INTRODUCTION
Mumps is usually a mild viral infection in which parotitis is the most common manifestation. About 30% of mumps infections are asymptomatic and severe manifestations are recorded more frequently among adults than children [Reference Gupta, Best and MacMahon1]. Complications of the disease may include encephalitis, aseptic meningitis, orchitis in post-pubertal young men, oophoritis in post-pubertal young women [Reference Gupta, Best and MacMahon1, 2] and permanent disabilities such as sensorial hearing loss, hydrocephalus and cerebellar ataxia [Reference Gupta, Best and MacMahon1, Reference Nussinovitch, Volovitz and Varsano3].
Death due to mumps is rare; however, the disease burden in unvaccinated communities is high [Reference Galazka, Robertson and Kraigher4] and justifies routine vaccination. Indeed, after introducing vaccination against mumps in national immunization programmes, the incidence of the disease decreased dramatically in countries where high vaccination coverage is reported [Reference Galazka, Robertson and Kraigher4–Reference Peltola9] and thousands of cases of mumps meningoencephalitis, orchitis and deafness were prevented [Reference Galazka, Robertson and Kraigher4, Reference Peltola8, Reference Peltola9]. In the United States the incidence of mumps decreased by 99% between 1968 and 1995 [Reference van Loon5, Reference Watson6]. In England and Wales, where the measles-mumps-rubella (MMR) vaccine was introduced in 1988, hospital admissions due to mumps decreased by 92% compared with the pre-vaccination era [Reference Gay7], while Finland is the first country to document elimination of indigenous mumps [Reference Peltola8].
However, during the past few years there are cumulative reports on the occurrence of relatively large mumps epidemics in countries with routine immunization against the disease. Examples are the epidemic in United Kingdom in 2004–2005 [Reference Gupta, Best and MacMahon1, Reference Donaghy, Cameron and Friederichs10] and the latest multi-state epidemic of mumps in the United States [Reference Donaghy, Cameron and Friederichs10]. A debate is rising regarding the vaccine-induced protective immunity and the level of herd immunity needed to prevent mumps epidemics.
In Israel, high incidence rates of mumps were recorded in the pre-vaccination era ranging between 79·8 and 157·9/100 000 during 1977–1984 with relatively frequent outbreaks (Fig. 1) [11]. In April 1984, the mumps vaccine in a bivalent measles-mumps (MM) vaccine formulation was introduced for the first time to the national Israeli routine childhood vaccination schedule and targeted at 15-month-old children. This programme was funded for only 16 months although the MM vaccine continued to be recommended by paediatricians and was commercially available [11, Reference Slater, Anis and Leventhal12]. The average vaccination coverage of the MM programme was 86% (Dr Moermann, personal communication). The incidence rates of mumps were relatively low during 1985–1987 ranging between 24·5 and 59/100 000. In 1988, however, a large epidemic of mumps occurred and the incidence rate increased dramatically up to 157·6/100 000. In the same year the mumps vaccine was re-introduced to the national Israeli childhood vaccination programme as a component of a single-dose MMR vaccine given to all 15-month-old children [11]. In 1994, a second dose of MMR vaccine was added to the immunization schedule and given to children by the age of 6 years while the age of administration of the first dose of MMR was changed to 12 months [11]. Before conducting the current study the vaccination coverage of the first dose of MMR vaccine was 91–93% [Reference Slater, Anis and Leventhal12, Reference Slater, Roitman and Costin13] and it reached a rate of 94% and 97% in Jewish and Arab children respectively, in 2001 [14]. The Jeryl Lynn strain was the mumps component in the MM and MMR vaccines used in Israel [Reference Slater, Anis and Leventhal12]. After implementing the two-dose MMR vaccination policy the incidence of mumps declined substantially to 0·1/100 000 in 2001, the lowest reported rate among selected Western countries [11, Reference Slater, Anis and Leventhal12]. In 2005 there was a threefold rise in the number of reported cases of mumps (109 cases) in Israel compared to 2004 (31 cases) with outbreaks in two different regions of the country occurring mainly among military recruits and high-school students.
