fan, Liu, Weng, Sun and Wang117 Co3O4, Reference Wang, Zhou, Li, Gao, Gao, Du, Liu and Guo118,Reference Cheng, Wang, Tao and Wang119 ZrO2, Reference Wan, Wu, Wu, Xu and Guan120,Reference Zhou, Zhou, Li, Yan, Han, Xia, Gao and Wu121 MoO2, Reference Qu, Gao, Zheng, Wang, Li, Li, Chen, Han, Shao and Zheng122 MoO3, Reference Ma, Liu and Wang123 Nb2O5, Reference Tao, Wei, Wang, Liu, Qiao, Ling and Long124 W18O49, Reference Zhang, Lin, Cong, Hou, Liu, Geng, Jin, Wu and Zhao125 MgO, Reference Tao, Wang, Liu, Wang, Yao, Zheng, Seh, Cai, Li, Zhou, Zu and Cui33,Reference Ponraj, Kannan, Ahn and Kim126 CeO2, Reference Tao, Wang, Liu, Wang, Yao, Zheng, Seh, Cai, Li, Zhou, Zu and Cui33,Reference Ma, Wen, Wang, Jin, Wu and Zhang127 Fe2O3, Reference Zhao, Shen, Xin, Sun and Han128 La2O3, Reference Tao, Wang, Liu, Wang, Yao, Zheng, Seh, Cai, Li, Zhou, Zu and Cui33,Reference Sun, Wang, Long, Qiao, Ling, Lv and Cai129 CaO, Reference Tao, Wang, Liu, Wang, Yao, Zheng, Seh, Cai, Li, Zhou, Zu and Cui33 In2O3, Reference Yao, Zheng, Hsu, Kong, Cha, Li, Seh, McDowell, Yan, Liang, Narasimhan and Cui130 etc. Most of them are coupled with conductive polymers or carbon materials to overcome the inferior conductivity of both themselves and sulfur. For example, Zhou et al., Reference Zhou, Zhou, Li, Yan, Han, Xia, Gao and Wu121 incorporated a fine amount of ZrO2 to the holey CNT–sulphur composites. The holey CNT contributed to the good conductivity of the h-CNT/S/ZrO2 cathode, while appropriate ZrO2 loading preserved the permselective channels for Li+ intercalation/deintercalation and trapped the soluble polysulphides.
These various metal oxides used in Li–S batteries provide a new strategy for anchoring polysulphides. However, which group of metal oxides as well as the appropriate size and morphologies of the metal oxides that are most beneficial to trap the polysulfides should be further investigated. Further research is also needed to find whether the capture mechanism by various metal oxides is achieved via either monolayered or multilayered chemisorption.
III. NANOSTRUCTURED METAL HYDROXIDES APPLICATION IN LI–S BATTERIES
Metal hydroxides, filled with copious functional polar/hydrophilic groups (e.g., hydrophilic groups, surface hydroxyl groups, and so on), Reference Jiang, Zhu, Ai, Wang, Wang, Zou, Huang and Yu131 are another promising class of encapsulation materials to build better Li–S cells.
More recently, thin layered metal hydroxides, including Co(OH)2, Reference Niu, Wang, Wang, Li, Zhang, Zhang, Yang, Yu and Tu132 Co(OH)2/layered double hydroxides, Reference Zhang, Hu, Li and Lou133 Ni(OH)2, Reference Gu, Tong, Wen, Liu, Lai and Zhang13,Reference Niu, Wang, Xie, Wang, Zhang, Li, Yu and Tu134 and Ni3(NO3)2(OH)4 Reference Jiang, Zhu, Ai, Wang, Wang, Zou, Huang and Yu131 have been used as effective encapsulation materials for sulfur cathodes. In one case, Lou’s group designed double-shelled nanocages with two shells of cobalt hydroxide and layered double hydroxides (CH@LDH) as a conceptually new sulfur host, as shown in Fig. 5(a). Reference Zhang, Hu, Li and Lou133 Such a hollow CH@LDH polyhedra with complex shell structures not only maximize the advantages of hollow nanostructures for encapsulating a high content of sulfur (75 wt%), but also provide sufficient self-functionalized surfaces for chemical bonding with polysulphides to suppress their outward dissolution. As a result, the resulted CH@LDH/S electrode with relatively high sulfur loading of 3 mg/cm2 showed excellent cycling stability at both 0.1 and 0.5 C over 100 cycles, much better than the reference C/S cathode as shown in Fig. 5(b).
