Child malnutrition is a major global health problem, contributing to increased morbidity and mortality, impaired intellectual development and working capacity, and increased risk of disease in adulthood( Reference Black, Allen and Bhutta 1 ). Twenty per cent of the 7·6 million deaths per year recorded among children under 5 years of age can be attributed to undernutrition( 2 ). Moderate wasting and severe wasting represent acute forms of undernutrition, and children suffering from them face a markedly increased risk of death. It is estimated that moderate acute malnutrition (MAM) and severe acute malnutrition (SAM) affect 52 million of children under 5 years of age worldwide. MAM is defined as weight-for-height between −3 sd and −2 sd below the median weight-for-height of the WHO child growth standards (weight-for-height Z-score (WHZ) between −3 and −2) without oedema( 3 ). Supplementary feeding programmes are designed to treat MAM and prevent the progression from MAM to SAM( 4 ), and thus have the potential to reduce child mortality and morbidity.
A wide range of nutritional products are currently used to treat MAM( Reference De Pee and Bloem 5 ). These include fortified blended flours, especially corn–soya blend (CSB) prepared as porridge; BP5 biscuits; and lipid-based nutrient supplements, especially ready-to-use supplements, both therapeutic and supplementary (RUTF and RUSF). CSB are the most commonly used( Reference Dewey and Adu-Afarwuah 6 ); in Cameroon, the national protocol for the management of acute malnutrition recommends the use of RUSF or CSB at the dose of 5021 kJ (1200 kcal)/person per d. However, concerns exist about their nutritional adequacy, issues around preparation at home (making the porridge too thin or inadequate boiling of water) and ration sharing( Reference Wood and Sibanda-Mulder 7 ).
There is some evidence that ready-to-use foods result in better outcomes for children with MAM than the standard CSB-based approaches( Reference Matilsky, Maleta and Castleman 8 – Reference Karakochuk, Briel and Stephens 11 ), although this has not always been found( Reference Maleta, Kuittinen and Duggan 12 ). In recent years, changes have been made to improve the composition of fortified blended flours to respond more appropriately to MAM treatment and two products have been developed: (i) CSB++ for children 6–24 months of age; and (ii) CSB+ for children above 24 months( 13 ). A recent study( Reference LaGrone, Trehan and Meuli 14 ) comparing the effectiveness of CSB++ with ready-to-use supplementary foods (RUSF) showed that CSB++ is not inferior to RUSF, although children receiving CSB++ required a longer time to recover and gained less weight than those receiving RUSF.
In the different published studies that have tested the effectiveness of nutritional products to treat MAM, the formulation of the products and the quantities given have varied; therefore there is no definitive consensus on the most effective way to treat children with MAM( Reference Lazzerini, Rubert and Pani 15 ). Most studies provided large quantities of supplementary foods (>2929 kJ/d (>700 kcal/d)), such that the energy content of the ration was either higher than or equal to the daily requirement for young children. In a recent study, Karakochuk et al.( Reference Karakochuk, Briel and Stephens 11 ) showed a significant benefit of RUSF given at complementary dose (2092 kJ/d (500 kcal/d)) over CSB given in large quantity (5912 kJ/d (1413 kcal/d)), which suggests that supplementary foods can be use at complementary dose for the treatment of MAM.
In the present randomized controlled trial, we compared CSB+ with RUSF in the treatment of MAM to test the hypothesis that supplementary foods given at complementary dose (about 50 % of the child’s energy requirement) result in high recovery rates. We assume the availability of some food in the household and caregivers were instructed on how best to use the food they have.
Methods
Study design
We conducted a comparative effectiveness trial study that assessed the treatment of MAM in children for a period of 56 d, using a controlled randomized design with parallel assignment for CSB+ or RUSF. Children were defined as having recovered when they reached a WHZ>−2; otherwise, they were categorized as having continued MAM despite 56 d of therapy, had developed SAM (WHZ<−3 and/or bilateral pitting oedema), were transferred to in-patient care, died, or defaulted (did not return for two consecutive visits). The primary outcome was to assess the recovery rate of the children receiving CSB+ or RUSF. Secondary outcomes included time to recovery and rates of gain in weight and mid-upper arm circumference.
