The global prevalence of anaemia is about 34 % affecting approximately 2·36 billion people, including 600 million children(Reference Vos, Allen and Arora1,2) . Nutritional anaemia is a common condition in primary school-aged children that results from a lack of certain vitamins and minerals, including iron(2). The potential consequences of nutritional anaemia in children include loss of appetite, growth retardation, reduced cognitive development and ability to concentrate, which ultimately has a negative impact on schoolwork performance(Reference Zimmermann and Hurrell3).
Food consumption is one of the most important factors in the aetiology of nutritional anaemia and iron deficiency (ID) in school-aged children(Reference Ahmed, Hossain and Sanin4,Reference Mwaniki and Makokha5) . The intake of a great variety of foods across and within the various food groups is needed to meet nutrient requirements and may help to alleviate multiple micronutrient deficiencies and reduce the risk of nutritional anaemia(Reference Labadarios, Steyn and Nel6–Reference Korkalo, Erkkola and Heinonen8). In low- and middle-income countries, including South Africa, the diets of the majority of children lack variety and consist mainly of plant-based foods (predominantly cereals, roots and tubers), with limited consumption of animal-source foods (ASF), fruits and vegetables(Reference Labadarios, Steyn and Nel6,Reference Faber, Laubscher and Laurie9–Reference Gewa, Murphy and Weiss11) . Individuals who consume mostly cereal-based diets are at greater risk of developing ID due to the high content of iron inhibitors, such as phytic acid, in the diets(Reference Heath and Fairweather-Tait12,Reference Hurrell and Egli13) . In contrast, increasing the intake of ASF, and fruits and vegetables rich in vitamin C and carotenoids may increase the absorption of iron from cereal-based diets(Reference García-Casal14). In addition, consumption of ASF contributes to dietary intake of nutrients such as highly bioavailable haem iron, vitamin B12 and vitamin A and plays an important role in reducing the risk of developing anaemia(Reference Balarajan, Ramakrishnan and Özaltin15–Reference Herrador, Sordo and Gadisa17). While some studies have reported an association between dietary diversity and biomarkers of iron status in different age groups(Reference Acham, Tumuhimbise and Kikafunda18–Reference Saaka and Rauf20), other studies showed no association between dietary diversity and anaemia(Reference Ali, Thaver and Khan21,Reference Saaka, Oladele and Larbi22) . Furthermore, in pre-schoolchildren in Ethiopia, a low prevalence of anaemia was observed even though children consumed a diet with low food variety(Reference Gashu, Stoecker and Adish23). Evidence on the relationship of dietary diversity with iron status and anaemia is therefore inconclusive.
Dietary diversity can be evaluated using simple tools such as dietary diversity scores (DDS), that is, the number of food groups consumed over a reference period(Reference Steyn, Nel and Nantel24). Several studies have shown that these scores are good proxies of overall dietary quality(Reference Arimond, Wiesmann and Becquey25–Reference Meng, Wang and Li27). However, the cut-off point for determining low DDS and the reference period on which the DDS is based may differ between studies. A DDS that was calculated based on a 1-day (1-d) reference period did not show an association with anaemia in pregnant women resident in rural areas of Northern Ghana(Reference Saaka, Oladele and Larbi22). Furthermore, a study in South African schoolchildren found no association between dietary diversity and anaemia using a 1-d reference period, but when a longer 7-day (7-d) reference period was used, low dietary diversity was significantly associated with anaemia risk(Reference Gwetu, Chhagan and Martin19). Thus, although dietary diversity may be important in preventing nutritional anaemia and ID, there is limited knowledge on how the diversity of diets as a whole and the consumption of specific food groups are related to anaemia and ID in school-aged children.