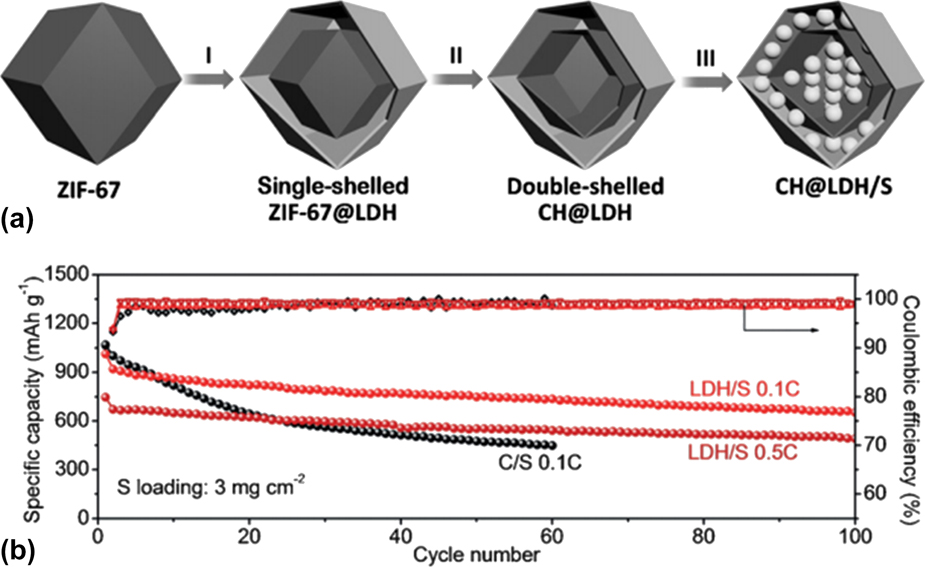
FIG. 5. (a) Schematic illustration of the synthesis of the CH@LDH/S composite. (b) Cycle performance comparison between CH@LDH/S and C/S. Reproduced with permission from Ref. Reference Zhang, Hu, Li and Lou133, Copyright 2016, Wiley-VCH.
The transition-metal hydroxides as coatings for the sulphur-based cathodes could effectively trap the polysulphides, but the mechanisms in which the transition-metal hydroxides bind with polysulphides should be further investigated to open a new avenue for future development of high performance Li–S batteries.
IV. NANOSTRUCTURED METAL SULPHIDES APPLICATION IN LI–S BATTERIES
A. TiS2
Titanium disulfide (TiS2) is known as a cathode material in the first generation rechargeable lithium batteries. Reference Garsuch, Herzog, Montag, Krebs and Leitner135 Due to its working voltage between 1.7 and 2.5 V, very similar to the voltage range of Li–S cells, it has become a second active component in sulfur cathode. Reference Garsuch, Herzog, Montag, Krebs and Leitner135 By adding the layered TiS2 into the carbon-S cathodes, the power capability of Li–S cells has obviously improved. Reference Su and Manthiram96,Reference Garsuch, Herzog, Montag, Krebs and Leitner135
Recently, Archer’s group developed a foam TiS2 and used it to encapsulate elemental sulfur. Reference Ma, Wei, Zhuang, Hendrickson, Hennig and Archer40 This 3D hybrid cathode demonstrated high areal specific capacity (9 mA h/cm2) and high retention even at a relatively large areal mass loading of approximately 40 mg sulfur per cm2 and high current density (10 mA/cm2). Additionally, Cui’s group used a two dimensional (2D) layered TiS2 to encapsulate Li2S. Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35 As a result, the core–shell Li2S@TiS2 nanostructure was obtained. It also showed a very high area capacity of 3.0 mA h/cm2 under high mass Li2S loading (5.3 mg Li2S per cm2) and high-C rate conditions (4 C). That is because the TiS2 possesses a combination of high conductivity and polar Ti–S groups that can potentially interact strongly with Li2S/Li2S n species. Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35,Reference Ma, Wei, Zhuang, Hendrickson, Hennig and Archer40 DFT analysis and ab initio simulations demonstrated the binding energy between Li2S and TiS2 was 10 times higher than that between Li2S and carbon-based graphene. Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35,Reference Ma, Wei, Zhuang, Hendrickson, Hennig and Archer40