A randomization list was created using a random number generator (Stat Trek). Allocation to either CSB+ or RUSF was performed by caregivers drawing from an opaque bag containing coded numbers corresponding to one of the two supplementary foods. The code was accessible only to the food distributor. Investigators performing the clinical assessment and nutrition education were blinded to the child’s assigned food group. If two children were from the same household, both children were given the same type of food to reduce the likelihood of confounding study foods.
Participants
Eight hundred and thirty-three children aged 6–59 months living in the health districts of Mvog-Beti (urban area) or Evodoula (rural area) in the Centre region of Cameroon were screened for eligibility. Children were excluded if they did not have appetite, had a chronic debilitating illness, or had a history of peanut allergy. Eighty-one children aged 25–59 months with MAM (WHZ<−2 and ≥−3 without oedema) were enrolled in the study from February to July 2012. With this number of children, a difference of 0·063 change in variance could be detected, assuming an expected variance of 0·01 with a power level of 80 % and a significance level of 5 % in two-sided tests, by using the formula of Chow et al.( Reference Chow, Shao and Wang 16 ):
where α is the probability of type I error (significance level), β is the probability of type II error (1− power of the test), μ 2−μ 1 is the the true mean difference between RUSF (μ 2) and CSB+ (μ 1) and σ 2 is the expected variance.
Informed written consent was obtained from each caregiver of children participating in the study. The protocol was approved by the ethics committee of the Institute of Medical Research and Medicinal Plants Studies (IMPM) and the trial was registered at www.clinicaltrials.gov as NCT01898871. Forty-one children received CSB+ and forty received RUSF (Fig. 1).
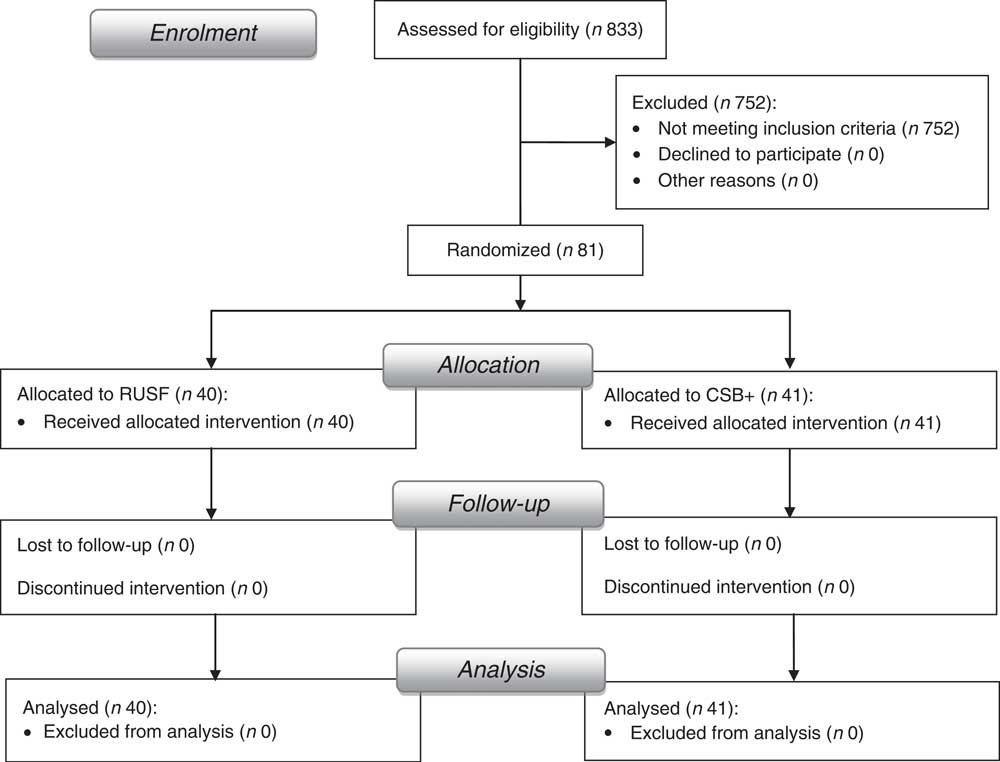
Fig. 1 Flowchart of study enrolment and completion
Food and treatment
On enrolment, children were examined by a paediatrician to assess their health status and they were de-wormed with one tablet of mebendazole 500 mg. Caregivers were interviewed regarding the child’s sociodemographic characteristics and to assess household food consumption score. Nutrition and general health counselling was also provided to all caregivers as well as information about the illness of their children and the benefit of supplementary feeding. Caregivers were instructed to continue to feed children their usual diet along with the supplementary food as medicine.
