Vitamin D deficiency is a major public health problem and an unrecognizable condition of epidemic proportions in both developed and developing countries, with widespread reports of 30–93 % prevalence in children and adults( Reference Hashemipour, Larijani and Adibi 1 – Reference Andıran, Çelik and Akça 3 ). Determination of vitamin D status in different age groups in a community and in different climates of a country is necessary and has important implications for general health. Recent studies suggest that vitamin D deficiency is no longer just a problem of the older generations but also is an important health concern among younger generations( Reference Shin, Kim and Lee 4 ). Studies in the last two decades have shown a high prevalence of vitamin D deficiency in healthy adolescents in different countries( Reference Melamed and Kumar 5 – Reference Neyestani, Hajifaraji and Omidvar 15 ). In Iran, Razzaghy-Azar and Shakiba and Moussavi et al. reported that 67 % of female children and adolescents in Tehran and 72·1 % of teenage girls in Isfahan had vitamin D deficiency, with higher rates of deficiency in girls than boys( Reference Razzaghy-Azar and Shakiba 16 , Reference Moussavi, Heidarpour and Aminorroaya 17 ). Vitamin D is known as the ‘sunshine vitamin’ because the mean exposure to sunlight should be sufficient for most people to produce their own vitamin D using UV light and cholesterol in the skin( Reference Cashman 18 ). Whenever sun exposure is insufficient, dietary intake of this vitamin becomes a necessity. However, there are few dietary sources of vitamin D available to provide the daily recommended requirements( Reference Khadilkar 19 ). Serum 25-hydroxyvitamin D (25(OH)D) is a commonly used marker of vitamin D nutritional status( Reference Alemzadeh, Kichler and Babar 20 – Reference Ganji, Zhang and Shaikh 24 ). Vitamin D is the most important determinant of growth and body development during childhood and adolescence( Reference Santos, Mascarenhas and Satler 25 ). Apart from its necessity for bone metabolism, vitamin D has multiple extraskeletal beneficial effects( Reference Saintonge, Bang and Gerber 12 , Reference Gannagé-Yared, Chedid and Khalife 26 ). Vitamin D plays a role in glucose homeostasis, insulin secretion and action, and regulation of lipolysis( Reference Cashman 18 , Reference Lu, Yu and Pan 21 , Reference Ashraf, Alvarez and Saenz 23 , Reference Khader, Batieha and Jaddou 27 , Reference Reis, von Mühlen and Miller 28 ). Low levels of serum 25(OH)D have been linked to some chronic diseases, including the metabolic syndrome (MetS), diabetes mellitus, CVD, myocardial infarction, peripheral arterial disease, hypertension, multiple sclerosis and cancers( Reference Ford, Zhao and Li 29 – Reference Ahonen, Tenkanen and Teppo 37 ). Even though the underlying mechanism has not been well understood, 25(OH)D appears to exert effects through direct modulation of gene expression via vitamin D receptors( Reference Lu, Yu and Pan 21 ). A possible role for vitamin D deficiency in the pathogenesis of MetS has been recently proposed( Reference Salekzamani, Neyestani and Alavi-Majd 38 ). MetS, a constellation of cardiometabolic disease risk factors, has become a global epidemic( Reference Lu, Yu and Pan 21 ). MetS is characterized by abdominal adiposity, dyslipidaemia and elevated glucose and blood pressure (BP)( Reference Ganji, Zhang and Shaikh 24 ). The prevalence and risk factors of MetS have been extensively studied in adults, while data from children and adolescents are more limited. In a national study conducted by Esmaillzadeh et al. on 3036 Iranian adolescents aged 10–19 years, the prevalence of MetS, according to the National Cholesterol Education Program Adult Treatment Panel III definition, was 10·1 %( Reference Esmaillzadeh, Mirmiran and Azadbakht 39 ). A relationship between 25(OH)D status and MetS was suggested in several epidemiological studies in Western populations( Reference Botella-Carretero, Alvarez-Blasco and Villafruela 40 , Reference Hjelmesaeth, Hofsø and Aasheim 41 ). Nevertheless, evidence from Asian populations, especially in adolescents, is limited( Reference Lu, Yu and Pan 21 , Reference Gannagé-Yared, Chedid and Khalife 26 , Reference Salekzamani, Neyestani and Alavi-Majd 38 , Reference Moy and Bulgiba 42 ). Because of ethnic differences in 25(OH)D metabolism and its nutritional status among different populations, it is not clear whether the findings from Western populations could be extrapolated directly to Asian individuals( Reference Lu, Yu and Pan 21 ). Considering that there is little information about vitamin D status in Iranian adolescents and that its probable role in MetS has not been investigated, we therefore initiated the present study to evaluate vitamin D status and its relationship with MetS components in Iranian adolescent girls attending high school in Boukan, a city of Western Azerbaijan, in north-west Iran.
