Systematic reviews have been performed to summarize vitamin D status among populations worldwide(Reference Hilger, Friedel and Herr1–Reference Cashman, Sheehy and O’Neill3). Data compiled on 168 389 participants of all age groups from 195 studies predominantly from North America, Asia Pacific and Europe showed that mean 25-hydroxyvitamin D (25(OH)D) level was below sufficiency (<50 nmol/l) in 37·3 % of the samples, with lower 25(OH)D values indicated among newborns and infants(Reference Hilger, Friedel and Herr1). Meta-analysed global information on maternal and newborn vitamin D status indicated sufficient mean 25(OH)D concentration for 46 % of pregnant women and only 25 % of newborns, but estimates were acknowledged as still poorly defined in African, South-East Asian and Eastern Mediterranean regions, and no studies from Latin America were included in the analysis(Reference Saraf, Morton and Camargo2). Reports properly informing on vitamin D status were in fact lacking for almost two-thirds of low- and lower-middle income countries(Reference Cashman, Sheehy and O’Neill3), which compromises the complete understanding of the magnitude of this problem. In Brazil, some cross-sectional studies among newborns and infants indicated prevalence of vitamin D <50 nmol/l at about 15–30 %(Reference Prado, Oliveira and Assis4, Reference Cobayashi, Lourenço and Cardoso5), but these investigations were rather small and could not cover participants at diverse latitudes.
There is an indication for tracking of vitamin D status in young children in relation to maternal characteristics and 25(OH)D level during pregnancy(Reference Marshall, Mehta and Ayers6, Reference Thorne-Lyman and Fawzi7). The importance of vitamin D for bone metabolism is widely known, along with increasingly recognized roles in immune response and cell differentiation as well as other potential health effects related to chronic conditions such as cancer, respiratory diseases and cardiovascular events(Reference Biesalski8). Specifically during the perinatal period, three trials provided evidence on the association of maternal vitamin D supplementation with a pooled 60 % reduction (95 % CI 0·23, 0·71) in low birth weight occurrence, but at a low level of evidence(Reference Thorne-Lyman and Fawzi7). Promotion of adequate vitamin D status, which is mainly influenced by sunlight exposure, along with some dietary sources and supplementation(Reference Holick, Chen and Lu9), should therefore be sustained starting early in life to favour proper child health and development.
Of note, some vitamin D functions seem to operate in synergy with other micronutrients, especially vitamin A(Reference Biesalski8). Both vitamins share the regulation of several immune processes, with hormone-like properties and effects mediated through nuclear hormone receptors(Reference Mora, Iwata and von Adrian10). Previous reports have pointed out a possible modification of the relationship between vitamin D below sufficiency and infection, such as respiratory tract infections among young children, according to vitamin A status(Reference Linday, Umhau and Shindledecker11). It is well known that inflammatory markers are associated with alterations in the serum concentrations of micronutrients(Reference Thurnham, McGabe and Northrop-Clewes12). These effects may be of particular interest in lower-income regions with the double burden of malnutrition and disease in childhood(Reference Victora and Rivera13).
We provide an early assessment of sociodemographic, nutritional and health conditions associated with vitamin D sufficiency among Brazilian children of up to 15 months of age, with the aim of contributing evidence for the promotion of optimal 25(OH)D status from the first years of life. Participants were selected at primary health-care units from diverse latitudes and regions across the country, and the present study appraises the potential relationship of 25(OH)D level with vitamin A status, accounting for the presence of inflammation.
Methods
Study design and participants
The present cross-sectional analysis is part of the ‘Estudo Nacional de Fortificação Caseira da Alimentação Complementar’ (ENFAC Study). Detailed information on sampling and field procedures has been described elsewhere(Reference Cardoso, Augusto and Bortolini14). Briefly, the ENFAC Study was a pragmatic controlled clinical trial designed to evaluate the impact of a multiple-micronutrient powder on anaemia and nutritional deficiencies among children seen at primary health-care units in Brazil. Four cities in different regions of the country participated in data collection: Olinda (7°59′26·9016″S), Rio Branco (9°58′31·3864″S), Goiânia (16°41′12·7752″S) and Porto Alegre (30°2′4·7292″S), located, respectively, in the north-eastern, northern, mid-western and southern region.
