Introduction
Milk is a complete food source for mammalian young. It is particularly important for marsupials, given their unique reproductive and developmental biology. Marsupials have a very short gestation period of up to 30 d, after which they give birth to altricial young that undergo the majority of development ex utero within the pouch(Reference Tyndale-Biscoe1). The development of pouch young is supported by a complex and extended lactation period, with examples such as koalas (Phascolarctos cinereus)(Reference Martin and Handasyde2) which can lactate for up to 12 months. Conversely, eutherian mammals, such as humans, have an extended in utero development and comparatively consistent milk composition throughout lactation(Reference Kuruppath, Bisana and Sharp3).
Lactation in marsupials studied to date is divided into three phases that differ in nutrients (protein, carbohydrate and fat) and bioactive compounds. For a thorough review of the nutritional components of marsupial milk, see Refs.(Reference Stannard, Miller and Old4,Reference Sharp, Lefèvre and Nicholas5) . The timing and definition of these phases differ between species. In this review, we will use the phases defined for tammar wallaby (Notamacropus eugenii) and brushtail possum (Trichosurus vulpecula) as their milk is the most well-studied amongst marsupials. Phase 1 represents pregnancy, phase 2A (early lactation) is when the joey is permanently attached to the teat, phase 2B (mid-lactation) is when the joey suckles intermittently, and phase 3 (late lactation) is when the joey starts to exit the pouch and is eventually weaned at approximately 9 months(Reference Demmer, Ross and Ginger6,Reference Joss, Molloy and Hinds7) .
Marsupial young are immunologically naïve at birth and lack mature immune tissues and cells for up to 120 d postpartum(Reference Old and Deane8). Given that the pouch is not sterile and contains a diverse range of microorganisms(Reference Maidment, Bryan and Pyne9,Reference Weiss, Taggart and Smith10) , the young require immunological support during development. Multiple mechanisms ensure the protection of joeys during this time, including passive immunity via the milk and bioactive compounds in the pouch skin. Numerous immune cells and proteins, such as immunoglobulins, have been identified in marsupial milk, which change in composition and abundance during lactation(Reference Cheng and Belov11,Reference Edwards, Hinds and Deane12) . Bioactive peptides within the milk and pouch, such as antimicrobial peptides (cathelicidins and defensins), lysozyme and marsupial-specific growth factors, also provide immune and nutritional support(Reference Cheng and Belov11,Reference Edwards, Hinds and Deane12) .
Marsupial joeys can be orphaned due to threats such as habitat loss and fragmentation, vehicle strike and fire(Reference Doherty, Geary and Miritis13). Orphan joeys in care are hand-raised using commercial colostrum and milk substitutes such as Wombaroo (Woombaroo Food Products, SA, Australia), Di-Vetelact (Lillelund Pty Ltd, NSW, Australia) and Marsupial Milk Replacer (Exotic Nutrition, Virginia, United States), which are tailored milk substitutes for kangaroos, wombats, possums and koalas of varying ages. A colostrum substitute is also commercially available (Woombaroo Food Products, SA, Australia) that contains bovine colostrum powder. Although these milk formulae meet the nutritional requirements (carbohydrate, protein, lipid) of joeys during development, they do not contain the diversity of bioactive immune compounds within marsupial milk. Given the essential role of milk immune proteins in marsupial development, it is not surprising that many orphaned joeys fail to thrive in care. For example, at one wildlife hospital (QLD, Australia), 31% of orphaned marsupials and 35·5% of orphaned koalas do not survive, compared with 25·7% mortality across all admitted orphaned wildlife species(Reference Taylor-Brown, Booth and Gillett14). Given the long evolutionary divergence between marsupials and eutherians(Reference Luo, Yuan and Meng15), there is a need for marsupial-specific knowledge of their milk’s bioactive components. In addition, the important role of the pouch in marsupial development compels investigation of possible bioactivity in pouch secretions.
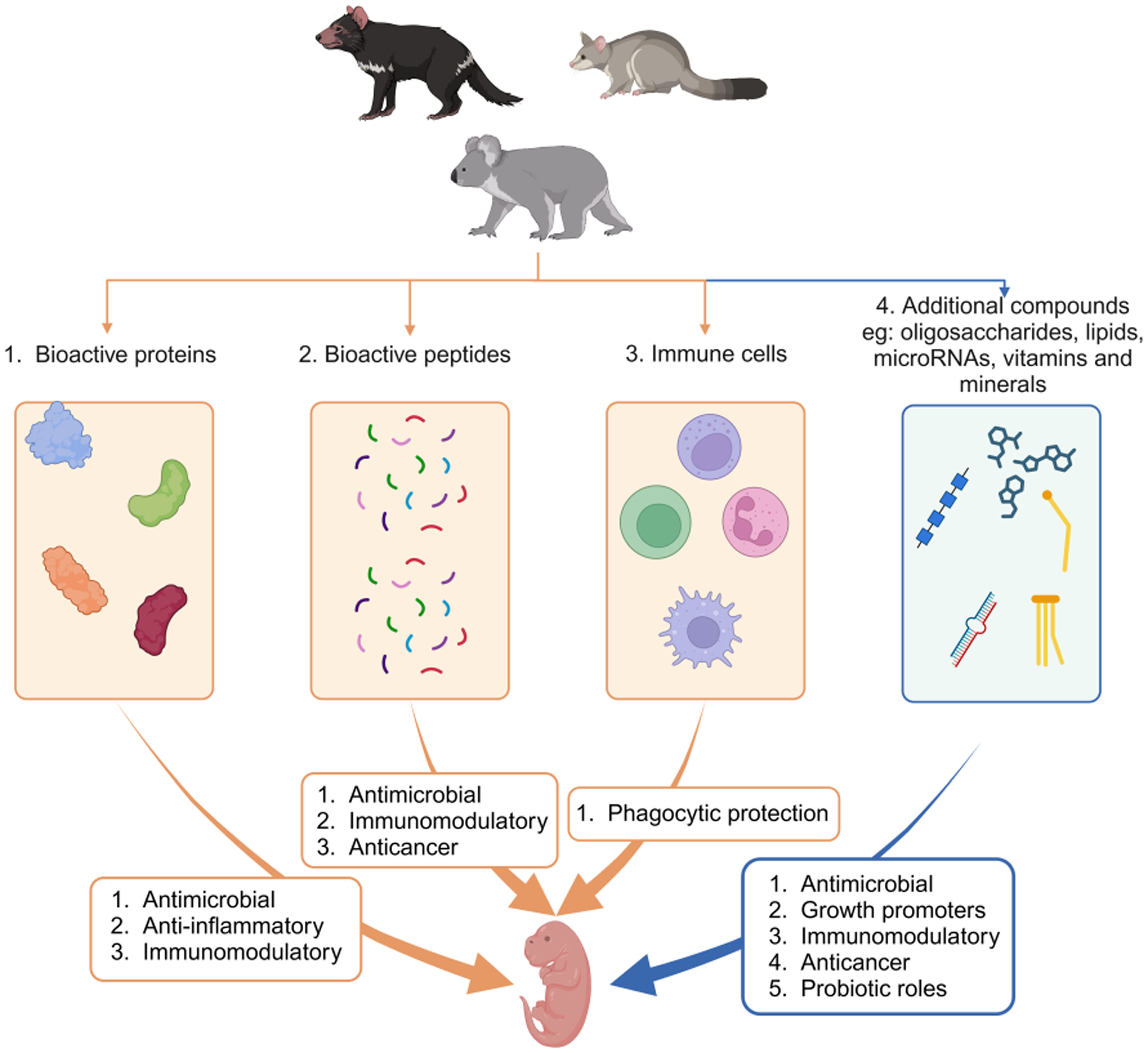
Bioactive compounds in the milk, mammary gland and pouch of marsupials have been investigated in multiple species. These include the koala, tammar wallaby, Tasmanian devil (Sarcophilus harrisii), brushtail possum, ringtail possum (Pseudocheirus peregrinus), woylie (Bettongia penicillata), quokka (Setonix brachyurus) and red kangaroo (Macropus rufus). A range of methods have been used to investigate bioactive compounds, including mammary gland and pouch skin transcriptomes(Reference Peel, Cheng and Djordjevic16–Reference Lefèvre, Digby and Whitley19); proteomes from milk(Reference Joss, Molloy and Hinds7,Reference Morris, O’Meally and Zaw20,Reference Ambatipudi, Joss and Raftery21) ; pouch secretion proteomes; and antimicrobial testing of pouch washes(Reference Ambatipudi, Joss and Raftery21–Reference Baudinette, Boontheung and Musgrave23).