B. Co x S y
Cobalt sulphides commonly show unique metallic or half-metallic characteristics, which means they exhibit particularly high room temperature conductivity. Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41,Reference Pang, Kundu and Nazar42,Reference Ma, Li, Hu, Liu, Huo and Wang136 Therefore the cobalt sulphides could afford efficient electron pathways and high electrocatalytic activity for polysulfide redox reactions in aqueous solutions. Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41 Simultaneously cobalt sulphides can significantly enhance the redox reactivity of lithium polysulphides due to their strong chemical affinity. Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41,Reference Pang, Kundu and Nazar42
For example, Zhang’s group selected a cost-effective, nonporous mineral and bulk CoS2 as the additive to the carbon/sulfur composite cathodes. Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41 The adsorption experiment as shown in Fig. 6(a) and the First-principle calculations based on DFT as shown in Fig. 6(b) both proved the CoS2 showed far stronger affinities to the Li2S4 compared to graphene. Meanwhile Nazar’s group reported a metallic Co9S8 with an interconnected graphene-like nano-architecture as the polysulphides host. Reference Pang, Kundu and Nazar42 The first-principles calculations coupled with spectroscopic evidence also demonstrated the synergistic strong dual-interactions of polysulphides with Co9S8.
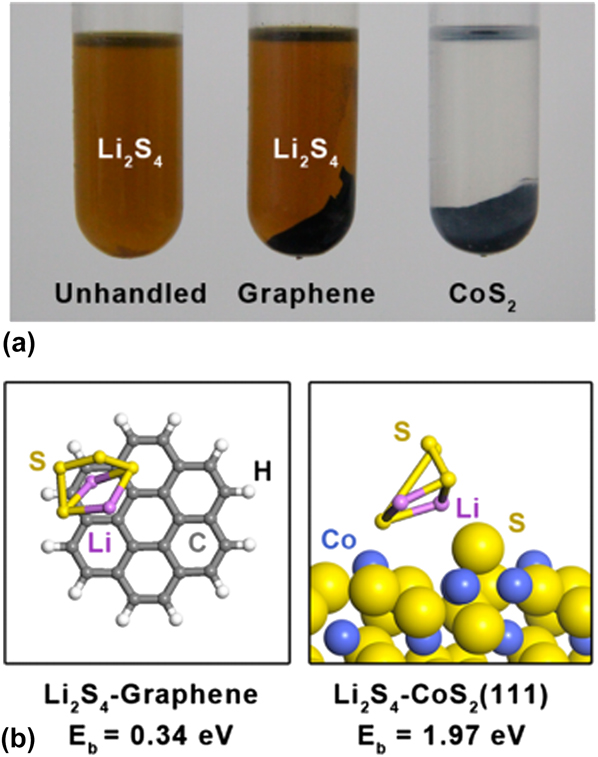
FIG. 6. (a) Visualized adsorption of Li2S4 on graphene and pristine CoS2 with the same surface area. (b) Binding geometries and energies of a Li2S4 molecule on graphene (left, modeled as coronene) and (111) plane of CoS2 with cobalt-terminated surface (right), which is derived from theoretical calculation based on DFT. Reproduced with permission from Ref. Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41, Copyright 2016, American Chemical Society.