The FAO uses a single 24-h recall period to calculate DDS because of less recall error compared with longer recall periods(Reference Kennedy, Ballard and Dop28). Collecting dietary data using 24-h recalls can be repeated on several non-consecutive days, with 3- to 7-d being the most widely used period for the purpose of an appropriate estimation of an individual’s usual intake(29). The aim of this paper is to describe the association of dietary diversity based on two different reference periods (a single 24-h recall v. three 24-h recalls) with anaemia and ID among primary school-aged children in South Africa.
Methods
An analysis was conducted with pooled individual data from the baseline surveys from three independent intervention studies in schoolchildren at different sites in South Africa, which have been previously reported(Reference Baumgartner, Smuts and Malan30–Reference Taljaard, Covic and Van Graan32). The original studies were conducted between April 2009 and June 2012 at primary schools in low socio-economic areas in KwaZulu-Natal (KZN) and North West (NW) provinces. These areas were malaria-free. At the time of data collection, all schools were participating in the National School Nutrition Programme of South Africa, which provided children with a daily meal on school days. Public schools in South Africa are grouped in quintiles according to the poverty level of the community where the school is located. Schools in quintile 1 are the poorest and all school funds come from the government; quintile 5 is the least poor and the bulk of the school funds are generated through school fees. The National School Nutrition Programme targets schools in quintile 1–3. All children received deworming medication immediately before the baseline survey. Parents or caregivers signed informed consent forms and the learners gave verbal assent. Data collection included socio-demographic data, biochemical measurements and 24-h dietary recalls. Dietary data were collected for all children in one of the studies(Reference Taljaard, Covic and Van Graan32) but only on a sub-sample of children in the other two studies(Reference Baumgartner, Smuts and Malan30,Reference Van der Hoeven, Faber and Osei31) . A short summary of the three independent intervention studies is presented in the online supplementary material, Supplemental Table S1. To increase sample size for the exploratory analyses, cases for the pooled analysis were extracted from the three original databases, using the following inclusion criteria: 5- to 12-year-old schoolchildren; socio-demographic information; availability of three biochemical values, namely Hb, plasma ferritin (PF) and C-reactive protein (CRP); and complete dietary intake data. Cases with missing data for any of these variables were excluded (see online Supplementary Material 1).
Biochemical measurements
In the original studies, blood samples collected from the children were used to analyse biochemical indicators. For the pooled data analysis, the Hb, PF and CRP concentrations were used. In the study in KZN, the Hb concentrations were measured in whole blood through the direct cyanmethemoglobin method (Bio Rad Laboratories [PTY] Ltd) by using Drabkin’s solution and a standard miniphotometer(Reference Baumgartner, Smuts and Malan30), while in the two studies in NW, a haematology analyser (Coulter® Ac·T™ 5diff CP; Beckman Coulter) was used(Reference Van der Hoeven, Faber and Osei31,Reference Taljaard, Covic and Van Graan32) . The PF and CRP concentrations were measured using an automated chemiluminescent immunoassay system (CLIA, IMMULITE) in the KZN study(Reference Baumgartner, Smuts and Malan30), while in the two studies in NW, a ferritin ELISA kit (Ramco Laboratories Inc.) was used for PF and an immunoturbidimetric method (Technicon RA-1000 auto analyser) for CRP(Reference Van der Hoeven, Faber and Osei31,Reference Taljaard, Covic and Van Graan32) . While the direct cyanmethemoglobin method and chemiluminescent immunoassay system used in the KZN study are the reference methods for Hb and PF (and CRP) measurements, respectively, the methods used in the two studies in NW are reliable and have comparable accuracy(Reference Karakochuk, Hess and Moorthy33,Reference Garcia-Casal, Peña-Rosas and Urrechaga34) . The PF values of children with a CRP concentration ≥5 mg/l were adjusted for inflammation by multiplying PF values with a correction factor of 0·65(Reference Thurnham, McCabe and Haldar35). Anaemia was defined as Hb < 115 g/ and ID as adjusted PF < 15 μg/l(36,37) .