The sample size in the ENFAC Study was defined as at least 105 children in each control and intervention group by study city. In the main trial, the primary outcome of interest was a detected increase of 6 g/dl in mean Hb concentration in the intervention arm (sd 12 g/dl), with a power of 0·95 and a two-sided significance level of 0·05(Reference Cardoso, Augusto and Bortolini14). To compensate for dropouts, the sample size was then increased by 30 %, resulting in 540 children expected for each control and intervention group. For the present analysis, the study population was composed of the children from the control group receiving routine paediatric care and aged 11–15 months. Primary health-care units were selected based on the largest enrolment in each city, and eligibility criteria comprised parental approval to participate and absence of current treatment for anaemia. Exclusion criteria included premature birth (<37 weeks’ gestation), twins, reported cases of HIV infection, malaria, tuberculosis or genetic Hb disorders, as well as fever (>39°C) on the day of blood sampling. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the School of Public Health, University of São Paulo, Brazil. The ENFAC Study was registered at www.ensaiosclinicos.gov.br as RBR-5ktv6b and written consent was obtained from the caregivers of all participants. Children with nutritional deficiencies diagnosed during the study were referred for treatment in the health centres.
Overall, 543 children were invited to participate; parents of twenty-two children (4·1 %) declined participation and one child did not provide a blood sample, resulting in a total sample of 520 children for the control group in the ENFAC Study. Of these, twenty-four children were older than 15 months of age at the time of blood collection, and for an additional twenty-eight participants, blood samples were not sufficient for vitamin D laboratory analysis, resulting in 468 children with complete data for the present analysis. Except for presenting older ages, children who were excluded (n 52) were not significantly different from those included in terms of distribution of the main exploratory variables considered in the present study.
Data collection and laboratory procedures
From June 2012 to January 2013, caregivers of children attending primary health-care units were invited to participate and, at enrolment, trained fieldworkers applied a structured questionnaire in face-to-face interviews to collect data on socio-economic, environmental, demographic and maternal variables, as well as information on the child’s birth, feeding practices, supplementation and morbidities. Children’s anthropometric measurements were obtained directly by trained research assistants using standardized procedures and calibrated equipment(15). Recumbent length was ascertained to the nearest 0·1 cm on a flat surface with a portable infant measuring board (Sanny model ES-2000, Los Angeles, CA, USA) and weight was measured with an electronic scale (Tanita model UM-061), accurate to 10 g. Measurements were taken in duplicate and the mean value was considered for analysis. BMI was computed as [weight (kg)]/[length (m)]2. BMI- and length-for-age Z-scores were calculated using the WHO Child Growth Standards(16).
Venous blood samples were collected by trained technicians up to a week after the interview, on the morning of a previously scheduled day and after at least 3 h of fasting. At the field laboratory, Hb concentration was determined with a portable haemoglobinometer (HemoCue Hb301, Angelholm, Sweden) and anaemia was defined as Hb concentration <11 g/dl(17). A separate blood sample was protected from light and centrifuged within 1 h of collection; serum and plasma samples were frozen at −20°C before being shipped to São Paulo on dry ice and maintained at −70°C until further analysis, within 6 months of collection. Serum concentrations of retinol and 25-hydroxycholecaliferol (25(OH)D3) were measured by standardized HPLC methods using a Chromsystems kit according to the manufacturer’s instructions (Chromsystems Instruments & Chemicals GmbH, Gräfelfing, Germany), as previously described(Reference Gomes, Alves and Sevanian18). To assess the presence of inflammation, α1-acid glycoprotein (AGP) and C-reactive protein (CRP) were determined using an IMMAGE Immunochemistry System (Beckman Coulter, Brea, CA, USA). The laboratory assayed internal and external blinded quality control specimens in each run. Based on the control specimens, the accuracy and inter-assay CV for all these analyses were within 7 %.
Correction factors for inflammation and assessment of micronutrient status
A four-level model of inflammation was used to identify children in the reference (AGP ≤ 1 g/l and CRP ≤ 5 mg/l), incubation (AGP ≤ 1 g/l and CRP > 5 mg/l), early convalescence (AGP > 1 g/l and CRP > 5 mg/l) and late convalescence (AGP > 1 g/l and CRP ≤ 5 mg/l) groups(Reference Thurnham, McGabe and Northrop-Clewes12). We approached potential impacts on micronutrient status by comparing serum concentrations of retinol and 25(OH)D3 for each stage of inflammation with Kruskal–Wallis tests.
