Introduction
The amount of obese individuals in the world today is steadily reaching epidemic proportions. Importantly, obesity is not only an adult issue, but children are also increasingly affected. This increase in frequency of sufferers of obesity is mainly attributed to high-energy diets and low activity levels. Because of increased knowledge regarding the effects of obesity, many products and quick-fix solutions are currently on the market. Many of these products are alternative medicines or vitamins, typically seen as harmless and able to improve health. Particularly, products that are known to have antioxidant value claim to address obesity. The effects of some of these products on children and adults have not been adequately tested, and particularly in a condition like obesity, which is a health concern that is multi-faceted, could potentially have very negative outcomes.
Obesity is not a health complication on its own, but brings with it significant associated co-morbidities. These include CVD and heart attacks, type 2 diabetes mellitus, non-alcoholic fatty liver disease, insulin resistance(Reference Avogaro and de Kreutzenberg1, Reference Baur, Pearson and Price2), hypertension, dyslipidaemia, impaired glucose homeostasis, sleep apnoea, accelerated skeletal and pubertal development (ageing processes), as well as cancer, orthopaedic disorders and psychological consequences(Reference Wellman and Friedberg3–Reference Naderali5). Although many of these conditions only develop later in life, obese individuals are already setting the scene for their future health concerns when they are young.
The adipose tissue of visceral obesity in particular has been found to release pro-inflammatory cytokines. Inflammation seems to be central to the multitude of obesity-related complications that shorten the lifespan of suffering individuals. Inflammation leads to endothelial dysfunction which ultimately results in CVD, either through atherogenesis or through more direct mechanisms. Inflammation also has a negative effect on glucose metabolism, leading to insulin resistance which is problematic either through resultant type 2 diabetes mellitus (impaired glucose homeostasis), or through causal relationships with endothelial dysfunction (insulin stimulates NO synthesis) which then eventually leads to CVD yet again. Insulin resistance is complicating in that it not only creates grounds for vascular effect impairment but also for metabolic impairment. Thus, obesity literally causes a cascade of interrelated health complications that ultimately shorten the lifespan of suffering individuals. For a schematic diagram of the inter-relationship of the obesity-related co-morbidities discussed here, see Fig. 1.

Fig. 1 Schematic diagram of the inter-relationship of most prominent co-morbidities of obesity. ROS, reactive oxygen species.
The link between obesity and endothelial dysfunction and insulin resistance is strengthened by the finding that endothelium-dependent vasodilation is impaired in proportion to insulin resistance and various indices of adiposity under baseline conditions(Reference Avogaro and de Kreutzenberg1, Reference De Jongh, Serne and Ilserman6).
One of the antioxidants/anti-inflammatories that is now seen as a being advantageous in the treatment of obesity is resveratrol, and, although it is typically used by adults, there are no restrictions against its use by children. Resveratrol is a naturally occurring phenolic substance, present in a variety of plants as a defence mechanism against bacterial and other infections(Reference Naderali5, Reference Breen, Sanli and Giacca7) as well as environmental stressors(Reference Das and Maulik8). A wide range of beneficial effects have been attributed to resveratrol, including anti-inflammatory, anti-cancer, anti-lipid, anti-ageing, as well as vasoprotective effects(Reference Naderali5, Reference Breen, Sanli and Giacca7). The substance has been reported to elicit numerous cellular responses including cell cycle arrest, differentiation and apoptosis(Reference Joe, Liu and Suzui9, Reference Palsamy and Subramanian10). It also displays anti-leukaemic, antiviral and neuroprotective properties(Reference Palsamy and Subramanian10–Reference Gao, Xu and Divine13). Resveratrol is able to function as an in vivo antioxidant(Reference Palsamy and Subramanian10, Reference Das, Sato and Ray14) and markedly reduces the risk of developing CHD; this property is probably due to its modulation of lipid metabolism and prevention of oxidation of the LDL(Reference Palsamy and Subramanian10, Reference Frankel, Waterhouse and Kinsella15), as well as inhibition of eicosanoid production and platelet aggregation(Reference Palsamy and Subramanian10, Reference Pace-Asciak, Hahn and Diamandis16). Additionally, resveratrol has been shown to inhibit several important enzymes involved in carcinogenesis(Reference Palsamy and Subramanian10, Reference Fontecave, Lepoivre and Elleingand17, Reference Chun, Kim and Guengerich18).