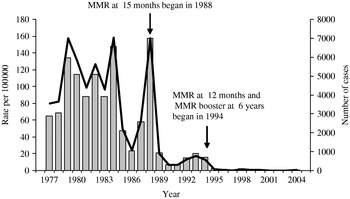
Fig. 1. The incidence of mumps in Israel based on reported cases 1977–2004. ![]() , Cases;
, Cases; ![]() , rates.
, rates.
Serosurveys have a valuable role in the assessment of the level of immunity to mumps following natural infection or vaccination and may lead to the identification of subpopulations at an increased risk for mumps outbreaks. Here we report and discuss the results of a seroepidemiological study that was carried out on sera collected during 1997–1998, to determine the seroprevalence of mumps antibodies in different age groups of the Israeli population in view of the vaccination policy and the recent mumps incidence data in Israel.
MATERIALS AND METHODS
Study population and collection of data
A serosurvey was conducted using archived serum samples collected by the Israel Center for Disease Control during 1997–1998. The serum bank comprised samples from all regions of Israel from both males and females. Collection of sera is ongoing and the sampling covers all the months of the year. The samples from the younger age groups (1–17 years) were residual sera from diagnostic laboratories and samples from the adult population (⩾18 years) were residuals of sera obtained from healthy blood donors living in Israel and screened before the use of the blood donations. Both sources excluded blood samples taken from subjects with confirmed or suspected immunological disorders. Each sample had a unique identifier plus details of the sex, age, place of residence (at the level of town) and the year in which the sample was drawn. For only a limited part of the samples, information was also available on country of birth, country of origin and nationality.
The targeted number of sera to be tested for each age group followed the European Sero-Epidemiology Network (ESEN) guidelines and was determined from power calculations using age-specific estimates of antibody seroprevalence [Reference Osborne, Weinberg and Miller15]. In total, 3330 samples were randomly selected from the serum bank using a stratified sampling design. The age intervals were re-grouped in relation to the MMR vaccination policy in Israel. The sample size of each age interval allowed detection of 10–15% differences between the age groups, assuming seroprevalences of mumps antibodies of 60–80% at α=0·05 and power of 80%.
Laboratory analysis
The mumps-specific IgG antibodies were determined at the Central Virology Laboratory of the Israeli Ministry of Health using the Behring ELISA System ‘Enzygnost’ (Dade Behring, Engygnost, Marburg, Germany) for quantitative measurement of specific IgG against mumps in human serum. The assay was carried out according to the manufacturer's instructions. The kit was re-evaluated within the framework of the European Sero-Epidemiological Network (ESEN 2) at the Robert Koch Institute, National Center for MMR, Berlin, Germany which served as reference laboratory for ESEN 2. A panel of 150 sera were tested in parallel by the Enzygnost ELISA kit and the mumps neutralization test which served as gold standard. The sensitivity and specificity for Enzygnost were 93% and 87% respectively [Reference Tischer16].
The multianalyte virotrol MuMZ (Mumps, Measles and VZV) for IgG (Blackhawk BioSystems Inc., San Ramon, CA, USA) were included as internal controls in every ELISA run. Results were expressed in titres, values of >1:500, between 1:230 and 1:500 and <1:230 were considered positive, equivocal and negative results, respectively.
Data management and analysis
The data were managed using Microsoft Excel and analysed with SPSS version 13 (SPSS Inc., Chicago, IL, USA).
Proportions and 95% confidence intervals (CIs) of seroprevalence to mumps were computed in the overall study population and in specific age and gender subgroups. Age-adjusted seroprevalence estimates were calculated for the whole population and for the two sex groups using the population of Israel in 2005 as the reference population. Geometric mean titres (GMT) of mumps antibodies and 95% CIs in selected age groups were also computed.
RESULTS
Serum samples from 3330 subjects (53·9% males) were tested. Using the cut-off titre of >1:500 for seropositivity to mumps, 2507, 366 and 457 subjects had sera positive, equivocal, and negative to mumps antibodies, respectively. The age- and sex-adjusted seropositivity to mumps was 77·0%, while among males and females the age-adjusted seropositivity rates were 76·7% and 76·8%, respectively.