A ration of supplementary food sufficient for 2 weeks was distributed at each visit. Children returned every 2 weeks for follow-up for up to four follow-up visits. At each follow-up visit, caregivers reported on the child’s clinical symptoms and tolerance of the study food, anthropometric measurements and nutrition education were repeated, and additional supplementary food was distributed for those who remained wasted. The daily supplementary rations were equivalent to 167 kJ (40 kcal) CSB+ or RUSF/kg body weight, to provide about 50 % of the child energy requirement set at 314 kJ (75 kcal)/kg body weight per d( Reference Torun 17 ).
The nutrient profile of each distributed ration is detailed in Table 1. The nutrient values for CSB+ and oil were referenced from the producer’s product sheet, and the nutrient values for RUSF were calculated using NutriSurvey for Windows.
Table 1 Nutrient composition of the supplementary foods per daily ration for a child weighing 8 kg

CSB+, improved corn–soya blend; RUSF, ready-to-use supplementary food.
The caregivers were instructed to give the supplement only to the enrolled child, in addition to the usual diet, and not to share it. Additional instructions were given to caregivers of children in the CSB+ arm about how to prepare the supplement properly, i.e. mix 40 g of CSB+ with 250 g of water and cook for 10 min.
CSB+ GMO-free was produced by Michiels Fabrieken n.v., Belgium, according to specifications from UNICEF. CSB+ contains corn, soya, sugar and concentrated minerals and vitamins. Before distribution, CSB+ was mixed with 10 % soya oil by weight. CSB+ and 10 wt% oil cost 0·93 €/kg, or 0·068 € for an average daily ration.
RUSF was formulated and produced by the Centre for Food and Nutrition Research, Yaoundé, Cameroon, by using pre-cooked soya and corn flours, peanut paste, sugar, soya oil and a premix containing concentrated minerals and vitamins (DSM, South Africa). RUSF costs 1·32 €/kg, or 0·080 € for an average daily ration.
The products underwent quality assurance and safety testing for aflatoxin and microbial contamination.
Nutrition education
Nutrition counselling aiming to improve the quality and quantity of food given to children was equally provided to all caregivers at enrolment and at each follow-up visit, during a training session preceding the distribution of supplementary foods. The key messages included: (i) continue to breast-feed your child until he is 2 years old; (ii) wash your hands and your child’s hands with soap and water before eating; (iii) wash your hands with soap and water after using the toilet or cleaning your child’s bottom; (iv) help your child eat and finish all food; (v) feed your child a variety of foods every day; (vi) vegetables are good for your child, it help him keep healthy and prevent illness; (vii) foods from animals help your child gain weight, grow strong and lively, give them every day; (viii) give fruits to your child every day; (ix) add beans, soya or groundnuts in your child’s porridge every day; and (x) keep food and water covered.
