Abdominal obesity is characterised by the accumulation of visceral adipose tissue, and it is a major risk factor for the development of hypertensionReference Freedman, Khan, Serdula, Galuska and Dietz1, Reference Thompson, Edelsberg, Colditz, Bird and Oster2. Abdominal obesity is also the principal risk factor for insulin resistance and the development of type 2 diabetesReference Meigs3. Hypertension in obese individuals is commonly complicated by the concomitant presence of dyslipidaemia, hyperinsulinaemia, impaired glucose tolerance and other components of the metabolic syndromeReference Haffner and Taegtmeyer4. Sodium retention, volume expansion and increased cardiac output are common findings in obese individuals. These changes are largely attributable to increased activity of the sympathetic nervous system and insufficient suppression of the renin–angiotensin system (RAS). Recent data show increased expression of angiotensin II-forming enzymes in adipose tissue, and increased activity of the RAS has recently been implicated in the development of insulin resistance and type 2 diabetesReference Sharma5. These evidences could explain the relevance of obesity as an important predictor of overall cardiovascular morbidity and mortalityReference Freedman, Khan, Serdula, Galuska and Dietz1, Reference Thompson, Edelsberg, Colditz, Bird and Oster2.
This review discusses the role of RAS in the relationship between obesity, essential hypertension and insulin resistance.
Renin–angiotensin–aldosterone system and obesity-related hypertension
Although excess weight gain is associated with marked sodium retention and expansion of extracellular fluid volume, obese subjects usually have increases in plasma renin activity, plasma angiotensinogen (AGT), angiotensin-converting enzyme (ACE) activity and plasma angiotensin (Ang) II levelsReference Hall6. Figure 1 summarises the mechanisms by which obesity increases renal tubular sodium reabsorption, impairs pressure natriuresis and causes hypertension as well as progressive glomerular injuryReference Hall6. A significant role for Ang II in stimulating sodium reabsorption, impairing renal-pressure natriuresis and causing hypertension in obesity is supported by the finding that treatment of obese dogs with an Ang II antagonist or ACE inhibitor blunts sodium retention and volume expansion, as well as elevated arterial pressureReference Hall, Henegar, Shek and Brands7, Reference Robles, Villa, Santirso, Martinez, Ruilope and Cuesta8. Also, ACE inhibitors are effective in reducing blood pressure in obese humans, particularly in young patientsReference Reisen, Weir, Falkner, Hutchinson, Anzalone and Tuck9.
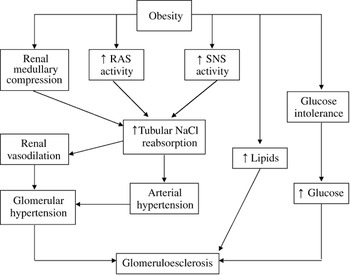
Fig. 1 Mechanisms involved in development of obesity-related hypertension and subsequent renal damage (modified from HallReference Hall6)
Activation of the RAS in adipose tissue may represent an important link between obesity and hypertensionReference Engeli and Sharma10. Adipose tissue is an important production site of AGTReference Ailhaud, Fukamizu, Massiera, Negrel, Saint-Marc and Teboul11, and several studies have reported correlations between plasma AGT concentrations, blood pressure and body mass indexReference Rotimi, Cooper, Ogunbiyi, Morrison, Ladipo and Tewksbury12, Reference Pratt, Ambrosius, Tewksbury, Wagner, Zhou and Hanna13. Overexpression of AGT exclusively in adipose tissue in AGT knockout mice not only resulted in measurable plasma levels of AGT but also resulted in an increase in blood pressure, restoration of sodium balance and augmented adipocyte sizeReference Massiera, Bloch-Faure, Ceiler, Murakami, Fukamizu and Gasc14, whereas RAS suppression by either an ACE inhibitor or deletion of the type-2 angiotensin-II receptor leads to a decrease of adipocyte sizeReference Furuhashi, Ura, Takizawa, Yoshida, Moniwa and Murakami15, Reference Yvan-Charvet, Even, Bloch-Faure, Guerre-Millo, Moustaid-Moussa and Ferre16. Ang II has also been shown to play a role in adipocyte growth and differentiationReference Ailhaud17. Furthermore, locally produced Ang II may directly stimulate leptin release from adipocytes, an effect that may be counterbalanced by increased sympathetic activityReference Cassis, English, Bharadwaj and Boustany18. Adipocytes also secrete adiponectin, a plasma protein that is downregulated in obese individualsReference Arita, Kihara, Ouchi, Takahashi, Maeda and Miyagawa19. Adiponectin enhances insulin sensitivity and prevents atherosclerosisReference Okamoto, Kihara, Ouchi, Nishida, Arita and Kumada20. Recently, it has been shown that blockade of the RAS with either an ACE inhibitor or an Ang II receptor blocker results in a substantial increase in adiponectin levels associated with an increase in insulin sensitivityReference Furuhashi, Ura, Higashiura, Murakami, Tanaka and Moniwa21. Further support for the involvement of RAS activation in obesity-associated hypertension came from a dietary intervention study in menopausal women. Engeli et al.Reference Engeli, Böhnke, Gorzelniak, Janke, Schling and Bader22 showed that 5% weight loss resulted in a 7 mmHg reduction of ambulatory blood pressure and that the decrease was accompanied by significant declines of serum AGT (27%), renin (43%) and ACE activity (12%) as well as AGT expression in adipose tissue (20%).