The mumps age-specific seroprofiles were different in birth cohorts of the pre- and post-vaccination era (Fig. 2). By the age of 1 year, the age of the first dose of MMR, the seropositivity was 49% but increased to 67–68% in the 2–3 years age group, probably following completion of administration of the first dose of the MMR vaccine. The seropositivity gradually decreased afterwards reaching the value of 51% by age 5 years (Fig. 2) and significantly increased again to a level of 73·8% (95% CI 69·1–77·9) among subjects in the 6–9 years age group who received a second dose of MMR at grade one (Fig. 2, Table). The seropositivity in the 10–13 years age group, who were not eligible for routine vaccination against mumps, was 59%, significantly lower than the seropositivity levels of both the younger (6–9 years) and older (14–17 years) age groups who received either two doses of the MMR vaccine or one dose of the MM vaccine, respectively (Table, Fig. 2). In the cohorts aged ⩾18 years, with probable previous exposure to the wild virus, the seropositivity levels were the highest ranging from 81% to 88%.

Fig. 2. Mumps seroprevalence by age and expected vaccination status in Israel 1997-1998. □, Equivocal; ![]() , positive and 95% CI.
, positive and 95% CI.
Table. Age-specific seropositivity of mumps antibodies by sex in Israel 1997–1998

In general, a higher percentage of equivocal results were observed in the younger subjects who comprised the birth cohorts of the post-vaccination era compared to older subjects who were mainly exposed to the wild virus (Fig. 2). In the post-MMR1 ages (2–5 years), a significantly higher percentage of 30·2% (95% CI 21·5–40·6) of equivocal results was found by age 5 years, about 3 years after receiving the first dose of MMR vaccine, compared to 11·6% (95% CI 8·3–15·9) in the 2–4 years age group (P<0·001 for difference in seroprevalence between groups). Thereafter, among birth cohorts of the post-MMR2 (ages 7–9 years) the equivocal results decreased to 13·3% (95% CI 9·9–17·6). The GMT of subjects aged 2–5 years who were eligible to receive the first dose of MMR was significantly lower than the GMT of mumps antibodies among children aged 7–9 years who were eligible to receive a second dose of MMR (624, 95% CI 534–729 vs. 908, 95% CI 784–1051).
DISCUSSION
The present seroepidemiological study of mumps is the first carried out to include both genders of all age groups in Israel. We conducted the present study on sera obtained during 1997–1998; 3–4 years after the introduction of a second dose of MMR at grade one. We found that the immune status to mumps of the Israeli population has been associated with a number of factors such as the year of introduction of the vaccine, the age at vaccination, the number of doses given and exposure to the wild virus.
Similarly to previous reports [Reference Amela, Pachon and de Ory17–Reference Matter19] the seropositivity varied among age groups being significantly higher in birth cohorts born before mumps vaccination was introduced in Israel and probably exposed to natural infections in comparison to the younger subjects who were immunized.
Of special interest are subjects in 10–13 years age group who were born during the period 1985–1987 when MMR was still not included in the routine vaccination schedule and after routine vaccination with MM was interrupted. In this age group we found that the seropositivity levels were substantially lower, ranging from 42% to 68%, compared to other age groups which were either immunized or exposed to natural infection. Most probably the low seropositivity is the result of less exposure to the wild strain due to its reduced circulation in the population after implementation of the MMR vaccination policy. Interestingly, the recent outbreak in Israel, in 2005, involved adolescents and young adults (high-school students and military recruits) belonging to these birth cohorts. We assume that the joint effect of two risk factors, namely the low level of immunity and a high degree of social mixing led to the onset and spread of the outbreak.