Marsupial milk contains many bioactive compounds, including bioactive proteins, antimicrobial peptides (AMP)(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) , marsupial-specific growth factors(Reference Sharp, Wanyonyi and Modepalli24) and lipids(Reference Crowleyac, Woodward and Rose25), oligosaccharides(Reference Durham, Wei and Lemay26) and microRNAs(Reference Sharp, Lefèvre and Nicholas5). Here, we expand upon recent milk reviews(Reference Stannard, Miller and Old4,Reference Cheng and Belov11,Reference Pharo27) by summarising research specific to components of the marsupial milk and pouch with demonstrable bioactive roles. We also highlight compounds with suspected bioactive roles based on evidence in other species as areas for future investigation in marsupials. A thorough understanding of bioactive components in marsupial milk will allow advances in the development of antimicrobials and the targeted development of marsupial-specific milk substitutes.
Bioactive compounds
Immune cells
Marsupial milk contains numerous immune cell types that provide passive immunity to the developing pouch young(Reference Smith and Keyte28) (Table 1). Macrophages, neutrophils and lymphocytes have been identified in the colostrum and throughout lactation in the tammar wallaby, quokka and koala(Reference Young, Basden and Cooper29–Reference Cockson and McNeice31). Neutrophils are the most abundant immune cells in koala milk, and their numbers increase until the end of lactation, highlighting their importance to phagocytic protection of the joey even at this late stage(Reference Young, Basden and Cooper29,Reference Young and Deane30) . Preference for a high level of neutrophils may be due to their superior capacity to deal with pathogenic bacteria and fungi(Reference Young, Basden and Cooper29). A range of other immune cells are likely to be present in milk, given the identification of immune receptors and cell markers in milk transcriptomes and proteomes(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) . These include dendritic cells, helper (CD4) and cytotoxic (CD8) T lymphocytes, and other granulocytes such as eosinophils and basophils(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) .
Table 1. Bioactive components present in mammary gland, milk and pouch environment

IgA, immunoglobulin A; IgE, immunoglobulin E; IgG, immunoglobulin G; IgM, immunoglobulin M; MM1, marsupial milk 1; VELP, very early lactation protein; WAP, whey acidic protein; WFDC2, WAP four-disulphide domain protein-2.
Immunoglobulins
Immunoglobulins (Igs) play an important role in adaptive immunity. There are four types of Igs in marsupials (IgA, IgE, IgM and IgG) that have different functions(Reference Morris, Prentis and O’Meally32) (Table 1). IgA is involved in mucosal immunity, IgE is involved in defence against parasites and hypersensitivity, IgG is the most abundant immunoglobulin in the extracellular fluid and blood, and IgM is the first immunoglobulin produced after exposure to an antigen(Reference Morris, Prentis and O’Meally32). All four Ig types are present in marsupial milk and mammary glands. Once transferred through the milk, they can provide passive immunity to the altricial young(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Hurley and Theil33) . IgA and IgG are the predominant Igs in marsupial milk, which is also the case in eutherians(Reference Deane and Cooper34–Reference Adamski and Demmer36).
Immunoglobulins are generally transferred via the milk during two periods in marsupials: in the colostrum immediately after birth at the start of phase 2A, and when the young first emerges from the pouch at the onset of phase 3(Reference Adamski and Demmer36,Reference Daly, Digby and Lefèvre37) . At these times, high levels of Igs provide passive protection as young are exposed to new environments and microorganisms(Reference Cheng and Belov11). However, the expression of Ig types at these two timepoints can differ between species. IgG is absorbed across the pouch young gut epithelium, facilitated by the neonatal Fc receptor, and enters peripheral circulation, thereby protecting against infection(Reference Yadav38). IgG was detected in wallaby, kangaroo and possum colostrum, although the concentration was low compared with eutherian colostrum(Reference Deane and Cooper34,Reference Deane, Cooper and Renfreec35) . In possums, IgG was significantly elevated during late lactation compared with early lactation(Reference Adamski and Demmer36), whereas in koalas, IgG levels remained constant through early and late lactation, although the neonatal Fc receptor was expressed only during early lactation(Reference Hewavisenti, Morris and O’Meally18,Reference Adamski and Demmer36,Reference Daly, Digby and Lefévre39) .
