Henry(Reference Henry1) suggested over 20 years ago that the ‘salt (NaCl) consumption of a society is a measure of the social stress to which it is exposed’, and that stress, through its effect on increasing salt intake, is an important factor in the development of hypertension. Henry's hypothesis was initially based on ecological studies in human subjects, which suggested that salt intake may be driven by exposure to stress(Reference Henry1, Reference Denton2). These ecological studies relied on observational analyses, rather than on direct measures, and included a comparison of stress exposure in two different Polynesian communities: one which reflected a modern day, Westernised society that was likely to have higher stress exposure, had higher salt intakes and exhibited a blood pressure (BP) rise with age; and the other was the traditional community, with potentially low exposure to stress, and was associated with low salt intakes and minimal increases of BP with age(Reference Henry1–Reference Prior, Evans and Harvey3).
Hypertension is a well-established risk factor for CVD(Reference Bennett and Magnus4, 5), and is a contributing factor to the development of cerebrovascular disease, IHD, and cardiac and renal failure(Reference Whitworth6). There is overwhelming evidence that salt intake is linked to the development of hypertension(Reference Elliott, Stamler and Nichols7–Reference Jurgens and Graudal11). It also appears that stress plays a role in increasing risk of CVD(Reference Bunker, Colquhoun and Esler12). This raises the question – could stress actually induce a salt appetite and increase salt consumption in human subjects?
In this review, we will address the question – does stress increase salt intake in human subjects? Firstly, we describe salt appetite, salt preferences and the physiological responses to stress. Secondly, we assess the effect of stress on salt intake, reviewing evidence from animal models and human studies, which has been the recent focus of the present research. Thirdly, we discuss the physiological mechanisms that may link stress to salt intake using data from animal studies.
Salt appetite and salt preference
Salt appetite is the strong motivation for ingesting salt in situations of salt wasting(Reference Stricker, Kare, Fregly and Bernard13), whereas salt preference is a liking for salt in a Na-replete state(Reference Mattes14); both salt appetite and salt preference can be induced or innate, the latter may be determined by epigenetic influences on the foetus's genotype(Reference Nicolaidis15). Herbivores characteristically exhibit a salt appetite, which is likely to be due to a low Na diet, but omnivores, such as human subjects, do not typically exhibit this behaviour due to a high salt diet(Reference Denton2). Of interest is the finding that taste cells expressing the epithelial Na channel, which mediates behavioural attraction to salt, have been identified in mice, and may be present in human subjects(Reference Chandrashekar, Kuhn and Oka16). Salt appetite in human subjects is rare, but it has been documented in Addison's disease, a condition caused by deficiency of adrenal cortex hormones(Reference Kumar and Clark17), although it only appears to occur in 15 % of the patients with this disease(Reference Thorn, Dorrance and Day18). Gitelman's syndrome is a genetic disorder that affects the renal system, and is characterised by hypomagnesaemia, metabolic alkalosis, hypocalciuria and renal losses of Na(Reference Knoers and Levtchenko19). Cruz et al. (Reference Cruz, Shaer and Bia20) evaluated symptoms in fifty patients with Gitelman's syndrome, and found that 64 % of the patients reported salt cravings, which included drinking pickle brine and consuming salted cucumbers. In a clinical study which induced Na depletion, subjects reported a greater appetite for salty foods, although the authors duly acknowledge that this degree of Na depletion is unlikely to occur in free-living individuals(Reference Beauchamp, Bertino and Burke21). Overall, Na appetite in human subjects is rare, but it has been documented in clinical conditions and can potentially be induced in situations of Na depletion.
Salt preference has also been documented in human subjects. One small study that was conducted in twelve 4–6-month-old exclusively breast-fed infants with apparently no exposure to added salt indicated a preference for salted v. unsalted cereals(Reference Birch22). This may suggest that salt preference may be due to a genetic predisposition, as there was no prior exposure to salt as breast milk is low in salt and the cereal was the first food offered to the infants; the sample size was small and the results should be interpreted cautiously. Studies conducted in twins have determined that salty taste perception and preference are mostly caused by learned experiences rather than by genetics(Reference Wise, Hansen and Reed23). There is also evidence in adults that exposure to highly salty foods results in an increased preference for these foods(Reference Bertino, Beauchamp and Engelman24). Similarly, consumption of a low salt diet increased the perceived saltiness of foods and resulted in a preference for foods with lower salt concentrations(Reference Bertino, Beauchamp and Engelman25). It appears that salt preference is more likely to be influenced by environmental factors (repeated exposure to salt) rather than by genetic factors.