Methods
Study population
In the current cross-sectional study, a sample of 216 girls (14–17 years old) was selected from high schools in Boukan city during winter (in February) 2012 by a multistage stratified random sampling technique. Sample size was determined using as primary end point the relationship between fasting blood glucose (FBG) and vitamin D. Considering a 95 % confidence level and a power of 80 %, in two-tailed tests using G-Power 3·1·2 software the sample size was calculated to be at least 210 subjects. In the first stage, eight high schools were selected through systematic random sampling from all four districts (two schools per each district) of Boukan. In the second phase, total determined samples (n 240) were allocated to schools and then classes, the number of students was determined proportionately based on the schools’ populations and also by student grade, then classes in each school (two to four classes in schools for each grade) were randomly selected by using even and odd numbers of each grade. Finally, in each class the participants were selected based on a simple random sampling scheme. The final sample included 216 adolescent girls. The ethical committee of Tabriz University of Medical Sciences approved the study protocol. Girls with a history of disease (including diabetes, heart disease, hepatic, kidney and gastrointestinal diseases, and cancer) or with any use of medication or nutritional supplements (such as vitamin D, calcium, etc.) were excluded from the study. Written informed consent was obtained from the students and their parents prior to the study. Information about general characteristics was obtained by interviewing the participants.
Anthropometric assessments
Body weight was measured using a calibrated beam scale and was approximately recorded to 0·5 kg. The participants were measured barefoot wearing light clothing. Height was measured using a mounted tape with the participants’ arms hanging freely at their sides and was approximately recorded to 0·5 cm. BMI was calculated as the weight in kilograms divided by the square of height in metres. Underweight, healthy weight, overweight and obesity were defined according to the international BMI cut-off points for adolescents. At risk or overweight was defined as ≥85th to <95th percentile of BMI for age and sex, and obese was defined as ≥95th percentile of BMI for age and sex( Reference Cole, Flegal and Nicholls 43 , Reference Cole, Bellizzi and Flegal 44 ). Waist circumference (WC) was approximately recorded to 0·1 cm by a measuring tape. WC was determined at the midpoint between the lowest rib and the iliac crest while the participant was standing and after expiration( Reference Esmaillzadeh, Mirmiran and Azadbakht 39 ).
Blood pressure measurements
Systolic BP (SBP) and diastolic BP (DBP) were measured in the morning by a mercury sphygmomanometer with an adult cuff. BP was measured on the upper right arm, with the arm horizontally on a table, while the participant in the sitting position and after 5 min rest. SBP was measured as the first detectable sound (phase 1) and DBP was measured as the disappearance of Korotkoff sounds (phase 5). Two readings were recorded at an interval of 1–2 min, and the cuff was completely deflated between readings. The mean of the two readings was calculated for analysis. Sex-, age- and height-specific percentile levels were defined using US normative BP tables for children and adolescents. Hypertension was defined as SBP and/or DBP ≥ 95th percentile( 45 ).
Energy intake
Information about daily energy intake was obtained by the 24 h recall method for 3 d, including two weekdays and one weekend day, and analysed by Nutritionist 4 software (First Databank Inc., Hearst Corp., San Bruno, CA, USA).