IgA and IgM are not absorbed across the gut epithelium but are involved in immune defence at these sites(Reference Woof and Kerr40). IgA was identified in the colostrum of the tammar wallaby and brushtail possum(Reference Deane, Cooper and Renfreec35,Reference Adamski and Demmer41) . Higher IgA levels were observed during late lactation compared with early lactation in the koala and brushtail possum(Reference Morris, O’Meally and Zaw20,Reference Adamski and Demmer41) . In the koala milk proteome, IgA accounted for 2% of peptides in late lactation, compared with only 0·08% in early lactation. Conversely, IgM was identified only during early lactation in the koala and was not found in a late lactation milk proteome(Reference Morris, O’Meally and Zaw20). Tasmanian devil milk has been investigated only during mid-lactation, and IgA levels were the highest of the four Ig types present(Reference Hewavisenti, Morris and O’Meally18).
The S100 family of calcium-binding proteins
The S100 protein family consists of S100A1–S100A16, S100A19, S100B, S100G, S100P and S100Z proteins(Reference Kwek, Wynne and Lefèvre42). These proteins have well-known antimicrobial and immunomodulatory functions in eutherians(Reference Ryckman, Vandal and Rouleau43,Reference Büchau, Hassan and Kukova44) . In humans, S100A8 and S100A9 are highly expressed in colostrum and at lower levels in mature milk(Reference Pirr, Richter and Fehlhaber45). Gene knockout experiments in mice have shown that S100A8 and S100A9 are absorbed across the neonatal gut, enter peripheral circulation and may protect against neonatal sepsis via direct antimicrobial activity(Reference Pirr, Richter and Fehlhaber45,Reference Wang, Song and Wang46) . In marsupials, S100A8 and S100A9 are expressed in the milk and mammary gland(Reference Peel, Cheng and Djordjevic17), whereas S100A9 and S100A15 are expressed in the pouch skin(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Kwek, Wynne and Lefèvre42) (Table 1). Interestingly, S100A8 and S100A9 were not present in the koala early lactation milk transcriptome or proteome and have not been investigated in the colostrum of any marsupial species. Instead, these proteins were highly abundant in mid- and late lactation in devils and koalas(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) . S100A9 was the seventh most highly expressed immune transcript in the Tasmanian devil mid-lactation milk transcriptome (0·07%)(Reference Hewavisenti, Morris and O’Meally18). Similarly, S100A8 and S100A9 were highly abundant in the koala late lactation milk proteome, being the eighth (1·67%) and twenty-fifth (0·59%) most abundant protein, respectively(Reference Hewavisenti, Morris and O’Meally18). The high abundance of S100A8 and S100A9 indicates that these proteins may have important functions in marsupial milk, likely having anti-inflammatory and antimicrobial properties similar to human S100A8 and S100A9. The marsupial S100A8–S100A9 complex may also enter peripheral circulation in the young and protect against systemic infection, as in humans(Reference Pirr, Richter and Fehlhaber45).
Marsupials also have a unique S100 protein not found in eutherians, S100A19. This protein is expressed in the tammar wallaby mammary gland during pregnancy and involution but not lactation(Reference Kwek, Wynne and Lefèvre42). S100A19 is also expressed in the foregut of joeys during early pouch life when their diet consists only of milk(Reference Kwek, Wynne and Lefèvre42). Marsupial S100A19 is closely related to eutherian S100A15 and A7(Reference Kwek, Wynne and Lefèvre42), both of which are antimicrobial(Reference Büchau, Hassan and Kukova44,Reference Gläser, Harder and Lange47) . As such, S100A19 likely provides antimicrobial protection to the mammary gland and pouch young gut during development.
Lysozymes
The lysozyme family comprises lysozyme C and calcium-binding lysozyme. Among these, lysozyme C is the most prevalent across mammals, while calcium-binding lysozyme is less common(Reference Ragland and Criss48,Reference Irwin, Biegel and Stewart49) . Lysozyme is widely expressed in milk and is renowned for its antibacterial activity and immunomodulatory roles within the innate immune system(Reference Ragland and Criss48). Variation exists between mammalian species in the number of lysozyme C genes, with one gene identified in humans, four in the grey short-tailed opossum (Monodelphis domestica) and two complete and six incomplete genes in the tammar wallaby(Reference Irwin, Biegel and Stewart49). Gene duplications in the lysozyme family have not been well characterised across marsupials despite the recent release of numerous high-quality reference genomes across the marsupial tree(Reference Peel, Silver and Brandies50–Reference Stammnitz, Gori and Kwon52). In marsupial milk, lysozyme has been identified in the early milk proteome of the tammar wallaby(Reference Joss, Molloy and Hinds53), mid-lactation milk transcriptome of the Tasmanian devil(Reference Hewavisenti, Morris and O’Meally18), mid-lactation milk of the ringtail possum(Reference Nicholas, Loughnan and Messer54) and late lactation milk proteome of the koala(Reference Morris, O’Meally and Zaw20), and in the whey proteins and mammary gland RNA of all lactation stages of the brushtail possum(Reference Piotte, Marshall and Hubbard55) (Table 1). Up-regulation of lysozyme gene expression in the mammary gland and greater lysozyme C abundance in the milk as the lactation cycle progresses(Reference Demmer, Ross and Ginger6,Reference Vander Jagt, Whitley and Cocks56,Reference Kuy, Kelly and Smit57) suggests an important role of lysozyme C as the joey detaches from the teat and starts to move out of the pouch. Lysozyme has also been identified by proteomics in post-reproductive tammar wallaby pouch secretions(Reference Ambatipudi, Joss and Deane58), and lysozyme C was the most highly abundant transcript in the woylie pouch skin(Reference Peel, Silver and Brandies50). Although lysozyme has well-characterised antibacterial activity in eutherians(Reference Irwin, Biegel and Stewart49), possible antimicrobial and immunomodulatory roles of lysozyme in marsupials remain to be confirmed.
Transferrin and lactoferrin
The glycoproteins transferrin and lactoferrin are both integral components of the transferrin family and major components of milk. Lactoferrin exists in three isoforms: lactoferrin-α, lactoferrin-β and lactoferrin-γ. Lactoferrin-α is the only isoform capable of binding iron. The iron-binding and sequestration capabilities of transferrin and lactoferrin-α are crucial in inhibiting bacterial growth(Reference Kell, Heyden and Pretorius59). Lactoferrin has demonstrated antibacterial, antiviral, antifungal, anti-inflammatory and anticarcinogenic activity(Reference Kell, Heyden and Pretorius59) (Table 1). Furthermore, enzymatic cleavage of the N-lobe of lactoferrin yields crucial peptides lactoferricin and lactoferrampin, which exhibit antimicrobial activity and are under positive selection in primates(Reference Barber, Kronenberg and Yandell60). Marsupial N lobe sequences from the opossum and Tasmanian devil cluster separately to eutherians(Reference Hughes and Friedman61), but neither antimicrobial function nor diversity within marsupials has been explored.