C. MoS2
MoS2, has a typical layered structure and the spacing between the neighboring layers for bulk MoS2 is about 0.615 nm, which is significantly larger than that of graphite (0.335 nm). Reference Zhang, Yu, Yu, Chen, Gao, Li and Zhu137 It is a good candidate to encapsulate the sulfur. Furthermore, the MoS2 showed strong interaction with polysulphides, Reference Li, Zhao, Zhang, Ding and Hu138–Reference Dirlam, Park, Simmonds, Domanik, Arrington, Schaefer, Oleshko, Kleine, Char, Glass, Soles, Kim, Pinna, Sung and Pyun141 which makes it more attractive for use in Li–S batteries.
Cui’s group made a significant breakthrough on how MoS2 binds with Li2S. They used ab initio simulations to choose the terrace and edge sites of MoS2 for binding Li2S. Reference Wang, Zhang, Yao, Liang, Lee, Hsu, Zheng and Cui140 The calculation results showed that the binding energy between Li2S with MoS2 terrace site was ∼0.87 eV, slightly higher than the case of graphene (0.29 eV). While the edge sites, including Mo-edge and S-edge, binding energies were 4.48 and 2.70 eV, respectively, suggesting a large selectivity of edge versus terrace sites. In addition the Li2S was superior to bind with Mo-edge sites. Such a new discovery becomes important in metal sulphides and metal oxides materials synthesis for Li–S batteries.
D. Other metal sulphides
Apart from the metal sulphides illustrated above, various other metal sulphides, such as SnS2, Reference Li, Lu, Hou, Zhang, Zhu, Qian, Liang and Qian142,Reference Li, Chu, Wang and Pan143 WS2, Reference Lei, Chen, Huang, Yan, Sun, Wang, Zhang, Li and Xiong144 MnS, Reference Liu, Zheng, Shi and Zhang145 FeS2, Reference Zhang and Tran146 FeS, Reference Zhou, Tian, Jin, Tao, Liu, Zhang, Seh, Zhuo, Liu, Sun, Zhao, Zu, Wu, Zhang and Cui36 Ni2S3, Reference Zhou, Tian, Jin, Tao, Liu, Zhang, Seh, Zhuo, Liu, Sun, Zhao, Zu, Wu, Zhang and Cui36 NiS2, Reference Lu, Li, Liang, Hu, Zhu and Qian147 VS2, Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35,Reference Zhou, Tian, Jin, Tao, Liu, Zhang, Seh, Zhuo, Liu, Sun, Zhao, Zu, Wu, Zhang and Cui36 CuS, Reference Sun, Su, Zhang, Bock, Marschilok, Takeuchi, Takeuchi and Gan148 Cu3BiS3, Reference Gao, Wang, Ma, Jiang, Zou and Lu149 ZrS2, Reference Seh, Yu, Li, Hsu, Wang, Sun, Yao, Zhang and Cui35 and so on, have also been investigated as polar hosts to reveal the key parameters correlated to the energy barriers and polysulfide adsorption capability in Li–S batteries. Even though most of the metal sulphides have better conductivity compared to the metal oxides, it still can’t meet the demand for addressing internal resistance in the electrode, which is highly related to effective use of active materials. Reference Liu, Huang, Zhang and Mai19 Thus various nanocarbon materials were combined with the metal sulphides to further improve the active materials utilization. For instance, WS2 nanosheets grown on the carbon nanofiber was used as the sulfur host. Reference Lei, Chen, Huang, Yan, Sun, Wang, Zhang, Li and Xiong144 By combining the advantages of carbon nanofiber (excellent electronic transport of the 3D structure) and WS2 (polar adsorption of polysulphides), the resulted C@WS2/S composite still maintained with a high specific capacity of 502 mA h/g at 2 C, about 90% of its initial specific capacity.
Although adding metal sulphides into S-carbon based cathodes has remarkably enhanced the electrochemical performance, there still exists a gap between practical applications. Additionally most of the adsorption mechanism for polysulphides on metal sulphides are still not clearly and need to be deeply investigated.