Measurements and assessments
Using a structured questionnaire, caregivers were interviewed regarding the child’s sociodemographic characteristics. Data were obtained on whether the child lived with his two parents, the education level of the mother, whether the mother was the primary caregiver, whether the mother had a job, whether the child lived in a rural or urban area, the child’s vaccination history and the child’s use of medicines during the previous 2 weeks. Adverse effects (transferred to in-patient care, defaulted, died) were recorded in a weekly tracking sheet.
Food consumption score, which is a proxy indicator of household food security, was measured at enrolment considering the frequency of food consumption (number of days on which each food group was consumed during the past 7 d) and the weight of each food group( 18 ). Cut-off points were used to categorize households into acceptable level of consumption (>35), borderline level (21·5–35) or poor level of consumption (0–21).
Children were evaluated for acute malnutrition by trained nutrition researchers. Standard methods for anthropometric measurements were used( Reference Cogill 19 ). The infants were weighed without clothes to the nearest 5 g using a portable electronic infant scale (Seca 416, Hamburg, Germany). Length was measured to the nearest 0·2 cm using a standardized infantometer (Seca 416). Mid-upper arm circumference was measured to the nearest 0·1 cm with a non-elastic metric measuring tape (Seca 201).
Anthropometric indices (WHZ, weight-for-age Z-score (WAZ) and height-for-age Z-score (HAZ)) were based on the WHO’s 2006 Child Growth Standards( 3 ), calculated by using Anthro version 3·2·2( 20 ). Weight gain (in g/kg per d) was calculated using the model described by Patel et al.( Reference Patel, Engstrom and Meier 21 ).
Data analysis
The analysis was intention-to-treat and involved all patients who were randomly assigned. Statistical analyses were performed using the statistical software package SPSS Statistics 17·0. Comparisons of baseline and outcomes characteristics between CSB+ and RUSF were made by using Fisher’s exact test for dichotomous variables and Student’s t test for continuous variables.
XLSTAT version 2013·3·01 (Addinsoft) was used to conduct survival analyses. Kaplan–Meier analysis was used to estimate and compare survival curves and the Cox proportional hazards model was used to assess the association between characteristics of children at the time of enrolment with the risk of failure to recover. Independent variables used in the model were enrolment WHZ and HAZ, whether the child lived with his two parents, education level of the mother, whether the mother was the primary caregiver, whether the mother had a job, food consumption score, the sex of the child, whether the child lived in a rural or urban area, the type of supplementary food eating by the child and vaccination history of the child. Two-sided P values <0·05 indicated significance.
Results
Table 2 displays the baseline characteristics of children. No significant differences (P<0·05) were noted between CSB+ and RUSF groups. All the children enrolled in the study were moderately wasted, moderately underweight and moderately stunted. Mean value of food consumption score indicated that children were living in a context of moderate food insecurity with about 65 % having borderline food consumption, 17–20 % having acceptable food consumption and 15–17 % having poor food consumption.
Table 2 Enrolment characteristics of children (n 81; aged 25–59 months) treated for moderate acute malnutrition, Centre region of Cameroon, February–July 2012

CSB+, improved corn–soya blend; RUSF, ready-to-use supplementary food; MUAC, mid-upper arm circumference; WHZ, weight-for-height Z-score; HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; FCS, food consumption score.
a,bValues in the same line not sharing a common superscript were significantly different (P<0·05) according to Fisher’s exact tests (dichotomous variables) or Student’s t test (continuous variables).
Table 3 displays the characteristics of children after treatment. No adverse reactions to any of the foods were reported. After 56 d of treatment, a total 85 % of children recovered from MAM in the RUSF group (95 % CI 73 %, 97 %) and 73 % in the CSB+ group (95 % CI 59 %, 87 %). According to Fisher’s exact test, there was no significant difference (P=0·276) between the two groups. No defaulting and death were recorded. We observed a non-response rate of 20 % among children in the CSB+ group and 15 % in the RUSF group; thus, these children did not recover but maintained their MAM status at the end of 56 d treatment. Of children in the RUSF and CSB+ group, 3 % and 5 % respectively deteriorated to SAM.