Complications of obesity
Obesity (Fig. 1) is characterised by an excess amount of adipose tissue. It has become recognised as an independent factor of cardiovascular risk, predisposing the obese individual to type 2 diabetes mellitus, hypertension, reduced endothelial function, as well as inducing insulin resistance(Reference Wellman and Friedberg3, Reference Naderali5). Noteworthy is the fact that obesity is a component of the metabolic syndrome, of which insulin resistance is the hallmark. Metabolic risk factors associated with obesity are serum TAG elevations, low levels of HDL, elevated blood pressure, insulin resistance due to elevated glucose levels, as well as prothrombotic and pro-inflammatory states(Reference Avogaro and de Kreutzenberg1, Reference Stern19).
Inflammation
Obesity is known to be accompanied by an inflammatory response (Fig. 1) in various tissues. Adipose tissue is being implicated, more and more, as a key regulator of inflammation through secretion of pro-inflammatory cytokines (i.e. TNF-α) which seem to play a chief role in affecting both endothelial function and glucose metabolism(Reference Avogaro and de Kreutzenberg1, Reference Yudkin20). The negative influence on endothelial physiology may lead to the formation of atherosclerotic plaques(Reference Avogaro and de Kreutzenberg1). Endothelial dysfunction and dysfunctional glucose metabolism (ultimately insulin resistance) seem to head the two main cascades which link obesity to CVD. In fact, growing evidence is pointing to a causative relationship between inflammation and insulin resistance, as TNF-α has been established to mediate insulin resistance as a result of obesity in a multitude of rodent obesity models(Reference Avogaro and de Kreutzenberg1, Reference Hotamisligil21).
Resveratrol inhibits monocyte chemo-attractant protein secretion and gene transcription via down-regulation of TNF-induced transcription. It also reverses secretion and expression of atherogenic adipokines, actually reducing the size, density and mean area of atherosclerotic plaques, as well as the thickness of the vessel intima layer of atherosclerotic lesions(Reference Naderali5, Reference Penumathsa, Koneru and Samuel22). Treatment with resveratrol, in addition to relieving glycerol-induced renal injury by suppressing the inflammatory process and by inhibiting lipid peroxidation(Reference Naderali5, Reference De Jesus Soares, Volpini and Francescato23), also significantly attenuates renal dysfunction and oxidative stress in diabetic rats(Reference Naderali5, Reference Sharma, Anjaneyulu and Kulkarni24); therefore, assuming a significant anti-inflammatory role (Fig. 2) for resveratrol is plausible(Reference Naderali5).
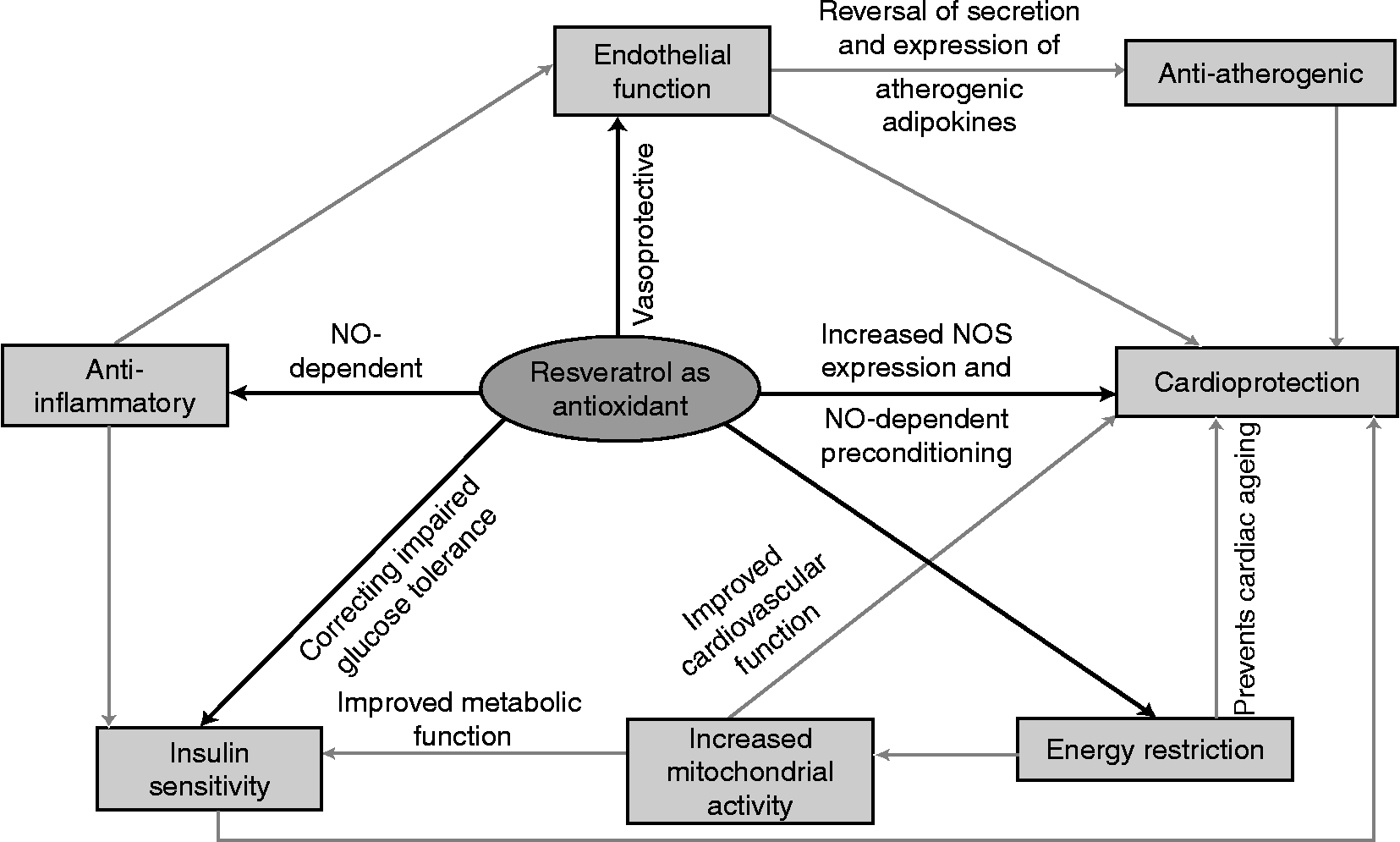
Fig. 2 Schematic diagram of benefits of resveratrol in prominent co-morbidities of obesity.