Responses to stress
Stress can be defined as ‘a complex physiological state that embodies a range of integrative physiological and behavioural processes that occur when there is a real or perceived threat to homoeostasis’(Reference Tilbrook and Fink26). Stressors (the noxious agents that threaten homoeostasis) can be physical, psychological or physiological(Reference Dobson and Smith27). Stress can be of short term (acute stress; lasting for seconds, minutes or up to a few hours), can occur repeatedly or on a daily basis (repeated acute stress) or can be continuous and prolonged (chronic stress; lasting for days, weeks or months)(Reference Turner, Hemsworth and Tilbrook28–Reference Turner, Tilbrook, Ashworth and Kraeling30).
Several physiological pathways are activated by stress(Reference Turner, Canny and Hobbs31). In brief, an immediate response to acute stress is the activation of the sympatho-adrenal medullary system, which results in the release of noradrenaline from sympathetic nerve terminals in peripheral tissues and the release of adrenaline and noradrenaline from the adrenal medulla into the systemic blood. This is a rapid response, with plasma concentrations of adrenaline and noradrenaline reaching their peak within about 10–15 min(Reference Tilbrook, Rivalland and Turner32). Activation of this system prepares the individual for a ‘fight or flight’ response to a stressor including increasing heart rate and a redistribution of blood flow to skeletal and cardiac muscle and away from gastrointestinal activities.
Another response to stress is the activation of the hypothalamo–pituitary–adrenal axis. When acute stress is encountered, corticotrophin-releasing hormone and arginine vasopressin are released from the hypothalamus into the hypophyseal portal blood vessels. These neuropeptides stimulate corticotrophs of the anterior pituitary gland to release adrenocorticotrophic hormone into the peripheral blood. In turn, adrenocorticotrophic hormone stimulates the adrenal cortex to synthesise and release glucocorticoids. The principal glucocorticoid synthesised and released in human subjects is cortisol. This is a less rapid response than that of adrenaline and noradrenaline, with plasma concentrations of cortisol reaching a peak within 20–60 min (depending on the type of stress encountered)(Reference Turner, Canny and Hobbs31–Reference Turner, Hosking and Parr33). Cortisol mobilises energy stores through processes such as glycogenolysis in the liver and lypolysis in adipose tissue, thus making substrates available for activities that may be required to respond to the stress. Physiological responses to stress are summarised in Fig. 1.
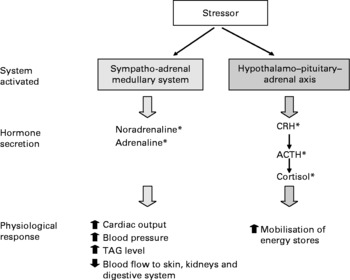
Fig. 1 Physiological systems activated in response to stress. * Any element of the physiological systems activated during stress may be involved in inducing a salt appetite, and there is evidence for some of these actions in animal studies (see text for details). Nevertheless, since human subjects are generally salt replete due to an excess of salt in the food supply, these mechanisms are not likely to be active in human populations. CRH, corticotrophin-releasing hormone; ACTH, adrenocorticotrophic hormone.
Stress-induced salt appetite and potential physiological mechanism
The effect of stress on salt intake
Studies in rats, mice, rabbits and hamsters have investigated the effect of stress on daily salt intake, with most demonstrating an increase in salt intake in response to stress. Stressors of varying severity and duration have been imposed, and the intake of a test NaCl solution has been measured (Table 1). Eight studies reported an increase(Reference Bourjeili, Turner and Stinner34–Reference Ely, Herman and Ely41), one study reported no change(Reference Howell, Harris and Clarke35) and two studies reported a decrease in salt intake(Reference Bensi, Bertuzzi and Armario42, Reference Niebylski, Bertuzzi and Bensi43). It appears that under these conditions, stress can increase salt intake.