Retinol concentration varied significantly across stages of inflammation (P < 0·001), with lower median value among children with raised inflammatory markers compared with those in the reference group. Internal correction factors were calculated as the ratios of the geometric mean of serum retinol among children in the reference group to each geometric mean of serum retinol among those in the incubation, early convalescence and late convalescence groups (equivalent to 1·33, 1·78 and 1·12, respectively). Based on the observed stage of inflammation, each child’s serum retinol concentration was multiplied by the corresponding factor to estimate a corrected retinol concentration(Reference Thurnham, McGabe and Northrop-Clewes12). After correction, retinol concentrations were similar between groups (P = 0·64). Vitamin A status was then classified as sufficient at serum retinol concentration of ≥1·05 µmol/l, marginal at 0·70–1·04 µmol/l and deficient at <0·70 µmol/l(19).
Serum 25(OH)D3 concentration was not significantly altered according to stage of inflammation (P = 0·13) and a similar approach for correction was not deemed necessary. Vitamin D sufficiency was defined as ≥50 nmol/l(20).
Statistical analyses
The main dependent variable of interest was vitamin D sufficiency. Exploratory variables comprised child’s sex, age and skin colour, latitude, maternal characteristics, birth weight, nutritional and health indicators, including duration of breast-feeding, presence of stunting or overweight, use of vitamin supplements, occurrence of diarrhoea or wheezing in the past 15 d, anaemia, vitamin A status and presence of inflammation.
First, we compared the distribution of basic characteristics by latitude with Pearson’s χ 2 or Fisher’s exact test and t tests or Mann–Whitney U tests to examine differences in proportions and in continuous variables, respectively. Differences in proportion of vitamin D sufficiency were further explored between categories of these variables at different latitudes.
A multiple model for vitamin D sufficiency was fitted with Poisson regression with robust variance. Prevalence ratios (PR) and 95 % CI for vitamin D sufficiency were initially adjusted for the child’s sex, age and skin colour. We considered a hierarchical conceptual framework(Reference Victora, Huttly and Fuchs21) adapted to the present analysis to further select variables at distal level (latitude), intermediate level (maternal education) and proximal level (presence of stunting, use of vitamin supplements, vitamin A status corrected for the presence of inflammation) in relation to vitamin D sufficiency.
Concerning latitude, estimates were equivalent when considering the classification of participating cities into two categories (northern/north-eastern regions, at lower latitudes, and mid-western/southern regions, at higher latitudes) or three categories (northern/north-eastern regions, mid-western region and southern region). Thus, we present results considering cities located at lower v. higher latitudes. The association with vitamin A was evaluated according to categories (sufficient, marginal, deficient) as well as continuous concentration. Other potential covariates were not independently associated and did not significantly affect the estimates of association with the dependent variable of interest at each hierarchical level. We additionally performed square-root transformation on 25(OH)D3 concentrations to run multiple linear regression models to observe the direction and significance of linear associations with the same covariates considered for vitamin D sufficiency. Finally, a control variable accounting for different seasons of blood collection in our study was also added to the final multiple model and interpretation of findings remained similar.
Missing observations (considering all covariates, n 70 or 14·9 %) were included in the multiple models by creating missing-value categories. Complete-case multiple models regarded analogous estimates. Statistical significance was set at P < 0·05. We used the statistical software package Stata version 11.2 for all analyses.