However, evidence has arisen showing an increase in both lipid peroxidation and DNA and protein carbonyl damage markers with resveratrol treatment in the kidneys, suggesting the possibility of kidney toxicity of resveratrol and its metabolites. Resveratrol forms extensive conjugated metabolites including glucuronides, sulfates and methylated products(Reference Meng, Maliakal and Lu25–Reference Wong, Gruber and Jenner27). Pharmacokinetic studies on acute resveratrol doses indicate significant distribution thereof to the kidneys(Reference Wong, Gruber and Jenner27, Reference Bertelli, Bertelli and Gozzini28), with rapid excretion in urine(Reference Meng, Maliakal and Lu25, Reference Wong, Gruber and Jenner27, Reference Walle, Hsieh and DeLegge29). These metabolites are capable of crossing glomerular membranes and accumulating in the kidney tubules where they may act as pro-oxidants or activate oxidative stress, as seen for various polyphenolic conjugates(Reference Wong, Gruber and Jenner27, Reference Monks and Lau30). Wong et al. (Reference Wong, Gruber and Jenner27) hypothesised that this may have led to the increase in lipid and protein damage observed in the kidneys of the resveratrol-treated mice in the study which they undertook. However, further in vivo studies on the biological activity of resveratrol metabolites are needed to investigate the chronic effects of low-dose resveratrol on the kidney.
Given the growing popularity of resveratrol as a supplement, the possibility of kidney damage is a cause for concern and warrants further investigation, especially before the supplement is recommended for usage in obesity treatment.
Reduced endothelial function
Vascular endothelial cells maintain cardiovascular homeostasis. Importantly, in addition to the physical barrier which they create, endothelial cells secrete a number of mediators that regulate platelet aggregation, coagulation, fibrinolysis and vessel tone(Reference Avogaro and de Kreutzenberg1). There is strong evidence indicating an interaction between endothelium and the secretory proteins of adipocytes (adipokines), suggesting that the ability of adipokines to directly affect vascular homeostasis may be of importance in the developmental mechanism of CVD (Fig. 1) in obese patients(Reference Avogaro and de Kreutzenberg1, Reference Caballero31).
Endothelium is a contributor to the regulation of blood pressure and flow, by releasing NO as well as other compounds which contribute to both vasodilation and vasoconstriction(Reference Avogaro and de Kreutzenberg1). NO is the major contributor to endothelium-dependent relaxation in conduit arteries(Reference Avogaro and de Kreutzenberg1, Reference Hwa, Ghibaudi and Williams32). Obesity has been shown to increase endothelium-dependent vasoconstriction in the absence of endothelial NO(Reference Naderali5, Reference Steinberg, Chacker and Leaming33).