Lactoferrin was the most abundant protein in the koala late lactation milk proteome (Fig. 1), accounting for almost half of all transcripts expressed(Reference Morris, O’Meally and Zaw20). Possible antimicrobial or other functions should be investigated to better understand the role of lactoferrin in marsupials.

Figure 1. Differential expression of lactoferrin between early and late proteomes. Lactoferricin accounted for 49·4% of all transcripts expressed in koala milk during late lactation, but just 0·19% of transcripts produced during early lactation.
Transferrin has been detected around the time of birth in the brushtail possum(Reference Adamski and Demmer36) and tammar wallaby(Reference Ambatipudi, Joss and Raftery21) (Table 1). Elevated levels of transferrin have also been observed at mid-lactation, when the joey begins exiting the pouch, in both the ringtail(Reference Nicholas, Loughnan and Messer54) and brushtail possums(Reference Adamski and Demmer36,Reference Adamski and Demmer41,Reference Grigor, Bennett and Carne62) . Transferrin was also identified in the early lactation milk proteome(Reference Joss, Molloy and Hinds53) and mid-lactation mammary gland transcriptome(Reference Lefèvre, Digby and Whitley19) of the tammar wallaby. Evidence for increased expression of transferrin coinciding with birth and first pouch exit suggests that it may play an important role in the immunological protection of marsupial young.
Whey proteins
The whey acidic protein (WAP) is a type of whey protein characterised by cysteine-rich domains, known as disulfide core (4-DSC) domains(Reference Wilkinson, Roghanian and Simpson63). The WAP is classified as antimicrobial and involved in inhibiting neutrophil serine proteases(Reference Wiesner and Vilcinskas64). WAP inhibits the growth of Staphylococcus aureus (Reference Iwamori, Nukumi and Itoh65) and also has non-immune functions, such as stimulating the proliferation of mammary epithelial cells(Reference Topcic, Auguste and De Leo66). WAP has been identified as a major whey protein in the milk of numerous eutherian and marsupial species, as well as monotremes(Reference Sharp, Lefèvre and Nicholas67) (Table 1). Marsupial WAP has an additional 4-DSC domain to the two 4-DSCs in eutherian WAP(Reference Sharp, Lefèvre and Nicholas67). WAP was one of the top 200 most highly expressed transcripts in the Tasmanian devil mid-lactation milk transcriptome(Reference Hewavisenti, Morris and O’Meally18) and has also been detected at mid-lactation in the red kangaroo(Reference Nicholas, Fisher and Muths68) and brushtail possum(Reference Demmer, Stasiuk and Adamski69). The tammar wallaby mammary gland WAP four-disulphide domain protein-2 (WFDC2) has demonstrated bactericidal activity against Salmonella enterica, Pseudomonas aeruginosa and S. aureus but no activity against commensal bacteria(Reference Watt, Sharp and Lefevre70). In koalas, WFDC2 was abundant in the early lactation milk proteome and present in the late lactation proteome(Reference Morris, O’Meally and Zaw20). The abundance of WFDC2 in early lactation may coincide with the need for increased immune protection around birth, while the expression of WAP in mid-lactation suggests a possible immunological role in protecting the joey as it begins to exit the pouch.
Whey proteins with specific primary roles can also have secondary bioactive functions as pre-proteins, aside from contributing to the macronutrient profile of milk. Prominent preproteins in marsupial milk include β-lactoglobulin, which is a lipocalin protein that transports iron and other hydrophobic molecules(Reference Roth-Walter, Pacios and Gomez-Casado71), and α-lactalbumin, which is intimately involved in lactose metabolism(Reference Brew72) and iron absorption(Reference Wang and Zhang73). Evidence from eutherians suggests possible bioactivity of these two whey proteins. Proteolytic digestion of bovine β-lactoglobulin and α-lactalbumin resulted in numerous sequences with activity against Gram-positive bacteria(Reference Pellegrini, Thomas and Bramaz74,Reference Pellegrini, Dettling and Thomas75) (Table 1). β-Lactoglobulin and α-lactalbumin have been widely identified in marsupial milk: both were among the top 200 most highly expressed transcripts in the Tasmanian devil milk transcriptome(Reference Hewavisenti, Morris and O’Meally18) and were the predominant proteins in the mature reproductively active tammar wallaby pouch(Reference Ambatipudi, Joss and Deane58). α-Lactalbumin was expressed in the mammary gland of the brushtail possum at all phases of lactation and increased in phase 3(Reference Demmer, Ross and Ginger6,Reference Piotte, Marshall and Hubbard55) and was present in all samples of ringtail possum milk tested(Reference Nicholas, Loughnan and Messer54). β-Lactoglobulin was the most abundant transcript in the koala mammary gland transcriptome(Reference Morris, O’Meally and Zaw20); was secreted at constant levels in red kangaroo mid-lactation(Reference Nicholas, Fisher and Muths68); was present in the early milk protein and expressed in the mammary gland at constant levels throughout lactation in the brushtail possum(Reference Demmer, Ross and Ginger6,Reference Kuy, Kelly and Smit57) ; and was present in the tammar wallaby mid- and late lactation mammary gland transcriptomes(Reference Lefèvre, Digby and Whitley19). The main chain of β-lactoglobulin was also identified in the milk proteins of the tammar wallaby throughout lactation, with a second isoform present in phase 2A and a third isoform at a high concentration in phase 2B(Reference Joss, Molloy and Hinds7). In pouch secretions of the tammar wallaby, β-lactoglobulin was present in high concentrations. Although there was no evidence of direct antimicrobial activity of β-lactoglobulin against Escherichia coli or S. aureus, the antimicrobial potential of cleaved peptides has been hypothesised(Reference Ambatipudi, Joss and Raftery21). Both α-lactalbumin and β-lactoglobulin are present in the milk, mammary glands and pouches of numerous marsupials. It is possible they provide immune protection when cleaved, but given that they have other functions, their expression likely has other effects, too, and further investigation is required to confirm their bioactivity.