Table 1 Summary of the effects of stress on salt intake in animals*
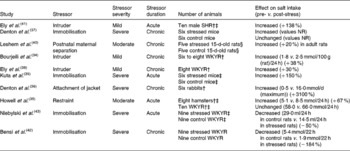
SHR, spontaneously hypertensive rat; WKYR, Wistar-Kyoto rats; NR, not reported.
* All differences are at P < 0·05 level.
† Own control.
‡ Intake measured after conclusion of stress.
§ Na depleted.
Physiological mechanism for a stress-induced salt appetite
Any element of the physiological systems activated during stress may mediate the effects of stress on salt intake. When rats that exhibited a stress-induced increase in salt intake were given a drug that blocked sympatho-adrenal medullary system activity and were then subjected to stress, salt intake from both food and fluids decreased(Reference Bourjeili, Turner and Stinner34, Reference Ely, Herman and Ely41). One possible explanation is that sympatho-adrenal medullary system activity may increase urinary Na excretion, resulting in a Na-depleted state which may increase Na appetite(Reference Light and Turner44, Reference Harshfield, Pulliam and Alpert45).
Corticotrophin-releasing hormone decreased salt intake when administered subcutaneously to sheep(Reference Weisinger, Blair-West and Burns46) and baboons(Reference Shade, Blair-West and Carey47) and when administered directly into the lateral parabrachial nucleus of the brain in rats(Reference De Castro e Silva, Fregoneze and Johnson48). In contrast, corticotrophin-releasing hormone increased salt intake after intracerebroventricular infusion in mice(Reference Denton, Blair-West and McBurnie37) and rabbits(Reference Tarjan and Denton49), and it had no effect after subcutaneous infusion in mice(Reference Denton, Blair-West and McBurnie37). Subcutaneous infusion of adrenocorticotrophic hormone stimulated salt intake in mice(Reference Denton, Blair-West and McBurnie37), sheep(Reference Weisinger, Blair-West and Burns46), rabbits(Reference Tarjan and Denton49) and rats(Reference Weisinger, Denton and McKinley50), although studies in baboons(Reference Shade, Blair-West and Carey47) and pigs(Reference Jankevicius and Widowski51) reported no effect of intramuscular injections of adrenocorticotrophic hormone. Glucocorticoids may also influence salt appetite, but possibly only when co-administered with mineralocorticoids. Shelat et al. (Reference Shelat, King and Flanagan-Cato52) found that glucocorticoids in isolation did not influence salt intake in rats, but when glucocorticoids were administered in combination with a mineralocorticoid, a salt appetite was induced, and this may have been mediated by the direct effects of angiotensin II in the brain. In another study conducted in rats, glucocorticoid co-administered with a mineralocorticoid was found to increase salt intake, and this may have been due to increased urinary excretion of water and Na(Reference Thunhorst, Beltz and Johnson53); a Na-induced appetite may have resulted from Na depletion(Reference Rowland, Farnbauch and Crews54). It is not clear why administration of elements of the hypothalamo–pituitary–adrenal axis resulted in different outcomes in different studies. The route of administration may be important since salt appetite-regulatory pathways(Reference Geerling and Loewy55) may be influenced in a stimulatory or inhibitory manner through different routes of administration of the hypothalamo–pituitary–adrenal axis hormones. There may also be species-specific differences related to the function of the hypothalamo–pituitary–adrenal axis hormones in salt appetite. Further studies would be needed to elucidate the precise mechanisms involved. A systematic approach in a single species would be best, with caution being exercised in the extrapolation of the findings to human subjects.