It is the increased oxidative stress in accumulated fat which plays a major role in the induction of endothelial dysfunction. Endothelium thus loses its physiological properties which include the tendency to promote vasodilation, fibrinolysis, and its anti-aggregation properties(Reference Avogaro and de Kreutzenberg1). NO has numerous important effects on the vasculature; these include the maintenance of basal tone by relaxing smooth muscle cells, inhibition of platelet adhesion, activation, secretion and aggregation, as well as the promotion of platelet disaggregation(Reference Avogaro and de Kreutzenberg1, Reference Anderson34). Additionally, NO is a potent inhibitor of mechanisms ultimately leading to neointimal proliferation and atherosclerosis, through preventing endothelial leucocyte adhesion and smooth muscle cell migration and proliferation(Reference Avogaro and de Kreutzenberg1).
Resveratrol has the ability to increase NO synthesis, which in turn functions as an in vivo antioxidant in the ischaemic–reperfused heart, brain or kidney, and lowers oxidative stress(Reference Das and Maulik8, Reference Hattori, Otani and Maulik35).
Cardiovascular effects
Resveratrol's protective mechanism (Fig. 2) includes its role as an intracellular antioxidant, anti-inflammatory agent, its ability to induce NO synthase (NOS) expression, as well as its ability to induce angiogenesis(Reference Das and Maulik8, Reference Bhat, Kosmeder and Pezzuto12). NO was thus identified to have a direct role in vasorelaxation when increased NOS activity was found in cultured pulmonary artery endothelial cells treated with resveratrol(Reference Klinge, Risinger and Watts36), suggesting cardioprotection by resveratrol through affecting the expression of NOS(Reference Das and Maulik8).
Much evidence suggests that resveratrol-mediated cardioprotection is achieved by preconditioning(Reference Das and Maulik8, Reference Hattori, Otani and Maulik35). Preconditioning cardioprotection is achieved by subjecting the heart to a therapeutic amount of stress, so as to disturb normal cardiovascular homeostasis and re-establish a modified homeostatic condition with increased cardiac defences that can withstand subsequent stress insult(Reference Das and Maulik8). Resveratrol's pharmacological action for preconditioning of the heart takes place in an NO-dependent manner. The anti-inflammatory action itself of resveratrol seems to take place through an NO-dependent mechanism(Reference Das and Maulik8).
Use of resveratrol improves post-ischaemic cardiac function regardless of the presence of glucose intake, and reduces the incidence of ventricular fibrillation and infarct size. Moreover, resveratrol increases GLUT-4 expression while reducing endothelin expression and cardiac apoptosis in ischaemic–reperfused hearts, also regardless of the absence of glucose intake, suggesting a direct protective effect on the heart(Reference Naderali5, Reference Das and Maulik8, Reference Sato, Maulik and Bagchi37, Reference Lekli, Szabo and Juhaz38). GLUT-4 translocation is not involved in resveratrol's action, but the mechanism of action appears to lead to stimulation of the intrinsic activity of the GLUT-4 glucose transporters themselves(Reference Breen, Sanli and Giacca7).
Insulin resistance
Insulin resistance (Fig. 1) is one of the pathophysiological mechanisms leading to diabetes mellitus(Reference Palsamy and Subramanian10, Reference McCarthy and Hattersley39) and the state of resistance is attained through a decreased sensitivity or responsiveness of peripheral tissues to the metabolic action of insulin. This, together with the other components of the metabolic syndrome, is associated with altered endothelial function, which ultimately leads to CVD(Reference Avogaro and de Kreutzenberg1). Insulin resistance occurs due to high glucose levels, and can contribute to reactive oxygen species production(Reference Avogaro and de Kreutzenberg1, Reference Inoguchi, Li and Umeda40) (oxidative stress then plays a role in endothelial dysfunction). The insulin-resistant state is additionally characterised by an increase in pro-inflammatory cytokines such as TNF-α, which may also contribute to hepatocellular injury(Reference Palsamy and Subramanian10, Reference Grove, Daly and Bassendine41). The process leading to insulin resistance leads to long-term damage, dysfunction, and failure of various organs particularly the eyes, kidneys, nerves, heart and blood vessels(Reference Palsamy and Subramanian10, Reference Schuster and Duvuuri42).