Additional immune proteins
Marsupial milk is also a rich source of other immune proteins, accounting for 9% and 6·6% of all proteins in koala and Tasmanian devil milk, respectively(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) (Table 1). These include immune receptors such as the major histocompatibility complex proteins (MHC) classes I and II, toll-like receptors (TLRs) and natural killer (NK) cell receptors(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Lefèvre, Digby and Whitley76) . The presence of these receptors indicates the diversity of immune cells present in the milk and mammary gland, and potential protective functions involving antigen recognition and binding.
Immune signalling molecules, such as cytokines and chemokines, are also present in marsupial milk and mammary glands. The chemokine CCL25 was one of the most highly expressed immune signalling molecules in both koala and Tasmanian devil milk(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) . CCL25 has only recently been identified in human milk and is highly expressed in colostrum(Reference Abe, Onoda and Furushima77). In humans, CCL25 is essential for thymocyte development(Reference Uehara, Hayes and Li78) and may play a similar role in pouch young. Other chemokines, such as CCL28, were also identified in the devil mammary gland transcriptome(Reference Morris, O’Meally and Zaw20). In humans, CCL28 and CCL25 facilitate the transfer of IgA into the milk by attracting IgA-antibody-producing immune cells to the mammary gland epithelium(Reference Wilson and Butcher79,Reference Bowman, Kuklin and Youngman80) . CCL28 is also antibacterial against Gram-negative and Gram-positive bacteria, and the fungus Candida albicans (Reference Hieshima, Ohtani and Shibano81). As such, these chemokines may be involved in both direct and indirect protection of the pouch young gastrointestinal tract.
Complement factors are present in both marsupial milk and the mammary gland. Proteins involved in the classical complement pathway (C2, C3 and C4A) were identified in the koala milk proteome and mammary gland transcriptome(Reference Morris, O’Meally and Zaw20). These factors were highly abundant during early lactation, accounting for 2·4% of peptides. Complement proteins bind to pathogens, thereby enabling phagocytosis by immune cells, and some are also bactericidal(Reference Janeway, Travers and Walport82).
Antimicrobial peptides
Cathelicidins and defensins
Cathelicidins and defensins are two major families of antimicrobial peptides in mammals. They form part of the innate immune system through antimicrobial and immunomodulatory activity and may also be involved in immune defence against cancer(Reference Kościuczuk, Lisowski and Jarczak83–Reference Petrohilos, Patchett and Hogg85). Cathelicidins and defensins are small, cationic, amphipathic peptides that are expressed in immune and epithelial cells(Reference Kościuczuk, Lisowski and Jarczak83,Reference Bals and Wilson86) . Both cathelicidins and defensins are encoded as pre-propeptides that undergo enzymatic cleavage to release the C-terminal active mature peptide(Reference Kościuczuk, Lisowski and Jarczak83,Reference Ganz87) . Defensin mature peptides contain six highly conserved cysteine residues that form three disulphide bonds that define the α-, β- and θ-subfamilies(Reference Yang, Biragyn and Hoover84,Reference Hazlett and Wu88) . Theta defensins are found only in Old World primates and so are not discussed further in this review. Both cathelicidins and defensins from eutherian mammals have antimicrobial activity against fungi, enveloped viruses, Gram-positive and Gram-negative bacteria and parasites(Reference Yang, Biragyn and Hoover84,Reference van-Harten, van-Woudenbergh and van-Dijk89) . They are also immunomodulatory; they enhance phagocytosis, are chemotactic, induce degranulation of neutrophils and mast cells, and induce migration of epithelial cells(Reference Yang, Biragyn and Hoover84,Reference van-Harten, van-Woudenbergh and van-Dijk89) .
Marsupials have a large repertoire of diverse cathelicidins, likely driven by the need for additional immune protection of altricial young during development in the pouch (Table 1). This differs from eutherians, such as humans and mice, that have only a single cathelicidin peptide(Reference van-Harten, van-Woudenbergh and van-Dijk89). Cathelicidins have only been characterised in the grey short-tailed opossum, koala, Tasmanian devil and tammar wallaby, with between six and nineteen cathelicidin genes identified in a single species(Reference Peel, Cheng and Djordjevic16,Reference Peel, Cheng and Djordjevic17,Reference Daly, Digby and Lefévre39,Reference Peel, Cheng and Djordjevic90,Reference Belov, Sanderson and Deakin91) . Similar to human cathelicidins, marsupial peptides have broad-spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria, including methicillin-resistant S. aureus, and fungi (Fig. 2)(Reference Peel, Cheng and Djordjevic16,Reference Peel, Cheng and Djordjevic17,Reference Peel, Cheng and Djordjevic90) . Koala cathelicidin PhciCath5 was active against Chlamydia pecorum, one of the aetiological agents that cause chlamydiosis that has severely impacted koala populations(Reference Peel, Cheng and Djordjevic17). Recent work has shown that Tasmanian devil cathelicidins have anticancer activity against devil facial tumour disease (DFTD) cells and may also have immunomodulatory functions (Fig. 2)(Reference Petrohilos, Patchett and Hogg85).

Figure 2. Bioactive roles of cathelicidin.
Marsupial cathelicidins are expressed in the milk and mammary gland throughout lactation(Reference Peel, Cheng and Djordjevic16,Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Carman, Simonian and Old92) . This is unlike other immune proteins that are generally expressed in only two periods as discussed in previous sections(Reference Edwards, Hinds and Deane12). In the tammar wallaby and koala, individual cathelicidins were differentially expressed during early and late lactation(Reference Joss, Molloy and Hinds7,Reference Morris, O’Meally and Zaw20,Reference Wanyonyi, Sharp and Khalil93) . Some of these peptides had antimicrobial activity, whereas others were not active against the strains tested and may have immunomodulatory functions(Reference Peel, Cheng and Djordjevic17,Reference Wanyonyi, Sharp and Khalil93) . In addition, cathelicidins may also be involved in mammary gland proliferation and remodelling during involution in the tammar wallaby (Fig. 2)(Reference Wanyonyi, Sharp and Khalil93). Cathelicidins are also expressed in the pouch skin(Reference Peel, Cheng and Djordjevic16,Reference Peel, Silver and Brandies50) and may provide direct protection. Tasmanian devil cathelicidins expressed in the pouch were active against bacteria identified in the pouch microbiome. Cathelicidins may also indirectly protect pouch young by contributing to modulation of bacterial communities in the pouch, although this hypothesis has not been proven(Reference Peel, Cheng and Djordjevic16).