Results
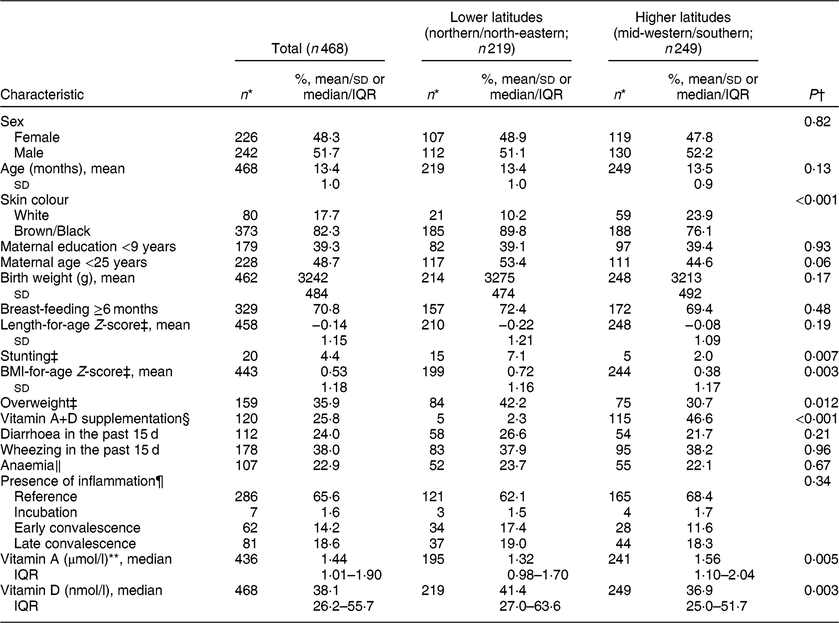
Among 468 children included in the present analysis, 48·3 % were girls and the mean age was 13·4 (sd 1·0) months. The distribution of some characteristics varied significantly according to latitude (Table 1). The northern/north-eastern regions, at lower latitudes, had a smaller proportion of white children (10·2 %) compared with the mid-western/southern regions, at higher latitudes (23·9 %, P < 0·001). Reported vitamin A+D supplementation was more frequent at higher latitudes, occurring among 46·6 % of the children (v. only 2·3 % of children living at lower latitudes, P < 0·001).
Table 1 Characteristics of study participants by latitude: children aged 11–15 months (n 468) from primary health-care units in four Brazilian cities, June 2012–January 2013
IQR, interquartile range.
Data are presented as n and %, unless specified otherwise.
* Totals may differ from the total number of participants due to missing values.
† Pearson’s χ 2 or Fisher’s exact test and t tests or Mann–Whitney U tests were used to examine differences in proportions and in continuous variables between latitudes, respectively.
‡ According to the WHO Child Growth Standards, stunting was defined as length-for-age Z-score < −2 and overweight as BMI-for-age Z-score > 1.
§ Ever used or is currently using vitamin A and D supplementation.
‖ Defined as Hb < 11 g/dl.
¶ Defined according to a four-level model of inflammation with α1-acid glycoprotein (AGP) and C-reactive protein (CRP) as reference (AGP ≤ 1 g/l and CRP ≤ 5 mg/l), incubation (AGP ≤ 1 g/l and CRP > 5 mg/l), early convalescence (AGP > 1 g/l and CRP > 5 mg/l) and late convalescence (AGP > 1 g/l and CRP ≤ 5 mg/l).
** Each child’s serum retinol concentration was multiplied by a correction factor according to the observed level of inflammation.
Overall, one-third of children were in the incubation, early convalescence or late convalescence stage of inflammation. After correcting retinol concentrations by the presence of inflammation, vitamin A deficiency was estimated in 12·6 % (v. 16·1 % before correction). Median concentration of vitamin A was higher in the midwestern/southern regions. On the other hand, median concentration of vitamin D was higher in the northern/north-eastern regions, consistent with the incidence of sunlight at these latitudes.
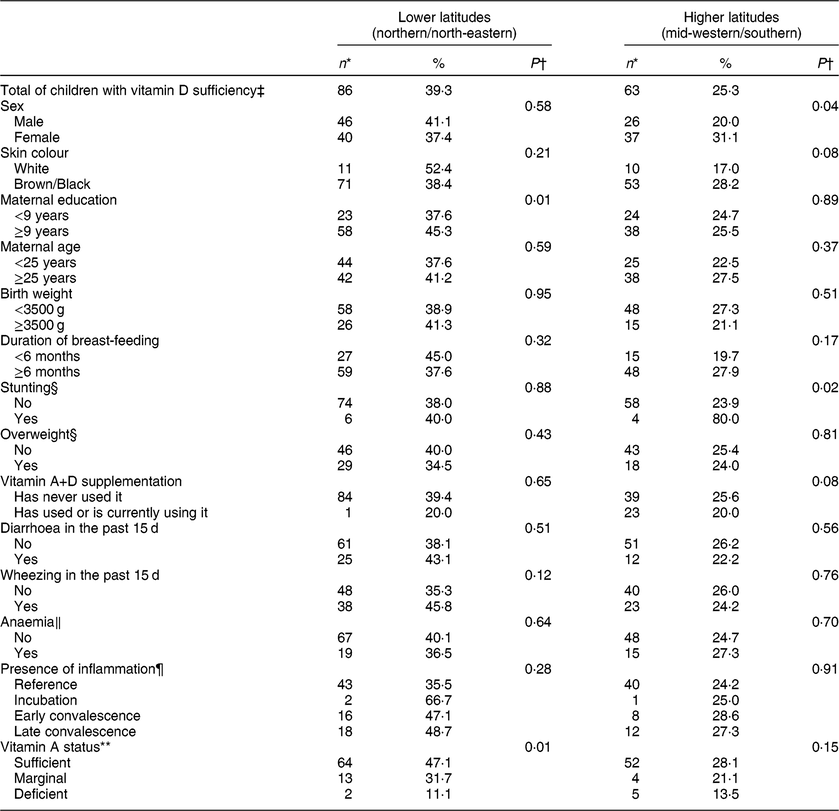
Considering usual cut-offs, vitamin D concentration <30 nmol/l was found among 32·9 % of children and <50 nmol/l among 68·2 %. A sufficient status of vitamin D was therefore present in 31·8 % of the study population and explored according to sociodemographic and health characteristics separately at lower and higher latitudes, as shown in Table 2. Maternal education was positively associated with vitamin D sufficiency among children from lower latitudes. At higher latitudes, the child’s sex and stunting were related to vitamin D sufficiency. In both regions, greater proportions of vitamin D sufficiency were noted with increasing vitamin A status. Birth characteristics, recent morbidities, anaemia and inflammation were not associated with vitamin D sufficiency in these children.