Resveratrol is capable of scavenging some intracellular reactive oxygen species(Reference Bhat, Kosmeder and Pezzuto12). Oral treatment with resveratrol significantly decreases the levels of glycosylated Hb (blood glucose indicator), suggesting that it may in fact prevent oxidative damage caused by the glycation reaction in diabetic conditions. Thus, resveratrol seems to be beneficial in preventing the pathogenesis of diabetic complications caused by impaired glucose metabolism(Reference Palsamy and Subramanian10). Impaired glucose tolerance serves as a marker for insulin-resistant states and predicts vascular complications(Reference Palsamy and Subramanian10, Reference Tominaga, Eguchi and Manaka43). With resveratrol treatment, the impaired glucose tolerance observed in various groups of diabetic animals was corrected to near normal, strengthening the data on resveratrol's insulin-stimulatory (Fig. 2) effects(Reference Breen, Sanli and Giacca7, Reference Palsamy and Subramanian10).
Data from a study by Breen et al. (Reference Breen, Sanli and Giacca7) indicate that resveratrol increases glucose uptake without stimulating the translocation of glucose transporters (i.e. GLUT-4) in skeletal muscle tissue. They demonstrated that resveratrol stimulates glucose uptake in skeletal muscle cells in a dose- and time-dependent fashion, independent of insulin.
Energy restriction
The purpose of energy restriction is to retard several aspects of ageing; these include age-related mortality, tumorigenesis, as well as physiological decline and the establishment of age-related transcriptional profiles(Reference Lee, Klopp and Weindruch44, Reference Barger, Kayo and Vann45). It is proposed that energy restriction confers health benefits by provoking an organismal defence response through mild stress(Reference Anderson, Bitterman and Wood46, Reference Howitz, Bitterman and Cohen47).
Animal studies have reported effects of energy restriction (Fig. 2) for resveratrol(Reference Baur, Pearson and Price2, Reference Naderali5, Reference Lekli, Szabo and Juhaz38, Reference Barger, Kayo and Vann45), ultimately resulting in weight reduction, through prevention of abdominal obesity and TAG accumulation in hepatic cells, and improvement of lifespan of animals fed a high-energy diet. This weight-reduction effect of resveratrol is speculated to be in part attributed to its effects on adipocytes(Reference Baur, Pearson and Price2, Reference Naderali5, Reference Lekli, Szabo and Juhaz38). Studies have reported a decrease in adipocyte size and decreased cell viability of adipocytes (both maturing and mature) through increasing apoptosis in these cells(Reference Naderali5).
Resveratrol mimics the effects of energy restriction to prevent cardiac ageing at both the transcriptional and functional levels. Additionally, mice on an energy-restricted diet or supplemented with resveratrol have improved insulin sensitivity (Fig. 2), as both these lower blood glucose levels without lowering plasma insulin(Reference Barger, Kayo and Vann45).
There is also a suggested up-regulation of gene expression regulating mitochondrial activity (Fig. 2) through resveratrol treatment as well as a proven increase in aerobic capacity. The increases in mitochondrial oxidative phosphorylation and aerobic capacity are associated with increased longevity, therefore improving animal survival time(Reference Naderali5, Reference Dasgupta and Milbrandt48).
Mitochondria are the principal energy sources of the cell that convert nutrients into energy through cellular respiration(Reference Wallace49, Reference Lagouge, Argman and Gerhart-Hines50). Compromised mitochondrial function has been linked to numerous diseases, including those of the metabolic and cardiovascular systems(Reference Lagouge, Argman and Gerhart-Hines50, Reference Petersen, Befroy and Dufour51). With impaired mitochondrial function, fatty acids are directed toward storage as opposed to oxidation. This may contribute to the lipid accumulation within muscle cells, which has been linked to insulin resistance in obesity and type 2 diabetes mellitus(Reference Lagouge, Argman and Gerhart-Hines50, Reference Virkamaki, Korsheninnikova and Seppala-Lindroos52–Reference Petersen, Dufour and Befroy54). In line with this, resveratrol significantly improves both muscle oxidative capacity and sensitivity to insulin in high-energy-fed mice(Reference Lagouge, Argman and Gerhart-Hines50).