Dietary diversity assessment
In all three intervention studies, dietary data were collected using the multiple-pass 24-h dietary recall method. In all studies, the 24-h dietary recalls were done approximately 1 week apart on three non-consecutive days, consisting of two weekdays and one weekend day, and thus were done over a period of 2–3 weeks. Children and their parents or caregivers were interviewed by trained fieldworkers and were asked to recall all foods and beverages items consumed the previous day. Similar dietary assessment aids to estimate portion sizes were used in the three studies which included plastic food models, household utensils, food packaging materials and ‘dish-up and measure’ (the participant used dry oats or rice to indicate the portion size for cooked food which was then quantified by the fieldworker using a measuring cup). Amounts reported in household measures or volume were converted to grams using the Medical Research Council Food Quantities Manual(Reference Langenhoven, Conradie and Wolmarans38). The energy and nutrient intakes for the pooled group and each of the study sites have been reported elsewhere(Reference Visser, Van Zyl and Hanekom39).
For the purpose of this study, foods reported during the 24-h recalls were categorised into the following groups: (i) starchy staples (combination of cereals, white roots/tubers), (ii) dark green leafy vegetables, (iii) other vitamin A-rich fruits and vegetables (including vitamin A-rich vegetables, tubers and fruits), (iv) other fruits and vegetables, (v) organ meat, (vi) meat, chicken and fish, (vii) eggs, (viii) legumes, nuts and seeds and (ix) milk and dairy products(Reference Kennedy, Ballard and Dop28). A score of ‘1’ was assigned to each food group if at least one food item within the specific food group was consumed during the reference period. A score of ‘0’ was assigned if the child did not consume any food item from a given food group. The DDS for each child was calculated as the sum of the scores, with a maximum possible score of ‘9’. According to the guidelines, the mean DDS value can be used as cut-off point to indicate low dietary diversity(Reference Kennedy, Ballard and Dop28). Based on the mean DDS for the pooled group, low dietary diversity was therefore defined as DDS ≤ 4. In addition, an animal-source foods score (ASFS) was calculated based on five ASF, namely: (i) organ meat, (ii) red meat (beef mince, beef and pork sausages, processed cold meat), (iii) white meat (chicken meat), (iv) fish and (v) eggs. A score of ‘1’ was assigned to each of these groups if at least one food item within the specific food group was consumed during the reference period. The ASFS for each child was calculated as the sum of the scores given, with a maximum possible score of ‘5’(Reference Kennedy, Ballard and Dop28). Two different DDS were calculated: one for a 1-d recall period (1-d DDS) and one for a 3-day (3-d DDS) recall period. The 1-d DDS was calculated as the number of different food groups consumed during the first 24-h recall period. The 3-d DDS was calculated as the number of different food groups consumed during the three 24-h recall days.
Statistical analyses
The statistical package SPSS version 25 (Inc.) was used for all statistical analyses. Pearson’s χ 2 test was used to compare the proportions of children consuming specific food groups, including the ASF group, and the percentage of children below the cut-off values for the DDS and ASFS. The paired sample t test was used to compare the mean DDS and ASFS between the two reference periods. The t test was used to compare the proportions of children who consumed specific food groups according to anaemia and iron status. Logistic regression analysis was done with the binary outcome (anaemic v. non-anaemic and ID v. non-ID) as the dependent variables and DDS and foods groups as independent variables. The results are presented as OR and 95 % CI only for food groups that showed statistically significant results in at least one of the reference periods. The potential confounders (age, gender and study site) were included in the model. Statistical significance was set at P < 0·05 and a trend as P < 0·1.
Results
Characteristics and anaemia and iron status of children in the pooled group
The mean age of the children (n 578) was 8·7 (sd 1·3) years, the gender distribution was 51·0 % boys and 49·0 % girls, and 13·8 % of the children were anaemic, 27·7 % were ID and 19·8 % presented with elevated CRP levels. Results on the biochemical indicators of anaemia and iron status for each of the study sites have been reported elsewhere(Reference Visser, Van Zyl and Hanekom39). In short, differences across study sites were observed. Compared with the other two study sites, the KZN study site had a significantly higher proportion of anaemic children (26·5 v. 14·0 and 10·4 %, respectively; P < 0·001) and ID children (42·2 v. 18·8 and 26·3 %, respectively; P < 0·001). The proportion of children with raised CRP levels was higher in NW2 (22·8 %) than in the other two study sites (19·6 and 8·0 %, respectively; P < 0·01).