Table 2 Proportion of vitamin D sufficiency by sociodemographic and health characteristics and according to living at lower and higher latitudes among children aged 11–15 months (n 468) from primary health-care units in four Brazilian cities, June 2012–January 2013
* Totals may differ from the total number of participants with vitamin D sufficiency due to missing values.
† Pearson’s χ 2 or Fisher’s exact test was used to examine differences in proportions of vitamin D sufficiency between categories of variables at each of lower and higher latitudes.
‡ Defined as 25-hydroxyvitamin D concentration ≥50 nmol/l.
§ According to the WHO Child Growth Standards, stunting was defined as length-for-age Z-score < −2 and overweight as BMI-for-age Z-score > 1.
‖ Defined as Hb < 11 g/dl.
¶ Defined according to a four-level model of inflammation with α1-acid glycoprotein (AGP) and C-reactive protein (CRP) as reference (AGP ≤ 1 g/l and CRP ≤ 5 mg/l), incubation (AGP ≤ 1 g/l and CRP > 5 mg/l), early convalescence (AGP > 1 g/l and CRP > 5 mg/l) and late convalescence (AGP > 1 g/l and CRP ≤ 5 mg/l).
** Each child’s serum retinol concentration was multiplied by a correction factor according to the observed level of inflammation. Vitamin A status was defined as sufficient at serum retinol concentration of ≥1·05 µmol/l, marginal at 0·70–1·04 µmol/l and deficient at <0·70 µmol/l.
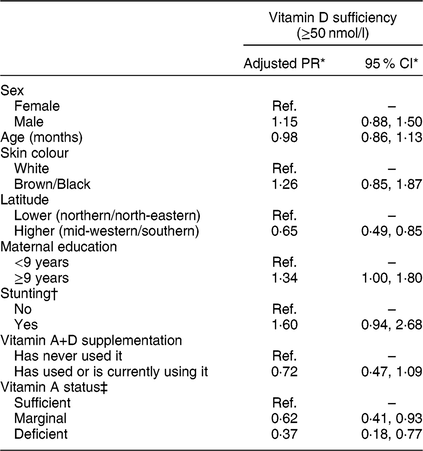
In adjusted analysis (Table 3), children living at higher latitudes had a lower prevalence of vitamin D sufficiency (PR = 0·65; 95 % CI 0·49, 0·85) than children from lower latitudes. Elevated maternal education positively influenced a sufficient vitamin D status in children (PR = 1·34; 95 % CI 1·00, 1·80), in comparison with those whose mothers reported <9 years of education. Compared with vitamin A concentration ≥1·05 µmol/l, marginal and deficient status of vitamin A were significantly associated with progressive reduction in the prevalence of vitamin D sufficiency (P trend = 0·001), accounting for proper correction for the presence of inflammation. Each increase of 1 µmol/l in vitamin A concentration was associated with a 1·38-fold higher prevalence of vitamin D sufficiency (95 % CI 1·18, 1·61), with additional adjustment for the child’s sex, age, skin colour, stunting and vitamin A+D supplementation. The direction and significance of all associations were similar when considering continuous vitamin D concentration in linear regression models. Specifically regarding the association with vitamin A corrected for inflammatory status, each 1 µmol/l increase of serum retinol was associated with a 0·43 nmol/l higher 25(OH)D3 concentration (95 % CI 0·18, 0·80), after controlling for all covariates (r 2 = 0·12).