Table 3 Outcome characteristics of the moderately wasted children (n 81; aged 25–59 months) who received supplementary food (daily ration of 167 kJ (40 kcal)/kg body weight for 56 d), Centre region of Cameroon, February–July 2012

CSB+, improved corn–soya blend; RUSF, ready-to-use supplementary food; SAM, severe acute malnutrition; MAM, moderate acute malnutrition; WHZ, weight-for-height Z-score; MUAC, mid-upper arm circumference.
a,bValues in the same line not sharing a common superscript were significantly different (P<0·05) according to Fisher’s exact test (dichotomous variables) or Student’s t test (continuous variables).
Children who received RUSF showed higher rates of weight gain compared with those receiving CSB+ (P<0·05).
Considering the number of children who recovered at any time of the study, the mean duration of treatment required to achieve recovery was 44 d in the RUSF group and 51 d in the CSB+ group. The proportion of children recovered in the RUSF group was always higher compared with the CSB+ group starting from day 28 of treatment and beyond (Fig. 2). The result of log-rank testing showed a significant difference between the recovery rates of CSB+ and RUSF (P=0·0048).

Fig. 2 Recovery of children with moderate acute malnutrition (n 81; aged 25–59 months) according to treatment (![]() , CSB+;
, CSB+; ![]() , RUSF), Centre region of Cameroon, February–July 2012. Log-rank test for trend, P=0·0048 (CSB+, improved corn–soya blend; RUSF, ready-to-use supplementary food)
, RUSF), Centre region of Cameroon, February–July 2012. Log-rank test for trend, P=0·0048 (CSB+, improved corn–soya blend; RUSF, ready-to-use supplementary food)
Most characteristics of children at the time of enrolment tested in the Cox proportional hazard model (Table 4) showed no significant association (P<0·05) with the risk of failure to recover; only the type of supplementary food received was a significant predictor of recovery (P=0·021).
Table 4 Cox proportional hazards model of factors associated with recovery from moderate acute malnutrition after supplementary feeding among children (n 81; aged 25–59 months), Centre region of Cameroon, February–July 2012

HR, hazard ratio; WHZ, weight-for-height Z-score; HAZ, height-for-age Z-score; CSB+, improved corn–soya blend.
The model constant=0·798; R 2=0·422 by Cox and Snell, R 2=0·422 by Nagelkerke, χ 2=10·401.
† HR<1 and 95 % CI not overlapping 1 indicate that as the independent variable increases, the risk of failure increases.
Discussion
In the present trial, 73 % and 85 % of children treated with CSB+ and RUSF respectively recovered from MAM, suggesting that both products were relatively successful for the treatment of MAM in children. The recovery rates achieved by children in both groups were comparable to or relatively higher than those observed in previous studies( Reference Matilsky, Maleta and Castleman 8 , Reference Karakochuk, Briel and Stephens 11 ) despite the lower quantity of supplement provided to children. This could be a reflection of the investment in education of caregivers on how best to use foods available in the house, since educational interventions have been shown to improve child feeding practices( Reference Caulfield, Huffman and Piwoz 22 ). In this regard, it is suggested that in the context of moderate food insecurity, nutrition education could improve the outcomes of the intervention and reduce the quantity of supplement generally provided.
Non-response rate was similar in the two groups. No characteristic of children, except the type of supplementary food received, was significantly associated with the risk of failure to recover (Table 4), suggesting that the non-response rate could be linked to the use of supplementary foods. Non-response rates have been associated with food sharing practice in previous studies( Reference Wood and Sibanda-Mulder 7 ). Children enrolled in the present study did not receive extra rations to accommodate presumed sharing and supplementary foods were promoted as special medicinal food for the child with MAM to discourage sharing. However, we cannot guarantee that our recommendation to not share the supplement was always followed, especially for CSB+ that requires preparation and looks like staple foods.