For the pooled group, the mean Hb concentrations for anaemic (n 80) v. non-anaemic (n 498) children were 111 (95 % CI 108, 113) and 128 (95 % CI 127, 129) g/l, respectively, and adjusted PF concentrations for ID (n 160) v. non-ID (n 418) children were 9·8 (95 % CI 7·8, 10·3) and 31·2 (95 % CI 27·9, 34·8) µg/l, respectively.
The proportions of children who consumed specific food groups, the mean DDS and ASFS as calculated for two reference periods
Table 1 presents the results of the proportions of children who consumed specific food groups, and the mean DDS and ASFS as calculated for two reference periods. All children consumed foods from the ‘starchy staples’ group for both recall periods, but a significantly higher proportion of children consumed foods from certain groups, in particular, ‘vegetables and fruits’ (other than vitamin A-rich), ‘organ meat’, ‘eggs’ and ASF groups, according to the 3-d recall period compared with the 1-d recall period (all P < 0·05). The mean DDS and mean ASFS were significantly higher for the 3-d recall period compared with the 1-d recall period, and the proportion of children with DDS ≤ 4 was significantly lower for the 3-d period compared with the 1-d period.
Table 1 Proportions (%) of 5- to 12-year-old schoolchildren who consumed foods from specific food groups, according to the 1-d and 3-d 24-h recall periods*

1-d, 1-day; 3-d, 3-day; DDS, dietary diversity score; ASF, animal-source foods; ASFS, animal-source foods score.
* Continuous data reported as mean and sd, categorical data presented as percentages. Paired t test was used to compare the means; χ 2 test was used to compare the proportions. Statistical significance was set at P < 0·05 and a trend as P < 0·1.
† Three non-consecutive days.
The proportions of children who consumed specific food groups over the two reference periods according to anaemia and iron status
Children were stratified according to their anaemia and iron status (Table 2), and the proportion of children who consumed different food groups and the means of the DDS and ASFS for the two reference periods were compared between these sub-groups. A significantly lower proportion of anaemic (v. non-anaemic) children consumed foods from the ‘vegetables and fruits (other than vitamin A-rich)’, ‘organ meat’ and the ‘ASF’ groups for both reference periods. Furthermore, for the 3-d reference period, it was found that a significantly lower proportion of anaemic (v. non-anaemic) children consumed foods from the ‘meat, fish, and seafood’ and ‘eggs’ groups, and a significantly lower proportion of ID (v. non-ID) children consumed foods from the ‘legumes, nuts and seeds’ group. Food groups consumed by the lowest proportion of children were ‘vitamin A-rich vegetables and fruits’ and ‘organ meat’. The proportion of children with DDS ≤ 4 was significantly higher in the anaemic sub-group (v. non-anaemic) for both recall periods. The means of the DDS were significantly lower in the anaemic and ID sub-groups (v non-anaemic and non- ID, respectively), according to both reference periods, but the mean ASFS was significantly lower for the 3-d reference period only.