The mechanism of salt appetite involves two processes: central regulation and regulation by the renal system. Mechanisms of central regulation of salt appetite are not fully resolved, but they are thought to include input signals from aldosterone and angiotensin II, from sensory inputs via baroreceptors and from detection of intracerebroventricular Na concentrations(Reference Geerling and Loewy55). Many different brainstem and forebrain regions (such as lamina terminalis and amygdala) are involved in the integration of these signals. These brain regions are involved in inducing motor responses such as salt-ingestive behaviours in the case of Na deficiency(Reference Geerling and Loewy55). Glucocorticoids are thought to enhance the salt appetite-promoting actions of aldosterone by increasing the concentration of mineralocorticoid receptors in the brain(Reference Ma, McEwen and Sakai56, Reference Zhang, Epstein and Schulkin57). The renal system is also involved in the regulation of Na levels via the renin–angiotensin–aldosterone system(Reference Sherwood58). When Na levels fall, renin is secreted by the kidney. Renin catalyses the conversion of angiotensinogen to angiotensin I and then to angiotensin II via angiotensin-converting enzyme. Angiotensin II results in the secretion of aldosterone from the adrenal cortex, which in turn increases Na reabsorption by the distal and collecting tubules of the kidney. Angiotensin II and aldosterone act directly on the lamina terminalis and amygdala to stimulate Na appetite.
Stress-induced salt intake: evidence from human studies
There are five laboratory studies conducted in human subjects, which allow close monitoring of food intake, that have examined the effect of stress on intake of salt and high salt foods (Table 2). One of these was a study that we conducted in men and women (n 20) with a mean age of 38·6 (sd 11·5) years and a mean BMI of 23·8 (sd 3·3) kg/m2, which investigated the effect of acute mental arithmetic stress induced in a laboratory setting on salt preference (Torres SJ & Nowson CA, unpublished results, 2008). Subjects were asked to indicate their preference for tomato juice with a range of salt concentrations (0, 109, 173, 240, 304, 370, 435 and 565 mmol/l) before and after acute mental stress. The mean perceived level of stress (range: 1 (no stress) to 10 (severe stress)) was 5·9 (sem 0·5). The mental stress test caused a significant increase in systolic BP (+13·8 (sem 2·2) mmHg), diastolic BP (+8·7 (sem 1·5) mmHg) and pulse rate (+11·2 (sem 7·9) beats per minute) (P < 0·05 for all). There was no significant difference in mean salt preferences in the non-stressed and post-stress states, 82 (sem 16) v. 96 (sem 13) mmol/l (P>0·05). All five laboratory studies reported significant increases in stress by either subjective or objective measures: three studies reported significant increases in BP and heart rate (Torres SJ & Nowson CA, unpublished results, 2008)(Reference Oliver, Wardle and Gibson59, Reference Miller, Friese and Dolgoy60); one study reported an increase in cortisol(Reference Epel, Lapidus and McEwen61) and three studies reported increases in self-reported stress (Torres SJ & Nowson CA, unpublished results, 2008)(Reference Oliver, Wardle and Gibson59, Reference Zellner, Loaiza and Gonzalez62). In summary, laboratory-based studies have found no effect of stress on salt intake in human subjects. Even though all the studies reported significant increases in stress, we may not see an effect on salt intake in laboratory studies as the response to acute stress induced may differ from chronic exposure to a stressful environment.
Table 2 Summary of the effects of stress on salt intake in human subjects*

TSST, Trier Social Stress Test.
* All differences are at P < 0·05 level.
† Torres SJ & Nowson CA, unpublished results, 2008.
‡ Own control.
§ Based on Buss-Durkee Hostility Inventory scores.
Naturalistic studies provide the opportunity to measure the effect of life stress on salt intake. The effect of self-reported stress on eating behaviour was examined in 212 undergraduate students (Table 2)(Reference Oliver and Wardle63). Snacking increased during periods of stress, and foods eaten in greater quantity included sweets and chocolate, cakes and biscuits, and savoury snacks (high salt foods). However, the observed increase in the consumption of savoury snacks in response to stress may be due to a drive for fat rather than for salt, or the perception that snacks are treats/rewards. In a previous review, we concluded that chronic life stress seems to be associated with a greater preference for energy- and nutrient-dense foods, namely those that are high in sugar and fat(Reference Torres and Nowson64).