V. NANOSTRUCTURED METAL CARBIDE APPLICATION IN LI–S BATTERIES
Metal carbide are an exciting family of transition-metal carbides, Reference Liang, Garsuch and Nazar37,Reference Huang, Zhang, Xu, Abouali, Akbari Garakani, Huang and Kim150,Reference Sim, Yi, Je, Lee and Chung151 which are inherently highly conductive and possess a highly active 2D surfaces to chemically bond to intermediate polysulphides by metal–sulphur interactions. Reference Liang, Garsuch and Nazar37
Nazar’s group first reported a new class of sulfur host materials—conductive MXene Ti2C. Reference Liang, Garsuch and Nazar37 The MXene Ti2C, not only displays the characteristic high 2D electron conductivity of transition-metal carbides (much higher than GO), but also its exposed terminal metal sites can bind to the sulphides as revealed by X-ray photoelectron spectroscopy (XPS) analysis, with the corresponding mechanism scheme shown in Fig. 7. Reference Liang, Garsuch and Nazar37 Due to these superiorities, Ti2C was highly effective as a sulfur host material for Li–S batteries, providing very stable cycling performance and high capacity even with 70 wt% S.
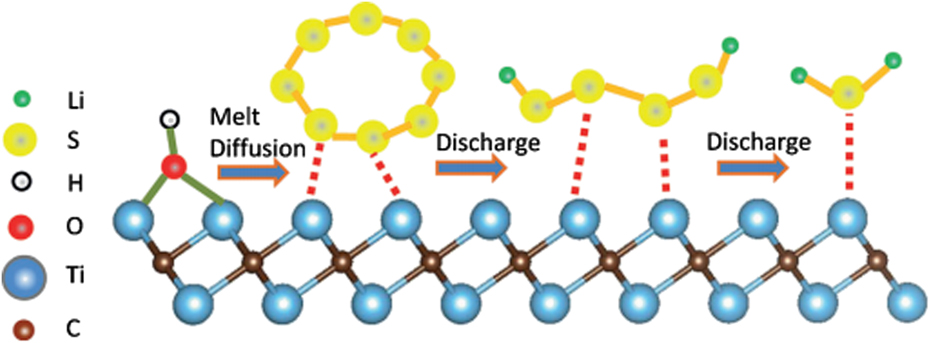
FIG. 7. Replacement of the Ti–OH bond on the MXene surface with a S–Ti–C bond on heat treatment or by contact with polysulfides. Reproduced with permission from Ref. Reference Liang, Garsuch and Nazar37, Copyright 2015, Wiley-VCH.
From then on, the MXene Ti3C2, Reference Zhao, Liu, Chen, Hou, Zhang, Chen, Yang, Chen, Li and An152 Fe3C, Reference Huang, Zhang, Xu, Abouali, Akbari Garakani, Huang and Kim150 and TiC Reference Peng, Zhang, Chen, Zhang, Xu, Huang and Zhang153 as the sulfur host have been subsequently reported. All these transition-metal carbides demonstrated the strong interatomic attraction for the polysulphides.
VI. NANOSTRUCTURED METAL NITRIDE APPLICATION IN LI–S BATTERIES
Metal nitrides couple the advantages of high electrical conductivity (batter than metal oxide and carbon) and the excellent chemical stability owing to the formation of an oxide passivation layer, Reference Cui, Zu, Zhou, Manthiram and Goodenough38 which enable them to become potential host materials of sulfur.
Goodenough’s group reported the use of mesoporous TiN to encapsulate sulfur by a melt-diffusion. Reference Cui, Zu, Zhou, Manthiram and Goodenough38 The resulting mesoporous TiN–S cathode delivered much better cycling stability and rate capability than both mesoporous TiO2–S and Vulcan C–S cathode. The excellent overall electrochemical performance of a TiN–S cathode can be attributed to the good conductivity, robust framework of TiN, and the strong interactions between TiN and polysulphides. Reference Cui, Zu, Zhou, Manthiram and Goodenough38 Not long ago, Li and his co-workers reported a 3D porous vanadium nitride/graphene (VN/G) composite as a chemical bridle for the polysulphides. Reference Sun, Zhang, Yin, Hu, Fang, Cheng and Li154 The anchoring effect of vanadium nitride was confirmed by both experimental and theoretical results as shown in Fig. 8. It can be clearly observed that the absorption peak of Li2S6 in the visible light remained in the solution with the addition of RGO but disappeared when adding the VN/G [Fig. 8(a)], indicating the strong adsorption of Li2S6 molecules to polar VN. The First-principles calculations based on DFT demonstrated the binding energy between Li2S6 and VN was 3.75 eV, Reference Sun, Zhang, Yin, Hu, Fang, Cheng and Li154 much higher than that on pyridinic N-doped graphene. And the strong polar–polar interaction between Li2S6 and VN resulted in an obvious deformation of the Li2S6 molecule [Fig. 8(c)] compared to that of pyridinic N-doped graphene [Fig. 8(b)].