Defensins are the second major family of antimicrobial peptides in mammals. Defensins have been characterised in the greater bilby (Macrotis lagotis), brown antechinus (Antechinus stuartii), common wombat (Vombatus ursinus), fat-tailed dunnart (Sminthopsis crassicaudata), eastern barred bandicoot (Perameles gunnii), red kangaroo, western ringtail possum, numbat (Myrmecobius fasciatus), woylie, grey short-tailed opossum, koala, tammar wallaby and Tasmanian devil(Reference Belov, Sanderson and Deakin91,Reference Jones, Yuanyuan and O’Meally94,Reference Peel, Hogg and Belov95) (Table 1). Marsupials encode multiple α- and β-defensin genes within their genomes, similar to eutherians. However, some α- and β-defensin lineages are specific to marsupials and, hence, may have novel functions(Reference Jones, Yuanyuan and O’Meally94). α-Defensins have not been detected in any marsupial milk, mammary gland or pouch skin transcriptome or proteome to date, so their bioactive role at these sites is unknown(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Jones, Yuanyuan and O’Meally94) . A small number of β-defensins were expressed during early lactation in the koala(Reference Morris, O’Meally and Zaw20,Reference Jones, Yuanyuan and O’Meally94) and mid-lactation in the Tasmanian devil(Reference Hewavisenti, Morris and O’Meally18). β-Defensins were also found in the transcriptomes of the koala’s lactating mammary gland, the bilby’s non-lactating mammary gland and the pouch skin of both bilby and the woylie(Reference Peel, Hogg and Belov95). Human defensins are expressed in the mammary gland and provide antibacterial defence against infections such as mastitis as well as neonatal gastrointestinal infections(Reference Baricelli, Rocafull and Vázquez96,Reference Tunzi, Harper and Bar-Oz97) . Given the function of marsupial defensins is unknown, future studies should focus on understanding the antimicrobial and immunomodulatory functions of these peptides, and their potential role in protecting marsupial pouch young.
Other antimicrobial peptides
Numerous antimicrobial peptides have been identified in marsupial milk, pouch and mammary gland, many due to availability of genomic and proteomic datasets and the comprehensive and in-depth nature of bioinformatic analysis (Table 1). Some are unique to marsupials, such as marsupial milk 1 (MM1), whereas others are orthologs of eutherian proteins, such as very early lactation protein (VELP)(Reference Morris, O’Meally and Zaw20).
VELP has been identified in the milk and mammary glands of the brushtail possum, tammar wallaby, koala and Tasmanian devil(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20,Reference Joss, Molloy and Hinds53,Reference Kuy, Kelly and Smit57) . VELP was initially thought to be unique to marsupials but has since been identified as an ortholog of the eutherian protein glycam 1(Reference Hewavisenti, Morris and O’Meally18). Glycam 1 is present in only some eutherians and is a pseudogene in humans(Reference Rasmussen, Johnsen and Petersen98). VELP abundance changes throughout lactation and differs between species. In the tammar wallaby, VELP is expressed in the mammary gland from pregnancy through to mid-lactation, but not in late lactation(Reference Joss, Molloy and Hinds7). VELP is also expressed at low levels during mid-lactation in the Tasmanian devil, being the only lactation phase investigated in this species(Reference Hewavisenti, Morris and O’Meally18). In koalas, VELP is highly abundant in early lactation, accounting for 13·3% of all peptides, which continues throughout late lactation(Reference Morris, O’Meally and Zaw20). The closely related eutherian protein glycam1 is also expressed in milk(Reference Groenen, Dijkhof and ban-der-Poel99), where it has antibacterial and antiviral functions(Reference Campagna, Mathot and Fleury100,Reference Inagaki, Nagai and Yabe101) . The function of VELP has not been tested, but it likely provides antimicrobial protection to the pouch young given the close evolutionary relationship to glycam 1.
Marsupial milk 1 (MM1) (previously called novel gene 1) is a putative antimicrobial peptide unique to marsupials. MM1 has been identified only in mammary gland transcriptomes and milk proteomes of the koala, devil and tammar wallaby and is specifically expressed in these tissues(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) . MM1 was highly expressed in the koala and devil, being the twenty-seventh most highly abundant transcript in the devil mammary gland, and twenty-sixth most highly abundant peptide in the koala early lactation proteome(Reference Hewavisenti, Morris and O’Meally18,Reference Morris, O’Meally and Zaw20) . The function of MM1 is unknown but is likely antimicrobial given genomic evidence. In the koala genome, MM1 is encoded in a genomic region that is syntenic to the region encoding antimicrobial proteins such as glycam 1 and dermcidin in humans(Reference Morris, O’Meally and Zaw20). The abundance and tissue specificity of MM1 suggests that this peptide may play an important role in the antimicrobial protection of the mammary gland and pouch young.
Dermcidin is an AMP secreted from sweat glands in the skin and is the major antimicrobial compound in human sweat(Reference Schittek, Hipfel and Sauer102). Dermcidin has broad-spectrum antimicrobial activity against bacteria and fungi across a range of pH and salt concentrations, which attenuate the activity of many other AMPs(Reference Schittek103). The pouch is essentially a fold of skin that undergoes significant structural changes leading up to birth and during lactation(Reference Tyndale-Biscoe1). As such, it is not surprising that dermcidin is present in the pouch secretions of tammar wallabies and wombats(Reference Ambatipudi, Joss and Deane58). Pouch wash protein fractions, which included dermcidin, displayed antibacterial activity(Reference Schittek, Hipfel and Sauer102). Dermcidin was not identified in woylie pouch skin, although this sample was from a female without pouch young(Reference Peel, Silver and Brandies50). As such, dermcidin in marsupials may be involved in regulating the pouch microbiome and preventing infections, similar to human dermcidin(Reference Schittek103).
The diversity and complexity of antimicrobial peptides (AMP) discovered thus far highlight their pivotal role in aiding immune protection to the developing pouch young. The array of capabilities AMP possess shows their relevance in governing immune protection and represents marsupials’ adaptive evolutionary methods to maintain the survival and health of their young.
Plurifunctional components with bioactive potential
Beyond their essential nutritive functions, milk components such as caseins and their derived peptides, as well as oligosaccharides, lipids, vitamins, minerals and microRNAs, demonstrate additional bioactivities in mammals (Fig. 3).

Figure 3. Other potentially bioactive maternal compounds.