Table 2 Proportions (%) of 5- to 12-year-old schoolchildren who consumed specific food groups over the two reference periods according to anaemia and iron status*
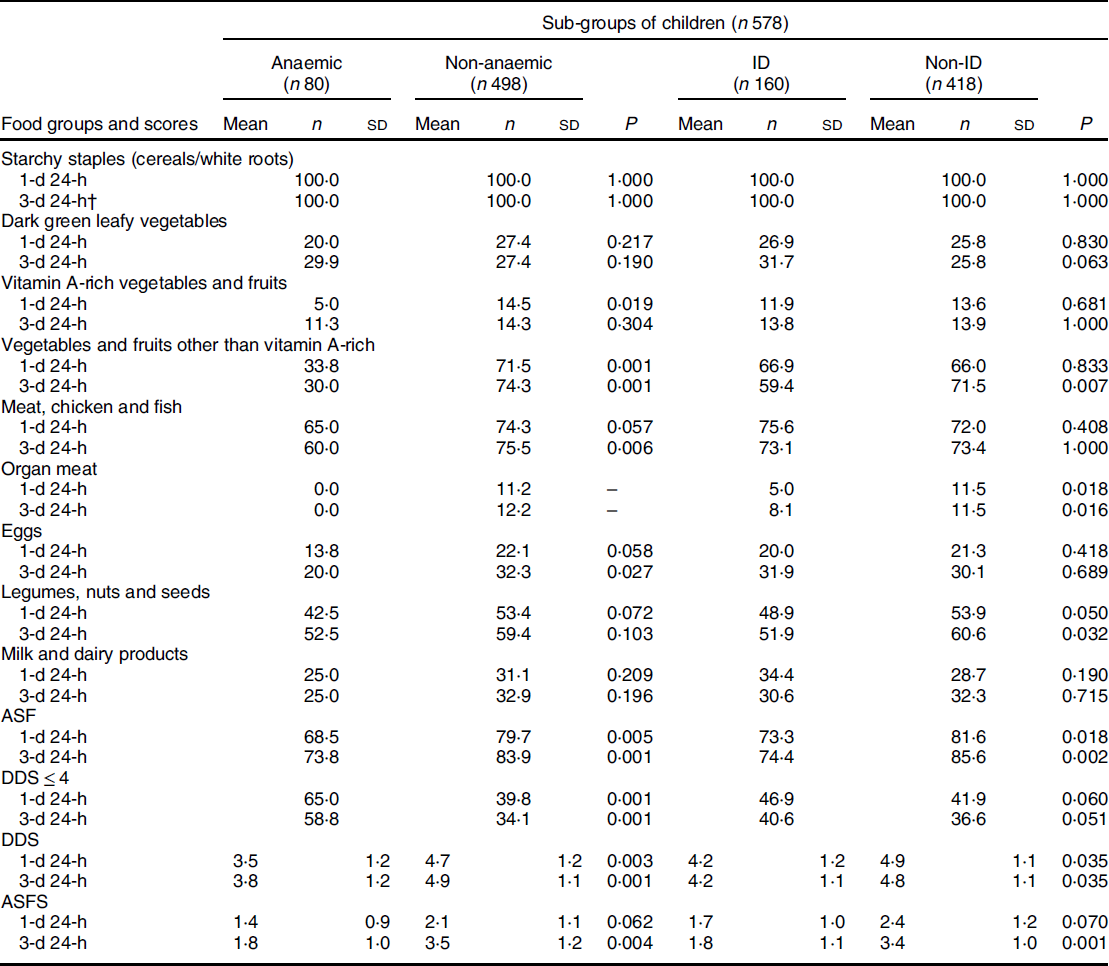
1-d, 1-day; 3-d, 3-day; ID, iron deficiency; DDS, dietary diversity score; ASF, animal-source foods; ASFS, animal-source foods score.
* Continuous data reported as mean and sd, categorical data presented as percentages. The t test was used to compare the mean and sd values; χ 2 test was used to compare the proportions. Statistical significance was set at P < 0·05 and a trend as P < 0·1.
† Three non-consecutive days.