Table 3 Multiple model for vitamin D sufficiency among children aged 11–15 months (n 468) from primary health-care units in four Brazilian cities, June 2012–January 2013
Ref., reference category.
* Prevalence ratios (PR) and 95 % CI for vitamin D sufficiency were estimated using multiple Poisson regression models with robust variance, with initial adjustment for the child’s sex, age and skin colour. Variables were subsequently selected according to a hierarchical conceptual framework for factors at distal, intermediate and proximal levels in relation to the outcome of interest.
† According to the WHO Child Growth Standards, stunting was defined as length-for-age Z-score < −2.
‡ Each child’s serum retinol concentration was multiplied by a correction factor according to the observed level of inflammation. Vitamin A status was defined as sufficient at serum retinol concentration of ≥1·05 µmol/l, marginal at 0·70–1·04 µmol/l and deficient at <0·70 µmol/l.
Discussion
In this early-life assessment of conditions related to vitamin D sufficiency among Brazilian children, 25(OH)D level ≥50 nmol/l was found in only one-third of study participants and was positively associated with living at lower latitudes. Independently of this proxy of sunlight exposure, maternal education and higher vitamin A concentrations contributed to vitamin D sufficiency in children up to 15 months of age.
Considering that, to date, meta-analyses have not been able to systematically include representative information on vitamin D status during the first years of life from several developing regions worldwide, our findings for vitamin D sufficiency in young Brazilian children may be compared with other individual studies on children of similar age groups from low- to middle-income countries. Among apparently healthy individuals selected from outpatient clinics of paediatric hospitals in northern India and Turkey, roughly half of children aged 1–3 years and 59 % of children aged 13–24 months, respectively, were considered to have sufficient 25(OH)D level(Reference Angurana, Angurana and Mahajan22, Reference Gülez, Korkmaz and Özkök23). While 64 % of Iranian healthy children 15–23 months of age attending health-care centres were vitamin D sufficient in a cross-sectional investigation conducted in different urban and rural areas of the country(Reference Olang, Naghavi and Bastani24), less than 10 % of children aged 12–60 months in rural Nepal recently exhibited 25(OH)D ≥ 50 nmol/l in a community-based study(Reference Avagyan, Neupane and Gundersen25).
Despite variations in estimates of vitamin D sufficiency, all the assessments mentioned above were carried out in locations at around the 30th parallel north (or somewhat higher). For Latin America and the Caribbean, a review of age groups throughout the lifespan suggested that vitamin D insufficiency is probably a public health concern in the region, but with undefined magnitude. For younger children, representative data were collected only from Mexico (15th–30th parallel north), where approximately one-quarter of pre-schoolers showed 25(OH)D concentration <50 nmol/l(Reference Brito, Cori and Olivares26). In Brazil, vitamin D level below sufficiency was found among 29·2 % of newborns from a probabilistic cross-sectional study in the city of Viçosa (20th parallel south; n 226)(Reference Prado, Oliveira and Assis4) and among 14·6 % of children <25 months of age in a population-based cross-sectional survey in the western Amazon area (20th parallel south; n 158)(Reference Cobayashi, Lourenço and Cardoso5). In view of the skin physiology involved in the provision of human vitamin D requirements, latitude may be interpreted as a proxy of exposure to sunlight and UV radiation(Reference Holick, Chen and Lu9). In this sense, our analysis was remarkable in its inclusion of children from cities in diverse regions and latitudes of Brazil, a country with continental dimensions in the southern hemisphere. Living at lower latitudes was an important factor associated with vitamin D sufficiency among generally healthy Brazilian children aged up to 15 months who attended primary health-care units. As has been well described, UV radiation is essential to the photochemical transformation of 7-dehydrocholesterol into previtamin D3, which undergoes hydroxylation reactions to later reach the physiologically active form of vitamin D(Reference Biesalski8, Reference Holick, Chen and Lu9).