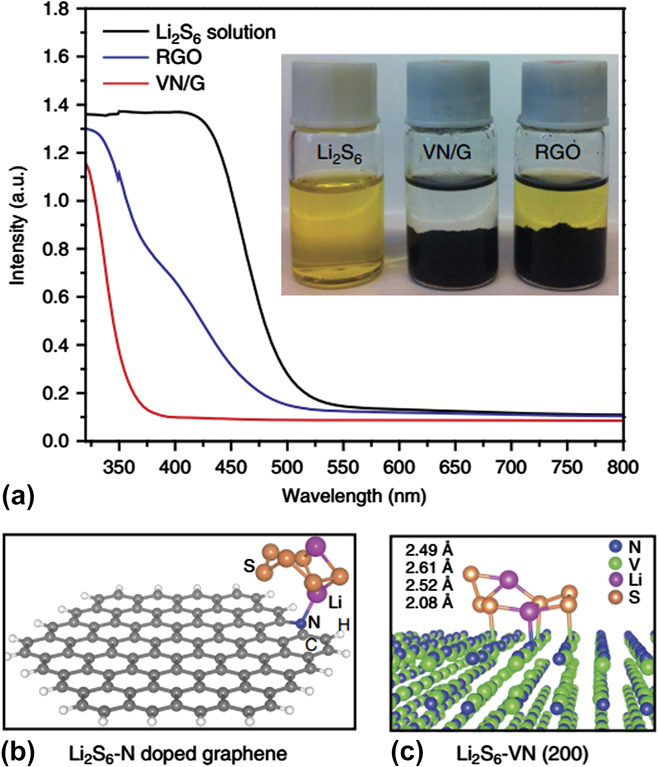
FIG. 8. Demonstration of the strong interaction of VN/G composite with polysulphides. (a) Ultraviolet/visible absorption spectra of a Li2S6 solution before and after the addition of RGO and VN/G. Inset image shows a photograph of a Li2S6 solution before and an 2 h after the addition of graphene and VN/G. (b) Side view of a Li2S6 molecule on a nitrogen-doped graphene surface, the binding energy between Li2S6 and pyridinic N-doped graphene is calculated to be 1.07 eV. (c) Side view of a Li2S6 molecule on VN(200) surface, the binding energy between Li2S6 and VN is calculated to be 3.75 eV. Reproduced with permission from Ref. Reference Sun, Zhang, Yin, Hu, Fang, Cheng and Li154, Copyright 2017, Nature Publishing Group.
The pioneered works on TiN, VN, and Mo2N have shown the improved performances of Li–S batteries by their high conductivity and strong chemical anchor functions, Reference Cui, Zu, Zhou, Manthiram and Goodenough38,Reference Sun, Zhang, Yin, Hu, Fang, Cheng and Li154,Reference Mosavati, Salley and Ng155 which opens a new direction of metal nitrides for energy storage.