OR and 95 % CI for having anaemia and ID for children according to the food groups consumed and DDS cut-offs
Results from binary logistic regression models with anaemia and ID as dependent variables and food groups and DDS categories calculated from both reference periods as independent variables are shown in Table 3. Consumption of ‘vegetables and fruits other than vitamin A-rich’ and ‘ASF’ was associated with lower odds of being anaemic, and consumption of ‘organ meats’ was associated with lower odds of being ID for both reference periods (all P < 0·05). Consumption of ‘meat, chicken and fish’ was associated with lower odds of being anaemic, and ‘vegetables and fruits other than vitamin A-rich’, ‘legumes, nuts and seeds’ and ‘ASF’ with lower odds of being ID for the 3-d reference period only (all P < 0·05). A DDS ≤ 4 was associated with higher odds of being anaemic for both reference periods (1-d P = 0·001 and 3-d P = 0·006) and higher odds of being ID for the 3-d recall period only (P < 0·001).
Table 3 OR and 95 % CI for having anaemia and iron deficiency (ID) for 5- to 12-year-old children according to the food groups consumed and dietary diversity scores (DDS) cut-off values*
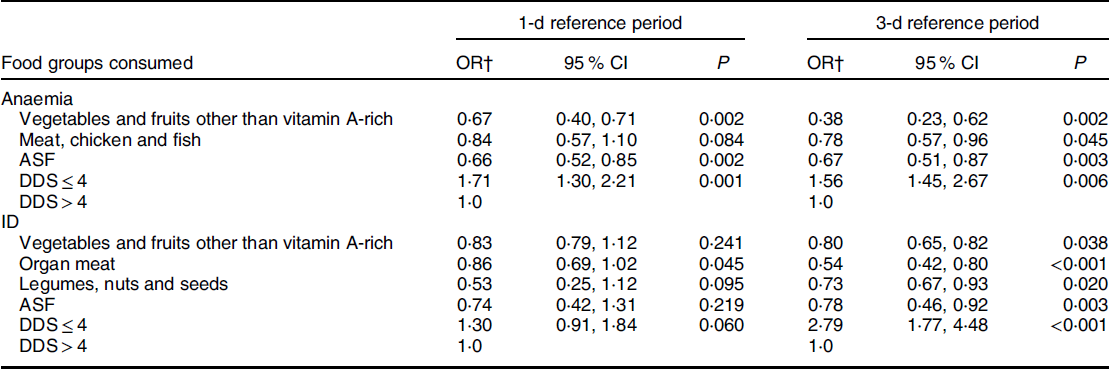
1-d, 1-day; 3-d, 3-day; ASF, animal-source foods.
* Statistical significance was set at P < 0·05 and a trend as P < 0·1. Food groups not indicated in the table showed no statistically significant results for either reference period.
† Adjusted for age, gender and study site.
Discussion
Associations of dietary diversity and consumption of different food groups with anaemia and ID were investigated in a pooled sample of primary school-aged children in South Africa. Results showed that children who consumed a diet with low variety (DDS ≤ 4) were more likely to be anaemic and ID. These results are similar to other studies that showed a positive association between dietary diversity and haematological indices(Reference Acham, Tumuhimbise and Kikafunda18,Reference Saaka and Rauf20) , and that a low dietary diversity was associated with anaemia(Reference Gwetu, Chhagan and Martin19). Furthermore, children who consumed foods from the ‘vegetables and fruits other than vitamin A-rich’ group and various ASF during the reference period were less likely to be anaemic and ID compared with non-consumers.