Baur et al. (Reference Baur, Pearson and Price2) hypothesised that resveratrol might shift the physiology of middle-aged mice on a high-energy diet towards that of mice on a standard diet and provide the associated health benefits of such a diet without having to reduce energy intake. After 6 months of treatment, they reported that there was a clear trend towards increased survival and insulin sensitivity. Also, it was found that resveratrol-treated mice on a high-energy diet displayed decreased organ pathology (liver, pancreas) when compared with untreated mice on a high-energy diet. The ability of resveratrol to improve motor function was also noted, indicating that the effects of resveratrol are not confined to the liver. Improvements in the morphology of the aortic elastic lamina were also apparent in resveratrol-treated mice. The livers of resveratrol-treated animals showed a considerable increase in the number of mitochondria when compared with untreated high-energy-fed mice, and were not significantly different from mice on a standard diet. The data collected by this group demonstrate quite clearly that resveratrol can alleviate the negative impact of a high-energy diet on the overall health and lifespan of middle-aged mice.
The findings of this group(Reference Baur, Pearson and Price2) showed that resveratrol does indeed shift the physiology of mice consuming high-energy diets towards that of mice on a standard diet. Resveratrol was also shown to modulate known longevity pathways and improve general health, including survival, motor function, insulin sensitivity, organ pathology, and mitochondrial number. These changes occurred, notably, without significant reduction in body weight.
It is, however, important to note that all experimental treatments have thus far been administered to middle-aged animal models; therefore, it has not yet been ascertained what the effects of resveratrol treatment would be on young animals. Although the present review focuses on the general effects of resveratrol on obesity, it cannot be ignored that children may prove to be more prone than adults to its potentially harmful effects. Thus, before any recommendation is made for the use of resveratrol in the treatment or prevention of obesity, studies will have to specifically test the product on adolescent animals, as well as compare differences in toxicity data with those of experiments performed on adult animals.
Multi-faceted effects of resveratrol
Resveratrol seems to present with two faces. It protects cells by potentiating a survival signal, but selectively kills cancer cells. It behaves as an antioxidant, yet can induce redox signalling. It is an anti-proliferative agent for cancer, inducing apoptosis in tumour cells and sensitising cancer cells by inhibiting cell survival signal transduction and anti-apoptotic pathways. In contrast, it also triggers a survival signal in ischaemic tissue by inducing anti-apoptotic genes and blocking apoptosis in the ischaemic heart. At low doses, resveratrol stimulates angiogenesis, but at higher doses it blocks the angiogenic response. At low concentrations, resveratrol scavenges reactive oxygen species, but at higher concentrations, it behaves like a pro-oxidant. Low concentrations of resveratrol are quite sufficient for cardiovascular preconditioning, whereas higher concentrations and chronic use thereof may, in fact, exert toxic effects in the heart and other organs(Reference Das and Maulik8).
In general, however, adverse effects of resveratrol have not been documented in humans, and a recommended dosage of 5–10 mg/d is suggested to be safe after a review of toxicological literature for resveratrol by the National Institute of Environmental Health Sciences(Reference Haneke, Carson and Gregorio55). However, the elevation in oxidative damage observed in the kidneys of mice in the study by Wong et al. (Reference Wong, Gruber and Jenner27) over an extended period took place at levels well below that for which toxicity has previously been reported(Reference Baur, Pearson and Price2, Reference Juan, Vinardell and Planas56, Reference Crowell, Korytko and Morrissey57). This raises concern regarding long-term, low-dose administration of resveratrol(Reference Wong, Gruber and Jenner27). Thus, it would not be considered wise to use resveratrol at this time for the prevention or treatment of obesity, especially in children, as long-term toxicity could in fact cause more detrimental harm to the systems of suffering individuals than currently anticipated.
Conclusion
Obesity is a growing problem worldwide, and treatment potentials are constantly under scrutiny. There is a prevalence of inflammation in obese patients, and this brings with it a cascade of problems including endothelial dysfunction and insulin resistance, ultimately resulting in cardiovascular complications. Resveratrol is a phenolic substance in which much interest has been placed. The substance is anti-inflammatory, vasoprotective, and increases glucose uptake, thus improving insulin sensitivity. These effects are the ones most applicable to obesity prevention and treatment. However, the use of resveratrol over the long term even in low doses has not yet been conclusively ascertained. Additionally, there are reports of kidney toxicity of resveratrol and its metabolites even at concentration levels currently approved by the National Institute of Environmental Health Sciences. It is thus wise not to recommend the use of resveratrol in the treatment of obesity, especially childhood obesity, as all experimental data available are from studies utilising middle-aged animal models, unless and until the safety of resveratrol and its metabolites have been scrutinised to a definitive level.
Acknowledgements
The authors contributed equally to the present paper and thank the National Research Foundation of South Africa (NRF) for funding E. P. (Indigenous Knowledge Systems (FA2004033100004)).
The authors have no conflict of interest and the authors have no financial interests in the products reviewed in this paper.