About 4 % of children enrolled in the study did not respond to supplementary feeding, but developed severe wasting. The reason for the development of SAM is unknown; development of SAM was not correlated to household food insecurity and could be explained by the hypothesis that these children had an untreated illness. Despite the fact that, at the beginning of the study, the children were de-wormed and excluded in the case of chronic illness, some children might have been affected by other infections such as malaria which, in Cameroon, is responsible of 40 % of mortality in children under 5 years of age( 23 ).
The weight gain of children in the RUSF arm was significantly higher than that in the CSB+ arm despite the fact that the total energy provided by the supplement in both groups was similar. However, the nutrient contents of CSB+ and RUSF were different (Table 1); besides, RUSF does not need any cooking, whereas CSB+ needs to be cooked. Because of CSB+ lower energy density and the large amount of water needed for cooking, children treated with CSB+ need to eat about eight times the mass of supplement compared with children treated with RUSF. It is possible that this affected the global dietary intake of the children and might account for the lower weight gain and recovery rate in the CSB+ group. However, the weight gain obtained in the present study was higher than the one reported in previous studies for children receiving regular CSB( Reference Stefanak and Jarjoura 24 , Reference Patel, Sandige and Ndekha 25 ). This is not the case with the improved fortified blended flour (CSB++), as a study in Malawi( Reference LaGrone, Trehan and Meuli 14 ), in comparison to the present study, showed higher weight gain (3·1 g/kg body weight per d) after the treatment. However, the Malawian study provided 46·6 % higher energy than the present study.
In Cameroon as in most African countries, national protocols for the management of MAM recommend the use of large doses of fortified blended flours which most of the time are imported. Based on the average time required for treatment in both groups, the cost to treat a child with CSB+ (3·48 €) was relatively lower than the cost with RUSF (3·52 €); this cost is for the product alone and does not include transport, storage or staffing costs, but suggests that the local RUSF could be more cost-effective if considering the operational limits of CSB+ as it requires preparation.
The main limitation of the current study was that children’s dietary intake during the treatment was not monitored and a non-supplemented control group was absent. Therefore, we could not estimate the contribution of home diet; we can only conclude on the relative effectiveness of CSB+ and RUSF on the recovery of children with MAM.
Conclusion
In conclusion, the present study showed that both CSB+ and RUSF were relatively successful for the treatment of MAM in children. It was interesting to note that despite the relatively low ration size provided (50 % of the child’s energy requirement), the recovery rates observed for both groups were comparable to or higher than those reported in previous studies, which could be a probable effect of nutrition education.
Acknowledgements
Acknowledgements: The authors are indebted to the participating children and caregivers who generously gave their time and commitment to fulfil the requirement for participating in this study. They gratefully acknowledge the contributions from Baleba Mbanga, Gondam Kamini, Thomas Ndanga and Philomene Emale who participated in the field activities. Financial support: This work was supported by the International Atomic Energy Agency (Technical Cooperation project CMR/6/010). The International Atomic Energy Agency had no role in the design, analysis or writing of this article. Conflict of interest: The authors confirm that they have no conflicts of interest in any company or organization sponsoring the research currently and at the time the research was done. Authorship: The authors’ responsibilities were as follows. G.N.M. initiated the study, drafted the research protocol, coordinated and led the research implementation in the field, analysed the data and drafted the manuscript. P.M.N. helped with data collection and analysis, had full access to all data in the study and was responsible of the integrity and accuracy of the data. A.C.A.N. was responsible for enrolment of the subjects and helped with clinical and nutritional evaluation of children. V.J.E., J.J.T.T. and H.T.D. helped with enrolment of the subjects and data collection. All authors edited the manuscript and approved its final contents. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of IMPM (Institute of Medical Research and Medicinal Plants Studies) and received administrative authorization from the Ministry of Public Health (number 631–19·10). Written informed consent was obtained from all caregivers. This trial was registered at www.clinicaltrials.gov as NCT01898871.