Casein
Compared with bovine milk, where protein is approximately 80% casein and 20% whey proteins(Reference Jäkälä and Vapaatalo104), marsupial milk has relatively less casein with a ratio of approximately 50:50. This is generally constant throughout lactation, although concentration tends to increase(Reference Nicholas105). The most abundant caseins are β-casein, followed by α-casein, then κ-casein(Reference Morris, O’Meally and Zaw20,Reference Vercruysse, Van Camp and Dewettinck106) . κ-Casein was once thought to be absent in marsupials until the genes encoding κ-casein were discovered in the brushtail possum(Reference Stasiuk, Summers and Demmer107). The amount of casein increases throughout lactation, predominantly owing to β-casein while α-casein remains relatively stable. In wallabies, caseins form micelles similar in structure to the well-studied bovine milk, despite the notable evolutionary distance(Reference Horne, Anema and Zhu108). Although caseins and whey proteins are well-known sources of nourishment, the peptides generated from these proteins can also play key roles in supporting an array of biological processes. Bioactive peptides generated from eutherian milk caseins have demonstrated an array of immunomodulatory and antimicrobial functionalities. Casocidin-1 generated from αs2-casein in cow has potent in vitro antimicrobial activity against E. coli and Staphylococcus carnosus. Isracidin is another casein (αs1-casein)-derived peptide that has displayed antibacterial activity in vivo against S. aureus and Candida albicans in cow and sheep(Reference Silva and Malcata109). Apart from these direct antimicrobial roles, β- and κ-casein-derived peptides in cow milk may promote lymphocyte proliferation, whereas β-casein identified from human milk induces chemokine production(Reference Nielsen, Liang and Rathish110). Peptides derived from casein have been found in peripheral circulation in neonates but not in adults, indicating that they can cross the gastrointestinal barrier and enter systemic circulation in infants. This finding highlights the possible age-specific functions that these peptides may have in promoting juvenile health and development(Reference Fox, Uniacke-Lowe and McSweeney111). However, a focus on milk peptides derived from caseins in marsupials has been notably insufficient or absent. Given that they likely represent a large proportion of the milk proteome in marsupials, a deeper understanding of casein-derived peptides will be essential for the development of tailored marsupial immune milk supplements to ensure orphaned joeys’ survival.
Vitamins and minerals
Milk provides essential dietary vitamins and minerals that are required for a variety of bioactive functions, including bone mineralisation, enzyme cofactors and hormonal/nutritive signalling. Marsupial milk mineral content has been reviewed recently(Reference Stannard, Miller and Old4), although the marsupial-specific literature on milk vitamins is limited and varies across species.
Kangaroo milk contains a similar diversity and concentration of vitamins to cow milk(Reference Poole, Sharman and Scott112), including lipophilic (vitamin A) and hydrophilic (B1, B2, B3, B5, B6, B7 and B12) vitamins(Reference Gaucheron113). Similar to other milk nutrients, the concentration of vitamins differs between lactation phases 2 and 3(Reference Poole, Sharman and Scott112). Folic acid (vitamin B9) is the most stable form of folate, which is an essential bioactive vitamin for neural tube closure and guides spinal cord development in mammals. In eutherians, this occurs early in gestation as folic acid is delivered via maternal blood. Suboptimal uptake of folic acid during pregnancy in eutherians leads to perinatal morbidity and mortality, signifying its importance(Reference Denny, Jeanes and Fathe114). In marsupials, neural development occurs ex utero within the pouch; hence, folic acid is delivered via the milk. This finding could explain why red and grey kangaroos’ (Macropus giganteus) early lactation milk contains elevated levels of folic acid, which then decreases throughout the lactation period(Reference Poole, Sharman and Scott112). The fluctuation of these vitamins based on their lactation stages has been descriptively studied in Poole’s 1982 study(Reference Poole, Sharman and Scott112).
Oligosaccharides
The carbohydrate fraction of milk contains oligosaccharides, which are present in higher proportions than lactose in marsupials compared with eutherians(Reference Urashima, Horiuchi and Sakanaka115). Human milk oligosaccharides have demonstrated prebiotic, anti-inflammatory and immunomodulatory activity(Reference Durham, Wei and Lemay26). Milk oligosaccharides in humans have proven to be incredibly resistant to hydrolysis by digestive enzymes in the small intestine and are known to pass through it unaltered. When they reach the colon, the endogenous microflora is known to secrete enzymes that allow them to utilise oligosaccharides as a source of energy, which leads to competitive inhibition of pathogenic microbial proliferation as illustrated in Fig. 4, thereby performing the prebiotic role(Reference Engfer, Stahl and Finke116). The beneficial microflora’s metabolic activity further lowers gut pH, which hinders pathogen proliferation(Reference Urashima, Fukuda and Messer117). They also function as receptor analogues that inhibit the attachment of pathogens to epithelial cells by facilitating their binding to oligosaccharides in the gut lumen. Pathogens may mistake free oligosaccharides for those bound to cell membranes and bind to them rather than attaching to joey tissue (Fig. 4)(Reference Newburg118). A database of oligosaccharides across mammals, MilkOligoDB, revealed predominantly linear oligosaccharide structures in the koala, wombat and brushtail possum(Reference Durham, Wei and Lemay26). Despite the variety of digestive systems and distinct microflora observed among marsupials due to their diverse dietary preferences(Reference Hume119), there remains a strong likelihood that oligosaccharides found in marsupial species will exhibit similar behaviour to human milk oligosaccharides in this context.

Figure 4. (1) Oligosaccharides (A) promote the proliferation of beneficial bacteria (B) while serving as receptor analogues and binding to invading pathogens (C), blocking their attachment to the gut epithelium. (2) Proliferation of beneficial microflora results in lower pH levels, which leads to competitive inhibition of potentially pathogenic microflora (1. D).
Lipids
Although milk fat has a reputation as a primary source of energy, it also has the potential to be bioactive. Bioactive lipids in mammalian milk comprise triacylglycerols, diacylglycerols, saturated and polyunsaturated fatty acids, and phospholipids, which have been shown to have anticancer, antibacterial, anti-inflammatory and immunosuppressive traits(Reference German and Dillard120).