Aldosterone has been implicated in the development of hypertension associated with obesityReference Rahmouni, Correia, Haynes and Mark23. Plasma aldosterone levels are elevated in obese hypertensives, especially in those with visceral obesityReference Goodfriend and Calhoun24. The mechanisms by which excess fat could increase aldosterone are unknown, but it may relate to the production by adipocytes of potent mineralocorticoid releasing factors Reference Ehrhart-Bornstein, Lamounier-Zepter, Schraven, Langenbach, Willenberg and Barthel25 or to the ability of oxidised derivatives of linoleic acid to induce aldosterone synthesisReference Goodfriend, Ball, Egan, Campbell and Nithipatikom26. The involvement of aldosterone in obesity-associated hypertension has been demonstrated by the blockade of mineralocorticoid receptors with the specific antagonist eplerenone in high-fat-fed dogsReference de Paula, da Silva and Hall27.
Insulin resistance and obesity-related hypertension
Resistance to insulin-mediated glucose uptake by the skeletal muscle, which is often linked with abdominal obesity, greatly increases the likelihood of developing abnormalities such as type 2 diabetes, hypertension and dyslipidaemiaReference Reaven28. The cluster of these independent cardiovascular risk factors is known as metabolic syndrome, described by the NCEP ATP III29, and it is well-recognised that hypertensive patients presenting metabolic syndrome show an increased cardiac, renal and vascular damageReference Cuspidi, Meani, Fusi, Severgnini, Valerio and Catini30–Reference Safar, Thomas, Blacher, Nzietchueng, Bureau and Pannier32. In the general US population, the age-adjusted prevalence is 24.0% for men and 23.4% womenReference Ford, Giles and Dietz33, and it rises continuously in an epidemic progressionReference Ford, Giles and Mokdad34, Reference Banegas and Ruilope35. Among hypertensive patients attended to in our hospital-based hypertension unit, the overall prevalence of metabolic syndrome rise to 49.4%, with no difference between genders (47.8% in men and 50.5% in women)Reference Segura, Campo, Roldan, Christiansen, Vigil and García-Robles36. Finally, in a population-based cohort of type 2 diabetes, a prevalence of metabolic syndrome of 75.6% has been recently describedReference Bruno, Merletti, Biggeri, Bargero, Ferrero and Runzo37. The International Diabetes Federation has recently described a new definition of metabolic syndrome, with more strict criteria for waist circumference (≥94 cm for Europid men and ≥80 cm for Europid women)Reference Alberti, Zimmet and Shaw38. According to this new definition, the prevalences described above would be increased.
The metabolic syndrome is associated with an increased risk of both diabetesReference Grundy, Hansen, Smith, Cleeman and Kahn39 and cardiovascular diseaseReference Isomaa, Almgren, Tuomi, Forsen, Lahti and Nissen40, Reference Lakka, Laaksonen, Lakka, Niskanen, Kumpusalo and Tuomilehto41. Abdominal obesity is a risk factor for cardiovascular disease worldwideReference Yusuf, Hawken, Ounpuu, Dans, Avezum and Lanas42, and it is becoming a dramatic issue for national health systemsReference Porier, Giles, Bray, Hong, Stern and Pi-Sunyer43. Specifically, overweight and obesity in childhood are highly associated with multiple comorbidities, elevated blood pressure values, dyslipidaemia, reduced insulin sensitivity and alterations of large and minor vesselsReference Schiel, Beltschikow, Kramer and Stein44, Reference Srinivasan, Myers and Berenson45. Obesity is often associated with insulin resistance and the components of metabolic syndrome. In fact, the degree of insulin resistance impacts the risk for obesity-related metabolic comorbidities, with a greater risk for type 2 diabetes and cardiovascular disease in patients who are severely insulin-resistantReference Bacha, Saad, Gungor and Arslanian46.