Of note, latitude distribution seemed to minimize the potential influence of skin colour on vitamin D sufficiency in the present study. In our study, as shown in Table 1, a significantly larger proportion of non-white children were living at lower latitudes in comparison to those at higher latitudes. With adjustment for latitude, however, such characteristic did not affect the estimates for vitamin D sufficiency. Interestingly, a small study conducted in the south-eastern region of Brazil with children aged 2–7 years (n 84) proposed a score accounting for favourable conditions for the skin synthesis of vitamin D (such as sunlight exposure, use of sunscreen, skin colour), which was in turn positively associated with 25(OH)D concentration(Reference Kurihayashi, Augusto and Escaldelai27). An ecological meta-regression study with data mostly from adults and elderly individuals worldwide detected an inverse relationship of latitude with 25(OH)D level solely among Caucasian subjects(Reference Hagenau, Vest and Gissel28).
We also found significant regional differences in practices regarding vitamin supplementation. Whereas only 2 % of children residing at lower latitudes (northern/north-eastern regions) received supplements, caregivers of almost half of the children from higher latitudes reported complying with the practice – in spite of the significantly lower prevalence of vitamin D sufficiency simultaneously detected. In contrast with other reports(Reference Dawodu, Zalla and Woo29, Reference Saggese, Vierucci and Boot30), vitamin D supplementation was not sustained as a major contributor to 25(OH)D status among children in our final multiple model. This may point to a variable range of factors to be acknowledged in understanding predictors and also planning actions aimed at optimizing vitamin D status from early life. Our adjusted analysis suggested a role for maternal education, which was positively associated with vitamin D sufficiency. Particularly with more extreme patterns of social inequities, one may speculate that diverse contexts encompass determinants of nutritional status such as environmental conditions, health knowledge and behaviours(Reference Leroy, Habicht and Cossío31).
As hypothesized, higher vitamin A concentration was positively associated with a sufficient status of vitamin D. This appeared to be especially pertinent for retinol concentration ≥1·05 µmol/l, in view of progressive decline in the prevalence of vitamin D sufficiency associated with marginal (0·70–1·04 µmol/l) and deficient (<0·70 µmol/l) status of vitamin A. Previous observational studies have found correlations among micronutrient status indicators, including vitamins D and A(Reference Schulze, Christian and Wu32, Reference Augusto, Cobayashi and Cardoso33). Controlling for favourable conditions for skin synthesis of vitamin D, serum retinol concentration was positively associated with 25(OH)D in a cross-sectional analysis with children aged 2–7 years (n 84) enrolled in a governmental fortified-milk programme in São Paulo, Brazil(Reference Kurihayashi, Augusto and Escaldelai27). Vitamin A supplementation (single-dose capsule of 5000 µg (200 000 IU)) was tested for its impact on physical growth in a randomized, double-blind, placebo-controlled trial in an urban community clinic in New Delhi, India, among children aged 12–59 months (n 900). There was an indication for modest improvement of weight gain specifically in the summer season(Reference Bahl, Bhandari and Taneja34), which could foster some discussion on a potential relationship of vitamin A with predictors of vitamin D status.
Among our generally healthy children, 34·4 % were identified in the incubation, early convalescence or late convalescence stage of inflammation. As a result, it is important to note that the association between these vitamins was ascertained after adequately correcting for the concurrent AGP and CRP concentrations. It is known that nutrition interrelates with immune function; retinol-binding protein is among the serum proteins whose concentrations are rapidly reduced during infectious processes, with a logical impact on vitamin A levels(Reference Thurnham, McGabe and Northrop-Clewes12), and there is much existing evidence that corroborates this link(Reference Thurnham, McGabe and Northrop-Clewes12, Reference Schulze, Christian and Wu32, Reference Lourenço and Cardoso35). Besides the well-known actions on bone health, a review of trials on the impact of vitamin D supplementation for preventing infections among children under 5 years of age was performed(Reference Yakoob, Salam and Khan36), but epidemiological evidence is still of restricted quality and no benefit has been proven. At the cellular level, examination of the joint effects of vitamins D and A on the regulation of immunity has in fact indicated a possible antagonistic dynamic between metabolites of these micronutrients, given that these compounds share common nuclear receptors in the retinoid X receptor family(Reference Mora, Iwata and von Adrian10). The characterization of larger-scale implications of specific anti-proliferative effects of vitamin D v. cytotoxic and proliferative roles of vitamin A, however, would require a prospective design of data collection along with the consideration of different levels of these vitamins’ availability to the organism, which was not possible in the present cross-sectional analysis.