Physical activity
The validated short form of the Iranian version of the International Physical Activity Questionnaire was used to estimate levels of physical activity( Reference Kelishadi, Rabiee and Khosravi 46 ). For each activity level, the metabolic equivalent of task (MET) value was multiplied by the time spent at that particular level. The MET-time at each level was added to obtain a total over 24 h MET-time, representing the physical activity level on an average weekday. In the current study, we categorized the physical activity level as sedentary (<3 MET), moderate (3–6 MET) or vigorous (≥6 MET)( Reference Kelishadi, Ardalan and Gheiratmand 47 ).
Laboratory methods
Venous blood samples (5 ml) were obtained for all participants after a 12 h overnight fast. Serum was separated by centrifugation and stored frozen at −70°C until assay. Serum 25(OH)D concentration was measured by an ELISA kit (EQ 6411-9601, lot E 120203 BL; EUROIMMUN AG, Lübeck, Germany) using an ELISA plate reader (model Stat Fax® 2100; Awareness Technology Inc., Palm City, FL, USA). The intra- and inter-assay CV were 2·4–4·4 % and 5·9–8·2 %, respectively. The following definitions were used for stratification of vitamin D status: deficiency, 25(OH)D level <20 ng/ml; insufficiency, 25(OH)D level <30 ng/ml; and sufficiency, 25(OH)D level ≥30 ng/ml( Reference Scharla 7 ). FBG was measured by an enzymatic colorimetric method (Alcyon 300 automated biochemistry analyser; Abbott Laboratories, Abbott Park, IL, USA) using a glucose oxidase kit (Pars Azmoon Inc., Tehran, Iran); inter- and intra-assay CV were both less than 2·2 %. For lipids, measurement kits (Pars Azmoon Inc.) and an biochemistry analyser (Alcyon 300) were used. TAG were assayed using an enzymatic colorimetric assay with glycerol phosphate oxidase. HDL-cholesterol (HDL-C) was measured after precipitation of the apoB-containing lipoproteins with phosphotungistic acid. All samples were analysed when internal quality control met the acceptable criteria. Inter- and intra-assay CV were 2·0 % and 0·5 % for HDL-C and 1·6 % and 0·6 % for TAG, respectively.
Description of the metabolic syndrome
MetS was defined according to the modified criteria of the National Cholesterol Education Program Adult Treatment Panel III. Participants with three or more characteristic of the following components were categorized as having MetS: (i) abdominal obesity (WC > age- and sex-specific 75th percentile for this population); (ii) elevated BP (SBP/DBP > age, sex- and height-specific 90th percentile; (iii) low HDL-C level (<50 mg/dl); (iv) elevated serum TAG (≥100 mg/dl); (v) elevated FBG (≥110 mg/dl)( Reference Kelishadi 48 – Reference de Ferranti, Gauvreau and Ludwig 50 ).
Statistical analyses
Data were expressed as mean and standard deviation for quantitative variables and as number and percentage for qualitative variables. Correlations between two sets of data were evaluated using Pearson's correlation. A multiple linear regression analysis was conducted to examine the relationship between serum 25(OH)D as independent variable and MetS components as dependent variables adjusting for energy, BMI and physical activity level. Data were analysed using the data entry and statistical software package IBM SPSS Statistics 17·0. A P value less than 0·05 was considered statistically significant.
Results
The general and clinical characteristics of the participants are shown in Table 1. Mean daily vitamin D intake of the participants was lower than the recommended dietary reference intake (15 μg). MetS was present in 10·6 % of the girls. Table 2 shows the vitamin D status and biochemical components of MetS in the studied population. Mean serum 25(OH)D was 7·26 (sd 2·81) ng/ml, ranging between 1·7 and 22·8 ng/ml. Prevalence of vitamin D deficiency and insufficiency was 95·8 % (95 % CI 93·2 %, 98·5 %) and 4·2 %, respectively. No one had vitamin D sufficiency. Characteristics of participants without and with MetS are presented in Table 3. Mean weight, BMI, WC and serum FBG and TAG were significantly higher, while mean serum HDL-C levels were significantly lower, in girls with MetS in comparison to girls without MetS (P < 0·001 for all except serum 25(OH)D, where P = 0·035). Table 4 shows the association between serum 25(OH)D and components of MetS. In the multiple-adjusted linear regression analysis, serum 25(OH)D was inversely associated with FBG (β = −0·143, P = 0·04). No significant associations were found between serum 25(OH)D and other risk factors of MetS. Age was entered into the model; however, there was no significant effect of age and therefore age was removed from the model. There was also no significant relationship (r = 0·066, P = 0·331) between serum 25(OH)D and daily vitamin D intake (data not shown).