It has been suggested that cortisol, a key hormone secreted during stress, may be a critical factor in the drive for hedonic, highly palatable foods(Reference Dallman, la Fleur and Pecoraro65) such as foods containing a high content of salt(Reference Yeomans, Blundell and Leshem66). Cortisol may increase appetite by affecting leptin and neuropeptide Y, key hormones that reduce(Reference Blundell, Goodson and Halford67) and stimulate food intake(Reference Levine and Billington68), respectively. A study with fifty-nine premenopausal women subjected to 45 min of stress (visuospatial puzzles, serial subtraction of a high number from a low number and delivery of a videotaped speech) found that women with high cortisol reactions (defined as the increase from baseline to stress levels of salivary cortisol) consumed significantly more high fat sweet foods but the same amount of salty foods (potato chips) compared with women with low cortisol reactions (Table 2)(Reference Epel, Lapidus and McEwen61). In a study with six men, administration of cortisol over 5 d, which significantly increased BP, did not alter salt preference(Reference Wong, Williamson and Brown69). Currently, there is limited evidence to support the suggestion that cortisol can increase salt intake in human subjects.
Thus, evidence from laboratory studies in human subjects, who are consuming salt in amounts in excess of physiological requirement, indicate that stress is not likely to influence salt intake.
Does stress induce salt intake in human subjects?
While animal models provide some evidence for a relationship between stress and salt intake, this has not been demonstrated in laboratory studies conducted in human subjects. We may not see an effect in laboratory studies in this acute stress situation, and this may differ from chronic stress that is experienced in the real world. This is supported by the findings of one study which found that during periods of life stress, consumption of highly salty snack foods increased(Reference Oliver and Wardle63); this finding will need to be confirmed in future studies. Importantly, an alternative explanation will need to be considered for why salty foods are consumed during periods of life stress, such as insufficient time to purchase and prepare foods and increased use of convenience foods which are typically high in salt, or as a learned response to a stressful situation.
The physiological requirement for Na in human subjects is 8·0–10·0 mmol/d (8·5–10·3 mmol salt/d)(70), yet many Westernised populations are consuming in excess of these requirements, up to twenty-four times of what is needed(71). In a study conducted in Australia, Na intake determined from 24 h urinary Na excretion was 118·0 mmol/d in women and 170·0 mmol/d in men, which is eighteen and seventeen times of what is needed in women and men, respectively(Reference Beard, Woodward and Ball72). These current high intakes of salt are due to the abundance of salt in the food supply, particularly in processed food products(Reference James, Ralph and Sanchez-Castillo73). Examples of population groups that have very low salt intakes are rare and limited to traditional communities(Reference Denton2). Therefore, it seems that in situations of Na depletion, human subjects could exhibit an increase in salt appetite, but this is generally not seen as salt is mostly consumed in amounts which exceed requirements. This was confirmed by two animal studies which found that rats on high intakes of salt, in excess of physiological requirements, exhibited no change in salt intake in response to stress(Reference Howell, Harris and Clarke35), whereas rats on lower intakes did increase their salt intake in response to stress(Reference Bourjeili, Turner and Stinner34). We may also not see a shift in salt preference in human subjects at these current high intakes of salt.
Conclusions
Data from animal studies suggest that stress might be a driver for salt intake, with evidence for a potential mechanism by the sympatho-adrenal medullary system and/or hypothalamo–pituitary–adrenal axis. In contrast, laboratory studies conducted in human subjects have found no effect of acute stress on salt intake. However, one study which measured the effect of life stress found that intake of snack foods including highly salty foods did increase, although there are likely to be a range of drivers for this behaviour other than a craving for salt taste. Stress could induce a learned response to consume comfort foods during stress which could include high salt foods. The majority of studies in human subjects have investigated the effect of acute stress. Studies investigating the influence of chronic stress on eating behaviours are required, including consumption of salty foods. In the current environment where most human subjects are consuming Na well in excess of physiological requirements, acute stress is unlikely to increase salt intake.
Acknowledgements
S. J. T. performed the literature review and wrote the manuscript except for the sections on ‘Responses to stress’ and ‘Physiological mechanism for a stress-induced salt appetite’, which were written by A. I. T.; C. A. N. provided expert input and guidance. All authors have read and approved all sections of the manuscript, and participated in the decision to submit for publication. The authors have no conflict of interest. The present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.