There are some limitations to our study. First, we did not collect data on the dietary intake of vitamin D among our participants. Nevertheless, past investigations among young Brazilian children(Reference Garcia, Granado and Cardoso37, Reference Hilger, Goerig and Weber38) found poor dietary variability in conjunction with widespread nutrient deficiencies in this age group, suggesting that no major contribution to 25(OH)D level would have arisen from food consumption. Second, time spent outdoors under sunlight exposure, clothing habits, and use of sunscreen or other types of barriers were not directly assessed, although we had information on other important parameters related to the skin synthesis of vitamin D, such as region of residence and child skin colour. We were not able to disentangle the effects of vitamin A and vitamin D supplementation as these micronutrients are often offered in combination, neither to precisely ascertain potential impacts on serum vitamin concentration because information on supplementation was collected jointly for its past or current use. Finally, we must interpret the present findings in view of the cross-sectional nature of data collection that prevents causal assumptions. Some strengths should be noted as well. We contribute multicentre information on vitamin D sufficiency among young children in low- to middle-income areas. Our study participants were selected at public primary health-care units across a wide range of latitude. Our findings also highlight the contribution of vitamin A, according to previous exposure to inflammation, to vitamin D status in this very young age group.
There is an increasing amount of longitudinal data showing a relationship of gestational vitamin D and perinatal outcomes(Reference Nobles, Markenson and Chasan-Taber39, Reference Lykkedegn, Beck-Nielsen and Sorensen40). Overall, in our analysis a relatively small proportion (31·8 %) of children had 25(OH)D ≥ 50 nmol/l already during their first months of life. In light of evidence for intergenerational pathways influencing vitamin D levels, there is substantial work ahead, therefore, to ensure proper status of vitamin D among young children during an important window of opportunity that favours adequate development and better health outcomes in later years.
Conclusion
In conclusion, vitamin D sufficiency was present in one-third of Brazilian children of up to 15 months of age attending primary health-care units and living at diverse latitudes. Living at lower latitudes, along with higher maternal education and vitamin A concentrations, after correction for the presence of inflammation, were positively associated with 25(OH)D level. Additional prospective surveys are necessary to elucidate underlying mechanisms for potential synergy of optimal status of vitamins.
Acknowledgements
Acknowledgements: The authors thank all the participants and health professionals involved in this study. Financial support: This work was supported by the Ministry of Health of Brazil, with administrative and financial management by the Brazilian National Council of Scientific and Technological Development, CNPq (grant number 552747/2011-4). Organizations involved in the financial support of this work had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: B.H.L. contributed with formulation of the present research question, performed statistical analyses, wrote initial the version of the article and had primary responsibility for final content. L.L.S.S. contributed with formulation of the present research question, data collection and analyses, and helped writing the article. M.A.C. designed the study, contributed with formulation of the present research question, data collection and analyses, and was involved in the review of the article. W.W.F. contributed with data interpretation and was involved in the review of the article. All authors have read and approved the final version of the article. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the School of Public Health, University of São Paulo, Brazil. Written consent was obtained from the caregivers of all participants. Children with nutritional deficiency diagnosed during the study were referred for treatment in the health centres. The ENFAC Study was registered at www.ensaiosclinicos.gov.br as RBR-5ktv6b.
Appendix
Members of the ENFAC Working Group
Marly Augusto Cardoso, Rosângela Aparecida Augusto, Fernanda Cobayashi (Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil); Maria Claret C.M. Hadler, Maria do Rosário G. Peixoto (School of Nutrition, Federal University of Goiás, Goiânia, Brazil); Pedro Israel C. Lira, Leopoldina Augusta S. Sequeira (Department of Nutrition, Federal University of Pernambuco, Recife, Brazil); Pascoal Torres Muniz, Cristiéli Sérgio de Menezes Oliveira (Department of Health Sciences, Federal University of Acre, Rio Branco, Brazil); Márcia Regina Vitolo, Daniela Cardoso Tietzmann (Federal University of Health Sciences of Porto Alegre, Porto Alegre, Brazil); Márcia Maria Tavares Machado (Department of Preventive Medicine, Federal University of Ceará, Fortaleza, Brazil); Patricia Constante Jaime, Eduardo Augusto Fernandes Nilson, Gisele Ane Bortolini, Sara Araújo da Silva (Coordenação Geral de Alimentação e Nutrição, Ministry of Health, Brasília, Brazil).