VII. NANOSTRUCTURED MOFs APPLICATION IN LI–S BATTERIES
MOFs are a class of porous materials assembled by connecting metal ions and organic linkers with tremendous extensiveness in variety and multiplicity. Reference Zhou, Li, Fan, Chen, Han, Li, Zheng, Wang and Li156 They usually have even richer pore structure and larger specific surface area than porous carbon. Reference Bao, Zhang, Qu, Zhou, Wang and Li157 Thus, Tarascon pioneered the use of the MOF named MIL-100(Cr) as host material for sulfur impregnation. Reference Demir-Cakan, Morcrette, Nouar, Davoisne, Devic, Gonbeau, Dominko, Serre, Ferey and Tarascon158 However, the confinement of polysulphides was mainly attributed to the bimodal pores of MIL100(Cr) rather than weak binding by the oxygenated framework even though the cycling performances of sulfur cathode were improved. Since then, various groups have demonstrated that other MOFs can be adopted. Reference Zheng, Tian, Wu, Gu, Xu, Wang, Gao, Engelhard, Zhang, Liu and Xiao39,Reference Zhou, Li, Fan, Chen, Han, Li, Zheng, Wang and Li156,Reference Bao, Zhang, Qu, Zhou, Wang and Li157,Reference Hou, Mao and Xu159–Reference Zhao, Wang, Liang, Li, Shi and Chen161
Among which, Xiao’s group proposed a mechanism of Lewis acid–base interactions between polysulphides and MOF. Reference Zheng, Tian, Wu, Gu, Xu, Wang, Gao, Engelhard, Zhang, Liu and Xiao39 They used the Ni-MOF with interwoven mesopores (∼2.8 nm) and micropores (∼1.4 nm) as an ideal matrix to confine polysulphides. The Ni-MOF is constructed with coordinated Ni(II), which is a moderate/soft Lewis acid. The Lewis acidic Ni(II) center is inclined to coordinate with soluble polysulphides (S x 2−) anion (soft Lewis base) as axial ligand, which could effectively capture the soluble polysulphides within the cathode. To better understand the mechanism, the First-principles calculations based on DFT were performed by their group. The calculation results showed that only the S atom on one end point of polysulfide chain bound to the Ni-MOF with the corresponding interaction mechanism scheme shown in Fig. 9(a). Further binding energy calculation demonstrated the binding energies between Ni-MOF and polysulphides were higher than those between Co-MOF and polysulphides as shown in Fig. 9(b), which is consistent with the experiment results.
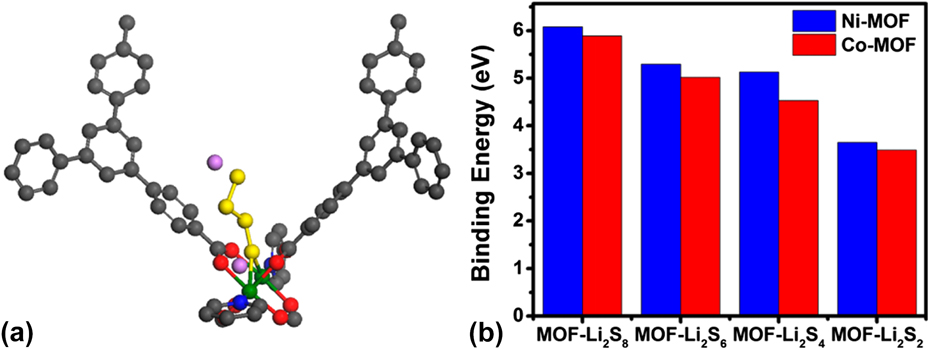
FIG. 9. (a) Schematic diagram illustrating the interaction between polysulfides (e.g., Li2S8/Li2S6/Li2S4, and so forth) and paddle-wheel unit in NiMOF. C, O, N, S, Li, and Ni atoms are represented by gray, red, blue, yellow, pink, and green spheres, respectively. (b) Comparison of binding energies of lithium polysulfides to Ni-MOF or Co-MOF. Reproduced with permission from Ref. Reference Zheng, Tian, Wu, Gu, Xu, Wang, Gao, Engelhard, Zhang, Liu and Xiao39, Copyright 2016, American Chemical Society.
The fundamental understanding from Lewis acid–base interactions may provide new insights and opportunities to develop MOFs application in Li–S battery technology.