Table 1 General and clinical characteristics of adolescent girls aged 14–17 years, Boukan, Iran, winter 2012 (n 216)
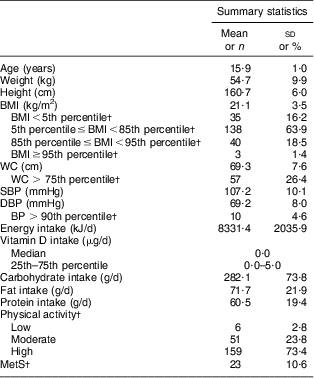
WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; MetS, metabolic syndrome.
†Data are expressed as n and %.
Table 2 Serum 25(OH)D and biochemical components of MetS in adolescent girls aged 14–17 years, Boukan, Iran, winter 2012 (n 216)
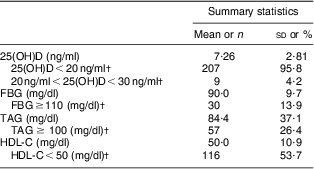
25(OH)D, serum 25-hydroxyvitamin D; MetS, metabolic syndrome; FBG, fasting blood glucose; HDL-C, HDL-cholesterol.
†Data are expressed as n and %.
Table 3 Comparison of general, clinical and metabolic characteristics of adolescent girls aged 14–17 years without and with MetS, Boukan, Iran, winter 2012 (n 216)
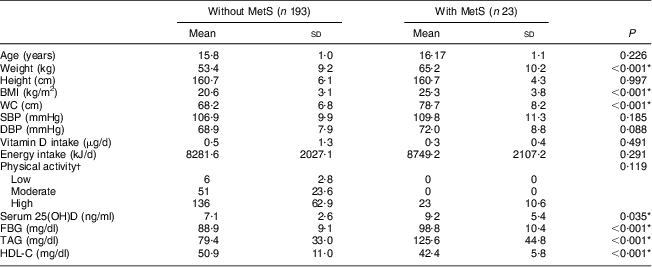
MetS, metabolic syndrome; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; 25(OH)D, serum 25-hydroxyvitamin D; FBG, fasting blood glucose; HDL-C, HDL-cholesterol.
*P<0·05 is significant.
†Data are expressed as n and %.
Table 4 Relationship between serum 25(OH)D and components of the MetS in adolescent girls aged 14–17 years, Boukan, Iran, winter 2012 (n 216)
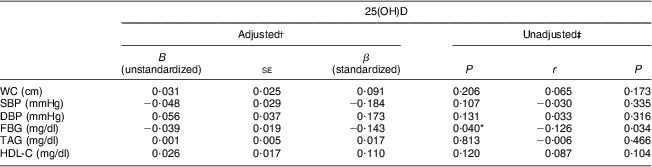
25(OH)D, serum 25-hydroxyvitamin D; MetS, metabolic syndrome; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-C, HDL-cholesterol.
*P < 0·05 is significant.
†Values were adjusted for energy intake, physical activity and BMI.
‡Pearson correlation.
Discussion
The present study is the first one to investigate vitamin D status among adolescent high-school girls in Boukan, Iran. Our data demonstrated that an unexpected proportion of healthy adolescents (96 %) had vitamin D deficiency (serum 25(OH)D ≤ 20 ng/ml). The prevalence of vitamin D deficiency in our study population is higher than that in other provinces of Iran, including 72·1 % for adolescent girls aged 14–18 years in Isfahan and 67 % for girls aged 8–18 years in Tehran( Reference Razzaghy-Azar and Shakiba 16 , Reference Moussavi, Heidarpour and Aminorroaya 17 ). It is even higher than that reported for adolescent girls of other countries, such as Turkey (64·8 %), the USA (54 %) and Brazil (36·3 %)( Reference Andıran, Çelik and Akça 3 , Reference Saintonge, Bang and Gerber 12 , Reference Santos, Mascarenhas and Satler 25 ). Similar to our results, Zhu et al. reported that 100 % of the adolescents (male and females) aged 12–16 years in Hanguzhou, China had low serum 25(OH)D levels in winter (<30 ng/ml)( Reference Zhu, Zhan and Shao 51 ). In a study conducted by Shin et al. on Korean adolescents aged 12–13 years, vitamin D insufficiency or deficiency was found in 100 % of girls( Reference Shin, Kim and Lee 4 ). Our finding clearly indicates that vitamin D deficiency is an important health problem in the studied adolescent girls.