Comparisons between anaemic v. non-anaemic and ID v. non-ID children showed that significantly fewer anaemic and ID children consumed ‘vegetables and fruits other than vitamin A-rich’; however, for ID children, this was only the case for the 3-d reference period. The low consumption of ‘vegetables and fruits other than vitamin A-rich’ by anaemic children corresponds to our findings on the nutrient intake of children(Reference Visser, Van Zyl and Hanekom39), which showed that vitamin C intake was lower in anaemic v. non-anaemic and ID v. non-ID children. Available evidence indicates that fruit and vegetable intake is associated with a reduced risk of many nutrition-related diseases in South Africa(Reference Naudé40). In low- and middle-income countries, low consumption of fruits and vegetables was shown to be a predictor of poor iron status, which may lead to nutritional anaemia(Reference Augusto, Cobayashi and Cardoso41,Reference Ghose and Yaya42) . A study of Brazilian children aged 4–10 years showed that children who did not consume fruits and vegetables had a higher prevalence of anaemia compared with consumers(Reference Augusto, Cobayashi and Cardoso41). Similarly, results of a recent study conducted in adult Ghanaian women showed that non-consumers of fruits and vegetables had a higher chance of being anaemic(Reference Ghose and Yaya42). The inverse relationship between the consumption of fruits and vegetables, other than vitamin A-rich, to anaemia and ID can, at least partly, be explained by the presence of nutrients that facilitate absorption of iron from the diet. The high vitamin C content in most fruits and vegetables may play an important role in enhancing the bioavailability of iron from plant-based foods, thus preventing nutritional anaemia(Reference Péneau, Dauchet and Vergnaud43).
The South African population generally has a low intake of fruits and vegetables(Reference Labadarios, Steyn and Nel6). Low consumption has also been reported for schoolchildren(Reference Faber, Laubscher and Laurie9). To thrive, a more frequent and regular intake of a variety of fruits and vegetables should be promoted. The South African Food-Based Dietary Guidelines recommend eating ‘plenty of fruit and vegetables every day’ as well as various types and colours(Reference Vorster, Badham and Venter44) in order to increase nutrient intake. Although consumption of vitamin A-rich fruits and vegetables was not associated with anaemia and ID in our study, these fruits and vegetables are rich in carotenoids, which are precursors of vitamin A, and consumption may increase the absorption of iron from cereal-based diets(Reference García-Casal14). The number of children who reported intake of vitamin A-rich fruits and vegetables during the recall period was low (<14 %). Strategies to increase intake of fruits and vegetables should thus be explored, as is recommended by the Centres for Disease Control and Prevention(45). For example, home gardening in rural low socio-economic areas could be promoted to improve the intake of fruits and vegetables, particularly those rich in vitamin A(Reference Faber, Venter and Benadé46). Also, the importance of fruit and vegetable consumption could be included in the school curriculum.
Consumption of various ASF also showed significant association with anaemia in primary schoolchildren. More than 70 % of the children in our study ate foods from the ‘ASF’ group, regardless of the reference period. However, significantly fewer anaemic v. non-anaemic and ID v. non-ID children consumed foods from the ‘ASF’ group. A number of studies in different age groups have shown a positive association between consumption of various ASF and haematological indices(Reference Wolmarans, Dhansay and Mansvelt47–Reference Tahir, Bukhari and Gillani51), including a study in school-aged children in Kenya(Reference Grillenberger, Neumann and Bwibo48). ASF, particularly organ meat (e.g., liver, kidney), followed by red and white meat, and fish, are rich in highly bioavailable nutrients such as iron, vitamins A and B12, which are important and effective for iron mobilisation and Hb synthesis and play an important role in managing nutritional anaemia(Reference Fishman, Christian and West52–Reference Pettit, Rowley and Brown54). Moreover, foods from ASF groups are effective enhancers of non-haem iron absorption when consumed in the same meal as plant-based foods and may increase iron absorption by 2- to 3-fold(Reference Hurrell and Egli13). The South African Food-Based Dietary Guidelines recommend that foods from the ASF group can be eaten daily, but to maintain a healthy balanced diet, consumption of a variety of foods from other food groups is recommended(Reference Vorster, Badham and Venter44).