VIII. SUMMARY AND PERSPECTIVE
In summary, this comprehensive review has systematically demonstrated nearly all of the recent metal compounds from single-functional conductive additives to multifunctional key components in the Li–S battery. Although it is hard to foresee what the best metal compounds for Li–S batteries exactly will be, some key features can still be predicted based on current research and knowledge. First of all, the metal compounds should have strong interactions with polysulphides. According to the relative research by Nazar, Reference Liang, Kwok, Lodi-Marzano, Pang, Cuisinier, Huang, Hart, Houtarde, Kaup, Sommer, Brezesinski, Janek and Nazar162 the metal oxides with a moderate voltage (versus Li/Li+), which form surface-bound thiosulfate via redox, are the most suitable for chemically binding polysulphides. TiO2, and MnO2 both display these characteristics. Secondly, when polysulphides are anchored on a conductive substrate, they receive electrons more easily which accelerates the kinetics of the polysulphide redox reactions. Thus, metal compounds with good conductivity, such as CoS2 (6700 S/cm), Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41 TiC (104 S/cm), Reference Peng, Zhang, Chen, Zhang, Xu, Huang and Zhang153 are better candidates for Li–S batteries. However, most metal compounds should still be coupled with conductive polymers or carbon materials to enhance the overall conductivity of the cathode rather than relying on the intrinsic conductivity to attain the best service performances in the Li–S batteries. Thirdly polar metal compounds, which can also serve as the electrocatalyst to facilitate the polysulphides redox reactions and increase the active materials utilization is preferable, of which VN, Reference Sun, Zhang, Yin, Hu, Fang, Cheng and Li154 CoS2, Reference Yuan, Peng, Hou, Huang, Chen, Wang, Cheng, Wei and Zhang41 and MnO2 Reference Liang, Hart, Pang, Garsuch, Weiss and Nazar81 have been reported. Finally, the nanostructures of metal compounds, i.e., surface area, pore size, pore volume, as well as particle size are also important factors which influence the performance of Li–S batteries. Reference Liu, Huang, Zhang and Mai19 Generally the metal compounds with smaller size, Reference Liang, Wu, Qin, Liu, Li, He, Kim, Li and Kang69 high surface area and pore volume, Reference Cao, Li, Hou, Mo, Yin and Chen98 and abundant micro/mesopores Reference Li, Li, Li, Zhang, Dong and Yin49,Reference Zegeye, Kuo, Wotango, Pan, Chen, Haregewoin, Cheng, Su and Hwang57 are beneficial to the cathode.
Even though tremendous progress in improving the electrochemical performance and understanding the reaction mechanisms of Li–S batteries have been achieved, Li–S batteries are far from being ready for practical use at this stage. Most of the reports on metal compounds-sulphur-based cathodes displayed an areal sulfur loading of 0.3–1.5 mg/cm2, Reference Liu, Huang, Zhang and Mai19 which is far less than the requirement of 5.0 mg/cm2 for practical cells with high energy density of more than 300 W h/kg. Reference Liu, Huang, Zhang and Mai19,Reference Hagen, Hanselmann, Ahlbrecht, Maça, Gerber and Tübke163,Reference Lv, Zheng, Li, Xie, Ferrara, Nie, Mehdi, Browning, Zhang, Graff, Liu and Xiao164 Although some of the carbon–S cathode with an areal sulfur loading has exceeded 5 mg/cm2, Reference Fang, Zhao, Hou, Cheng, Wang, Cheng, Liu and Li165–Reference Fang, Zhao, Pei, Qian, Hou, Cheng, Liu and Li167 the cycling stability is not ideal. Therefore discovering how to balance the high tap density of metal compounds with the good conductivity of carbon in the nanostructured sulfur cathode is required to realise the practical applications. The 3D hybrid electrode with heteroatoms doped/functioned carbon and porous/hollow polar metal compounds is promising to fabricate Li–S cells with high energy density and long cycle life. It is believed that Li–S batteries could be a promising and practical technology for the applications in transportation and large-scale grid energy storage in the near future.
ACKNOWLEDGMENTS
This work has been supported by the National Natural Science Foundation of China (Nos. 51572116 and 51202094); the Priority Academic Program Development of Jiangsu Higher Education Institutions.