Lipid content in marsupial milk gradually increases during early lactation, before rising substantially in late lactation to a peak during weaning(Reference Crowleyac, Woodward and Rose25,Reference Green, Merchant and Newgrain121–Reference Muths123) . Lipid content during late lactation can be five-fold higher than early lactation, which indicates a switch from carbohydrates to lipids as the major energy source(Reference Rose, Shetewi and Flowers124). However, in arboreal folivorous marsupials, such as koalas, brushtail possums and ringtail possums, the lipid concentration peaks in mid-lactation(Reference Cowan125,Reference Krockenberger126) . Koala milk has the highest milk lipid content of these three arboreal folivores(Reference Green, Merchant and Newgrain121,Reference Rose, Shetewi and Flowers124,Reference Krockenberger126) . Triacylglycerols are the major lipid type in both marsupial and bovine milk. Hydrolysis of triacylglycerol has yielded adequate levels of antimicrobial fatty acids and monoacylglycerols in human milk. The monoacylglycerols are known to exert antiviral, antibacterial and antiprotozoal effects(Reference German and Dillard120).
Fatty acids are known to have distinct biological roles(Reference Fox, Uniacke-Lowe and McSweeney111). Conjugated linoleic acid (CLA) is a geometric isomer of linoleic acid (LA) present in bovine milk and has anticarcinogenic, growth-stimulating, antiatherogenic and immunomodulatory activity(Reference Parodi127). Actinobacteria within the human gut microbiome convert LA to CLA. LA, a precursor for the formation of CLA, has been identified in potoroo (Potorous tridactyius) and kangaroo milk. Bacteria from the phylum Actinobacteria have also been discovered in the gastrointestinal tracts of kangaroos(Reference Li, Jin and Li128) and koala(Reference Alfano, Courtiol and Vielgrader129). As a result, LA in milk may be digested within the pouch young gut to generate CLA, which may have bioactive functions similar to the human counterpart. To date, minimal research has been conducted to link the marsupial gut microbiotas and LA metabolism.
Fatty acids from marsupial milk, such as linoleic, oleic and palmitic acids(Reference Crowleyac, Woodward and Rose25), have antiviral properties in vitro (Reference Welsh and May130). Arachidonic acids are a type of polyunsaturated fatty acid that have been identified in varying amounts in the milk of many marsupial species(Reference Green, Griffiths and Leckie131,Reference Green and Merchant132) . In humans, many biological processes and tissues depend on arachidonic acid and its metabolites to develop and function, including skeletal muscle, immunological, brain and neural functions(Reference Tallima and El-Ridi133). Arachidonic acid in marsupial milk may have comparable bioactive functions. Unlike in bovine milk, omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have not yet been detected in red kangaroo, eastern quoll (Dasyurus viverrinus) nor potoroo milk(Reference Green, Merchant and Newgrain121,Reference Green and Merchant132,Reference Griffiths134) . Given the importance of EPA and DHA for the development of the nervous systems of other mammals, their evasiveness from detection in marsupial milk warrants further investigation(Reference Harris, Dennison and Phoenix135–Reference Swanson, Block and Mousa137).
Milk fat is carried in globules that are encapsulated by a phospholipid bilayer called the milk fat globular membrane (MFGM). Lipids and embedded MFGM proteins are of particular interest due to their health-promoting functionalities in mammals(Reference Xu, Zhou and Liu138). However, the functionalities of MFGM proteins and their ability to generate bioactive peptides in marsupial milk are largely unexplored.
MicroRNAs
MicroRNAs (miRNAs) are known to have an array of biological activities, including organogenesis and morphogenesis, through gene regulation. They are most widely known in mammals for the role they play in lactation stage-dependent mammary gland growth. Recent research on the variable abundance of miRNA in milk suggests that they may also play a function in directing the development and immunological protection of joeys. Milk is thought to accomplish this by functioning as a carrier of miRNA and other bioactive components to growing young, resulting in exogenously generated miRNAs contributing to gene regulation in joeys. This hypothesis is supported further by the capacity of milk miRNAs to tolerate low pH and RNAse activity owing to the presence of exosomes that shield them from such extreme conditions. These miRNAs can enter the bloodstream alongside exosomes via the juvenile joey’s gut, or they might be digested, resulting in the release of miRNAs and subsequent absorption by gut cells(Reference Modepalli, Kumar and Hinds139,Reference Sharp, Modepalli and Enjapoori140) .
Conclusion
Recent advances in genomics, transcriptomics, lipidomics and proteomics have shed light on the array of bioactive components found in marsupial milk, mammary gland and pouch environment. Some of these are unique to marsupials and may support the healthy development of pouch young. Among several functions of these bioactive components, providing immunological protection to the young is of critical importance. These components play a pivotal role in safeguarding the developing marsupial against pathogens, thereby facilitating healthy growth and development within the pouch environment. Expression of these bioactive components can be either consistent throughout lactation or differentially expressed, and many show increased expression around birth and when the joey starts exiting the pouch at mid-lactation. Given their pivotal role, the uncharacterised potential bioactive components found in marsupials, which have demonstrated activity in eutherians, should be a focus for future studies. It is essential to conduct more investigations on both defined and uncharacterised beneficial compounds to determine their stability and functionality in the environmental conditions of the marsupials’ pouch and gastrointestinal tract. This approach will help us gain a better understanding of the resilience and efficacy of these compounds in the biological environments in which they function. However, large-scale studies on marsupial milk are not always feasible. Hence, methods require modification (down-scaling) to maximise the utility of rare samples. Current milk substitutes for orphaned joeys lack marsupial maternal immune compounds. This review examined current knowledge on maternal immune protection in joeys and identified potential avenues for future research to discover further potent bioactive components. These components could enhance existing milk supplement formulae, increasing the survival rates of orphaned, hand-reared marsupial joeys. Furthermore, different substitutes optimised for the different phases of lactation would be needed to meet the changing needs of the developing joeys. Therefore, although this review offers a preliminary understanding of the requirements of milk substitutes, further work is needed to design milk substitutes that meet marsupial joeys’ immune needs.
Acknowledgements
This manuscript was a collaborative effort between two nodes of the Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, specifically convened for the Marsupial Milk Project, namely the ‘Food and Agriculture Proteomics’ group at Edith Cowan University, Western Australia, and the ‘Australasian Wildlife Genomics’ group at The University of Sydney, New South Wales, Australia.
Authorship
M.W.J.M. and C.E.G. led the conceptualisation and writing of the original draft, with M.W.J.M. also handling the reviewing, editing and visualisation of the information. M.G.N. and K.A.F. contributed to conceptualisation, reviewing, editing and supervision. E.P. assisted with conceptualisation, reviewing and editing, while A.J. was responsible for reviewing, editing and supervision. K.B. supported the project through conceptualisation and funding acquisition. C.J.H. and M.L.C. provided essential support in conceptualisation, reviewing, editing, supervision, project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Financial support
This work was supported by the Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science (grant number CE200100012).
Competing interests
The authors declare none.