Poor 25(OH)D status in these adolescent girls likely results from low dietary intake, inadequate exposure to sunshine and skin covering. Unless fortified, most foods are a poor source of vitamin D, with the exception of fish oil, egg yolk, and certain types of fish and seafood( Reference Prentice 52 ). These food sources are limited in the Iranian diet( Reference Kalantari and Ghafarpour 53 ) and our findings confirmed low vitamin D intake in our study participants. There is also no vitamin D fortification programme ongoing in Iran now. Moreover, according to our religious regulations, females should wear long-sleeved clothes, so that only the face and hands (from wrist to fingers) are allowed to be exposed. This might also have contributed to the high occurrence of poor vitamin D status in our participants despite them having a relatively high physical activity level which included outdoor activities, too. Boukan is located at latitude of 37°N, where dermal synthesis of vitamin D in cold seasons is nearly minimal. During this time, the body inevitably relies on vitamin D body stores. The very high prevalence of vitamin D deficiency in our study participants reflected well their inefficient vitamin D storage during warm seasons.
According to our results, the frequency of MetS was found to be 10·6 % (n 23). Esmaillzadeh et al. demonstrated a 9·9 % prevalence of MetS in girls aged 10–19 years in Tehran, Iran( Reference Esmaillzadeh, Mirmiran and Azadbakht 39 ). In a study in Mashhad, another province of Iran, MetS was recorded in 6·5 % of girls aged 15–17 years( Reference Mirhosseini, Yusoff and Shahar 54 ). Results of a study by Kelishadi et al. in a national sample of youths in Iran indicated that MetS was present in 11·7 % of girls aged 14–18 years( Reference Kelishadi, Gouya and Adeli 55 ). No other published data are available about MetS prevalence in Iranian adolescents. In studies conducted in other countries, presence of MetS was reported in 8·6 %, 2·5 % and 6·5 % of US adolescent girls and Korean and Mexican adolescents (girls and boys), respectively( Reference Johnson, Kroon and Greenway 56 – Reference Rodríguez-Morán, Salazar-Vázquez and Violante 58 ). Behavioural factors such as poor dietary habits (including high intakes of carbohydrate, fat and processed foods) and other environmental factors, as well as ethnic predisposition to MetS, may implicated in developing this health problem in different populations( Reference Park, Hilmers and Mendoza 59 ).