There are different assessments regarding the herd immunity threshold for mumps. Anderson & May [Reference Anderson and May20] considered that a vaccination coverage of 90–92% would be needed to prevent circulation of the virus while Fine [Reference Fine, Plotkin and Orenstein21] estimated the herd immunity threshold for mumps to be in the range of 75–86%. It follows that the age- and sex-adjusted seropositivity for mumps antibodies found in our study for the Israeli population (77·0%), is lower or around the lower limit of the mumps herd immunity threshold range needed to prevent mumps epidemics.
It has been suggested that the arrival of large numbers of new immigrants from the former Soviet Union with relatively lower seropositivity could have contributed to the decrease in the immunity to mumps of the Israeli population [Reference Huerta22]. Against the background of high vaccination coverage of MMR vaccine in Israel the seropositivity found in our study population may slightly underestimate the real level of immunity to mumps of the Israeli population. One reason may be related to the serological assay used. The sensitivity of Enzygnost was found to be 93% when the assay was evaluated within ESEN 2 against the neutralization test. The recent study of Backhouse et al. [Reference Backhouse23] has also indicated that the Enzygnost ELISA had a sensitivity around 80% in comparison with the neutralization test used as gold standard [Reference Backhouse23]. It might also be possible that the epitopes of the mumps antigen employed by the Enzygnost kit do not entirely recognize antibodies induced by various vaccine strains used to immunize immigrants in their countries of origin and especially those from the former Soviet Union. Another reason could be related to the source of serum samples tested. Many healthy blood donors among the young adult age groups are soldiers serving in the Israel Defense Force, with fewer Arab and orthodox Jewish citizens than their respective proportions in the total Israeli population. These subpopulations are characterized by high fertility rates compared with the general Jewish population, large families and living in crowded conditions. These features increase the risk of transmission of mumps in childhood. It could, therefore, be expected that the under-representation of these segments of the general population in the study sample might lead to a certain underestimation of the seroprevalence of mumps antibodies when extrapolated to the adult Israeli population.
Larger mumps outbreaks involving thousands of cases of disease recently occurred in the United Kingdom, the United States, Scotland and other countries with routine immunization against the disease [Reference Gupta, Best and MacMahon1, 2, Reference Donaghy, Cameron and Friederichs10, 24]. The cases of disease involved in these epidemics [Reference Gupta, Best and MacMahon1, 2, Reference Donaghy, Cameron and Friederichs10] included young adults, born in the pre-vaccination era but also subjects who received one or even two doses of MMR [24] raising questions related to the mumps vaccine-induced protective immunity. As already suggested, pronounced waning immunity after vaccination with the mumps vaccine in the MMR formulation [Reference Davidkin, Valle and Julkunen25] and/or possible genetic changes of the wild virus [Reference Jin, Beard and Brown26] could have been the cause of the occurrence of new cases of mumps among previously vaccinated subjects.
Against the background of a consistently high documented vaccination coverage rate for the first dose of MMR [14] we also observed a trend of possible waning immunity in subjects aged 2–5 years who received their first MMR dose by the age of 1 year (1–4 years before this study was carried out). This assumption is supported by the parallel increase of the percentage of equivocal results in the same age groups and the decrease in older ages who received a second dose of MMR vaccine.
The decay observed 1–4 years after MMR1 was not seen in the corresponding period after MMR2. Moreover, it appears that the immune response after the second dose of MMR was more vigorous and sustained as reflected by a significantly higher GMT of mumps antibodies in the 7–9 years age group compared to the 2–5 years age group. Similar findings were reported by Davidkin et al. from a serological cohort study in Finland [24]. We believe that a longer serological follow-up will be needed to detect any decay in the level of mumps antibodies after the second MMR dose.
In summary, in view of the possible waning immunity following mumps vaccination it will be essential to carry out periodical seroepidemiological studies to assess the population's immune status. Special attention should be paid to the age groups which received one dose of MMR vaccine but have not yet received the second dose and to those who were immunized with the second vaccine dose a long time before.
ACKNOWLEDGEMENT
The authors thank R. Bassal, S. Goren and R. Dichtier for technical help in data management and for statistical assistance.
DECLARATION OF INTEREST
None.