Recently, we published the first study in Spain to report on the degree of blood pressure control achieved in hospital-based hypertension units across the whole nation in light of recommendations contained in international guidelinesReference Banegas, Segura, Ruilope, Luque, García-Robles and Campo47. We performed a survey covering 4049 patients, aged 18 years or older, who had a diagnosis of essential hypertension, had been using antihypertensive therapy at least for 1 year, had been seen at 47 hospital-based hypertension units nationwide and had been regularly followed up by the same medical team in each unit. Baseline data for the 4049 hypertensives studied are shown in Table 1. It should be highlighted the increased mean body mass index 29.5 ± 5 kg m−2 and the high prevalence of overweight (42.3%) and obesity (41.1%) among hypertensive patients attended in hospital-based hypertension unitsReference Banegas, Segura, Ruilope, Luque, García-Robles and Campo47. The univariate analysis showed that poorer blood pressure control occurred in older patients, females, obese patients, diabetic patients and in those treated with two or more antihypertensive drugs, and all variables, except for gender, remained statistically significant in the multivariate analysisReference Banegas, Segura, Ruilope, Luque, García-Robles and Campo47. Table 2 shows the percentages of systolic and diastolic blood pressure control according to body mass index, which is significantly lower among obese hypertensive patients. From the whole group, 63.7% were using two or more antihypertensive drugs. ACE inhibitors, Ang II receptor antagonists and calcium channel blockers were the drugs most frequently prescribed. These data confirm that hypertensive patients followed up in hospital hypertension clinics exhibit an increased cardiovascular risk linked to a high prevalence of target organ damage, associated clinical conditions, diabetes or other major cardiovascular risk factors, with a great relevance of overweight and obesity.
Table 1 Baseline demographic and clinical characteristics of the sample (from Ref. 47 with permission)
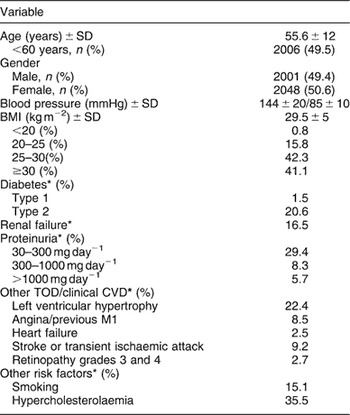
SD – standard deviation; BMI – body mass index; TOD – target organ damage; CVD – cardiovascular disease; Ml – myocardial infarction.
For definitions of clinical characteristics see Methods.
* The percentages listed are those for the population with the characteristic divided by the population in whom the condition was determined.
Table 2 Systolic and diastolic blood pressure control according to BMI (from Ref. 47 with permission)

BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure.
Clinical management of obesity-related hypertension
Despite the fact that an increasing number of hypertensive patients now present with a body mass index in excess of 30 kg m−2, there are currently no specific recommendations or treatment algorithms for obesity hypertensionReference Chobanian, Bakris, Black, Cushman, Green and Izzo48, 49.
The lack of an established approach to the management of obesity hypertension is perhaps largely caused by the lack of data from prospective intervention studies on obese hypertensivesReference Sharma, Pischon, Engeli and Scholze50. Despite the fact that many recent intervention trials comparing different antihypertensive drugs have been reported, they have not been designed specifically for obese hypertensive patients. Although some patients participating in these trials may have been obese, general extrapolation of these data may not be justifiedReference Sharma5. Because obesity hypertension results in significant cardiovascular, neurohormonal, renal and metabolic changes, a comprehensive approach to treatment including both weight loss and pharmacological approaches would be warrantedReference Sharma5. Antihypertensive drugs prescription should be based on guidelines recommendations for management of hypertensionReference Chobanian, Bakris, Black, Cushman, Green and Izzo48, 49, taking into account the growing evidences about the relationship between some antihypertensive drugs and the development of new-onset diabetes. Compared to diuretics or ‘conventional’ (diuretic and/or beta blocker) therapy, blockers of the RAS, and to a lesser extent calcium antagonists, reduced the risk of new-onset diabetes substantiallyReference Segura, Campo, Ruilope and Rodicio51. Physicians should take notice of the presence of several characteristics related to the development of diabetes (prediabetic state, metabolic syndrome, glucose intolerance, overweight–obesity) when they select long-term medications devoted to cardiovascular protection for hypertensive patientsReference Segura, Campo, Ruilope and Rodicio51.
Combination therapy for multiple cardiovascular risk factors is critical for the successful management of patients presenting obesity-related hypertension. Either ACE inhibitors or Ang II receptor blockers are preferable antihypertensive drugs as initial therapy of these patients, unless contraindications or compelling indications for other drugs. Considering that most patients will need more than one antihypertensive drug to achieve the blood pressure goal, a calcium-channel blocker, α-blocker or thiazide diuretic could be considered for combined therapy52.
Acknowledgements
Conflict of interest declaration:The authors had no conflicts of interest to report.
Authorship contribution:J.S. wrote the manuscript and L.M.R. reviewed it.