The link between 25(OH)D status and MetS has been, and still is, under debate( Reference Lu, Yu and Pan 21 , Reference Salekzamani, Neyestani and Alavi-Majd 38 ). Serum 25(OH)D levels in our study were shown to be inversely correlated with FBG. In the present study, participants with MetS had significantly lower 25(OH)D levels than participants without MetS. The inverse relationship between serum 25(OH)D and FBG was reported in other studies, too( Reference Alemzadeh, Kichler and Babar 20 , Reference Lu, Yu and Pan 21 , Reference Gannagé-Yared, Chedid and Khalife 26 , Reference Reis, von Mühlen and Miller 28 , Reference Delvin, Lambert and Levy 60 ). However, in a study by Ashraf et al. on obese African-American adolescent females, no relationship was found between serum 25(OH)D and FBG( Reference Ashraf, Alvarez and Saenz 23 ). Although the exact mechanisms linking 25(OH)D insufficiency with hyperglycaemia and subsequent diabetes risk are not completely understood, there is accumulating evidence that 25(OH)D may directly influence pancreatic β-cell secretory function through nuclear 25(OH)D receptors and may influence insulin sensitivity through insulin receptors’ expression regulation of intracellular calcium( Reference Reis, von Mühlen and Miller 28 ). The finding of no significant associations between serum 25(OH)D and other components of MetS, including WC, serum TAG, serum HDL-C and BP, in our study is similar to the results of studies conducted on women in Iran and on adults (men and women) in Jordan( Reference Khader, Batieha and Jaddou 27 , Reference Salekzamani, Neyestani and Alavi-Majd 38 ). Another study performed among adolescents of French origin in Canada failed to reveal an association between serum 25(OH)D and the existence of at least two components of MetS( Reference Delvin, Lambert and Levy 60 ). Erdönmez et al. found no association of serum 25(OH)D with components of MetS in Turkish adolescent girls( Reference Erdönmez, Hatun and Çizmecioğlu 61 ). On the other hand, the results of the 2001–2004 National Nutrition and Health Survey in the USA showed a strong association between low 25(OH)D level and MetS( Reference Reis, von Mühlen and Miller 28 ). The discrepancies seen in the results of various studies may be due to the different study populations, polymorphism of the vitamin D receptor, ethnic differences in vitamin D metabolism, the season of the studies and the methods used for determination of 25(OH)D( Reference Saintonge, Bang and Gerber 12 , Reference Reis, von Mühlen and Miller 28 , Reference Salekzamani, Neyestani and Alavi-Majd 38 ). It should be noted that we found a remarkably high prevalence of vitamin D deficiency among the 14–17 years age group included in the present sample. It is possible that the narrow range of serum 25(OH)D might contribute to mask any significant relationships between vitamin D and most of the components of MetS in our sample.
In present study, there was no significant relationship between serum 25(OH)D and BMI. In the literature, some reports showed no interaction of BMI and 25(OH)D, but particularly studies on adults and a few on children and adolescents demonstrated a relationship between obesity status and serum 25(OH)D( Reference Neyestani, Hajifaraji and Omidvar 15 , Reference Gannagé-Yared, Chedid and Khalife 26 , Reference Smotkin-Tangorra, Purushothaman and Gupta 62 ). In the study by Çizmecioğlu et al. in obese and overweight adolescents with hypovitaminosis D, serum 25(OH)D level decreased as BMI increased( Reference Çizmecioğlu, Etiler and Görmüş 6 ). It is believed that extra body fat, by sequestration of 25(OH)D, may reduce its bioavailability( Reference Wortsman, Matsuoka and Chen 63 ). As mentioned above in connection with MetS components, the narrow range of serum 25(OH)D in our participants might also have limited the possible relationship of serum 25(OH)D with BMI, too.
Our study had some limitations. Blood samples were drawn during the cold season, when dermal synthesis of vitamin D is negligible, so the vitamin D status of the study participants did not necessarily reflect their status for the whole year. Other limitations include the relatively small sample size and the lack of information about sun exposure and use of sunscreen. The cross-sectional nature of our data also impedes the ability to infer causation between vitamin D and MetS risk factors.
Conclusion
The results of the present study indicated the problem of vitamin D deficiency in adolescent Iranian girls. Low serum 25(OH)D was associated inversely with FBG. Future studies with a long-term longitudinal design are warranted to determine whether low vitamin D status during adolescence may impact MetS and related disorders such as diabetes and CVD during adulthood. At the present time, development and implementation of national polices and interventions towards improving vitamin D status among adolescent girls are needed.
Acknowledgements
Sources of funding: The present study was funded by the Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. The Nutrition Research Center had no role in the design, analysis or writing of this article. Conflicts of interest: The authors declare no conflicts of interest, both financial and non-financial, for this study. Ethical approval: The study was approved by the ethical committee of Tabriz University of Medical Sciences, Tabriz, Iran. Authors’ contributions: The whole project was designed and conducted by M.R., as a supervisor; and S.K.-H. contributed to data collection and on-site study management. Sampling methodology and statistical analysis were performed under the direction of M.A.-J. All laboratory work was carried out at the Drug Applied Research Center, Tabriz, Iran. All authors contributed to manuscript preparation. Acknowledgements: The authors thank all the school directors and students who participated in the study, and the parents who permitted their children to do so.