Significantly fewer ID than non-ID children in our study consumed legumes during the 3-d period, and the logistic regression analysis showed an inverse association between consumption from the ‘legumes’ group and ID for the 3-d reference period. Adding foods from the legumes group (i.e., dry beans, split peas, lentils and soya) to the diet will increase the nutrient content of meals, and the South African Food-Based Dietary Guidelines recommend that such foods are eaten regularly(Reference Venter, Ochse and Swart55). A study showed that adding legumes can slightly improve the iron content of plant-based diets, although the bioavailability of non-haem iron from these foods is low(Reference Saunders, Craig and Baines56). Legumes are generally rich in iron absorption inhibitors (i.e., phytate), which may affect the absorption of iron derived from both animal and plant sources(Reference Hurrell and Egli13). Other studies, however, could not find an association between the intake of legumes and ID(Reference Leonard, Chalmers and Collins50,Reference Asakura, Sasaki and Murakami57) . In meals containing legumes, non-haem iron bioavailability may be enhanced by adding sufficient amounts of foods rich in vitamin C, which has been shown to reverse the inhibitory effects of phytate(Reference Hallberg, Brune and Rossander58).
Our results indicate that estimated consumption of specific food groups may vary depending on the reference period. In dietary assessment studies, associations between less frequently consumed food groups and anaemia and ID as outcome might be missed if only a 1-d reference period is used. For instance, no association was found between intake of foods from the ‘meat, chicken and fish group’ and anaemia, and foods from the ‘vegetables and fruits other than vitamin A-rich’, ‘ASF’ and ‘legumes’ groups and ID in the 1-d reference period. However, the associations between intake of these foods and anaemia and ID, respectively, were found when the 3-d reference period was used. Nevertheless, for frequently consumed foods, there was an association regardless of the reference period. When using the DDS to describe dietary variety, there was a statistically significant difference in the mean DDS between the two reference periods, although the difference in the score values was relatively small.
This study contributes to the knowledge about dietary diversity and aetiology of nutritional anaemia in South African primary school-aged children. Considering that South Africa has a National Food Fortification Programme in place, which also aims to address nutritional anaemia and ID, and that all children in this study consumed starchy staples, of which maize meal and bread are fortified, our finding strengthens the point that a diverse diet should be promoted despite or in addition to fortification efforts.
For this study, baseline data from three independent intervention studies were pooled. The study sample is therefore not representative of the study population, and the prevalence figures for anaemia and ID cannot be generalised. It should however be noted that the focus of this paper is on associations of dietary diversity with anaemia and iron status, rather than anaemia and iron status per s e. Although a limitation of the pooled data is the different tests that were used for the biochemical indicators, there is evidence that these different methods are comparable(Reference Karakochuk, Hess and Moorthy33,Reference Garcia-Casal, Peña-Rosas and Urrechaga34) . The data are cross-sectional, and the results therefore reflect associations, but causality cannot be established.
Conclusion
Low dietary diversity was associated with higher odds of being anaemic and ID in South African school-aged children. Our results emphasise the importance of obtaining consumption data for multiple days when studying associations of specific foods groups with anaemia and ID, particularly for foods that are not frequently consumed by the study population. The promotion of dietary diversification, with a focus on greater consumption of vegetables, fruits and ASF, including organ meats, may have a positive effect on improving anaemia and iron status in children of primary school age.
Acknowledgements
Acknowledgements: The authors are grateful to all the research team members and all the participants of the single studies we obtained the data from. Financial support: This article was part of the first author’s doctoral thesis, which was supported by study grants from the South African National Research Foundation (grant no. UID 101720), Nestlé Nutrition Institute Africa (grant 22/06/2016) and Association of African Universities (grant PC/6). The funders had no role in the design, analysis or writing of this article. Conflict of interest: There are no conflicts of interest. Authorship: T.V.Z. and M.F. conceptualised this study and contributed to the writing of the paper; M.V. analysed the data and wrote the draft article; S.M.H., J.B., M.H., C.T.-K. and C.M.S. were responsible for data collection in the original studies and contributed to the writing of the paper. All authors read and approved the final manuscript. Ethics of human subject participation: This study did not involve human subjects and was conducted with pooled existing data derived from three independent intervention studies. All original intervention studies were conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving research study participants were approved by the appropriate ethics committees before data collection. Additional approval from the Health Research Ethics Committee was obtained to perform the present study (NWU-00027-16-A1).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020000543






