Vitamin A deficiency (VAD) is a serious public health problem among pre-school children in Sub-Saharan Africa, contributing to night blindness, xerophthalmia, permanent blindness, increased severity of morbidity and increased risk of mortality(1). A 2003 national survey in Zambia indicated that VAD (plasma retinol ≤0·70 μmol/l) among children was 54 %, a modest decrease from 66 % in the 1997 national survey(2).
VAD in Zambia persists despite the introduction of vitamin A supplementation and fortification programmes. In the 2003 national survey, no significant response of serum retinol was observed when measured before and 1 month after a round of vitamin A capsule distribution(2), suggesting there were other important determinants of vitamin A status. Further, only 59 % of households reported buying vitamin A-fortified sugar on a regular basis, and only 18 % of sugar samples contained the minimum of 10 mg vitamin A/kg(2). Dietary vitamin A intakes were not quantified.
Childhood infections are often associated with vitamin A status and may contribute to increased vitamin A requirements. Acute infections are associated with increased urinary excretion of vitamin A(Reference Stephensen, Alvarez and Kohatsu3, Reference Alvarez, Salazar-Lindo and Kohatsu4) and recent fever is associated with decreased absorption and retention of a vitamin A dose(Reference Aklamati, Mulenga and Dueker5). In addition, current infection reduces plasma retinol concentration by reducing hepatic synthesis and circulation of retinol-binding protein(Reference Gieng, Raila and Rosales6). As a result, the prevalence of VAD is overestimated in populations where infections are prevalent(Reference Thurnham, McCabe and Northrop-Clewes7).
The modified retinol dose-response (MRDR) test may be a more sensitive indicator of subclinical vitamin A status than plasma retinol(Reference Tanumihardjo, Muherdiyantiningsih and Permaesih8, Reference Tanumihardjo9). However, the relationship between plasma retinol and the dehydroretinol:retinol molar ratio following the MRDR test is not linear(Reference Verhoef and West10), such that when the prevalence of low plasma retinol is high, the MRDR test would be expected to predict a lower prevalence of deficiency than retinol when applying accepted cut-offs for defining VAD. The relative dose-response test may also underestimate VAD during the acute-phase response to infection(Reference Stephensen, Franchi and Hernandez11). If so, the difference in estimated prevalence of VAD by these two indicators is likely to be magnified when the prevalence of infection is also high.
Inadequate dietary vitamin A is accepted as the primary cause of VAD. However, in Zambia, vitamin A intakes from the usual diet or from fortified sugar have not been quantified among rural populations. This information is important because high-dose vitamin A capsules may not adequately maintain vitamin A stores in between twice-annual doses if dietary vitamin A is inadequate(Reference Allen and Haskell12) and VAD may thus persist. There is an absence of information on the adequacy of vitamin A intakes and its relationship to vitamin A status among Zambian children.
We conducted a cross-sectional survey in a maize-dependent, rural population of children in Zambia, with the objectives of (i) determining the contribution of dietary vitamin A intake and infection to vitamin A status and (ii) comparing plasma retinol and the MRDR test as indicators of vitamin A status. We hypothesized that the MRDR test would predict a lower prevalence of VAD even after adjusting serum retinol for the presence of infection, and that either of these indicators would be associated with dietary vitamin A intakes.
Experimental methods
The study was a cluster-designed, cross-sectional survey of vitamin A intakes and status of children in a rural, maize-consuming population in Zambia. The sample was designed to be representative of the combined population of two districts: Nyimba District in Eastern Province and Mkushi District in Central Province. These sites were selected based on characteristics that made them likely beneficiaries of an intervention to introduce provitamin A-biofortified maize. The main survey was conducted in the harvest/early post-harvest season (May–June 2009), while dietary assessment was repeated in the late post-harvest season (October–December 2009). The institutional review boards of the Tropical Diseases Research Centre (TDRC; Ndola, Zambia) and the International Food Policy Research Institute (Washington, DC, USA) approved the survey protocol.
Sampling methods and participants
Clusters were represented by standard enumeration areas (i.e. convenient administrative units defined from the 2000 population census) and were randomly selected from a list of all standard enumeration areas in the selected districts. Households with a resident child 24–59 months of age and an adult female caregiver were enumerated, and a subset was randomly selected to participate. If multiple eligible children resided in the household, one was randomly selected. Severely malnourished (i.e. weight-for-age or weight-for-height Z-score <−3) or severely anaemic (Hb < 7·0 g/dl) children were excluded.
Sample size
The primary outcomes were prevalence of low plasma retinol and prevalence of inadequate vitamin A intake. For serum retinol, a sample size of n 774 was determined using the following parameters: expected prevalence of low plasma retinol = 50 %; precision = 6 %; design effect = 2·9(2); β = 0·90 and α = 0·05. For vitamin A intakes, a sample size of n 387 was determined using the following parameters: expected prevalence of inadequate intake = 50 %; precision = 8 %; design effect = 2·5; β = 0·90 and α = 0·05. For comparison of the MRDR test and plasma retinol, n 275 were required to detect a difference of 13 percentage points with β = 0·95 and α = 0·05. In total, twenty-six clusters (thirteen per district) with thirty households per cluster were randomly selected to derive 780 children. A subset was randomly selected from each cluster for the dietary survey (n 390); from the latter, an additional subset was selected for the MRDR test (n 275).
Field methods
District health staff carried out sensitization and social mobilization of the communities. A supervisor obtained informed consent from the participating female caregiver. Field staff received specialized training. Data were checked on a daily basis by supervisors, problems were corrected immediately and field staff were retrained as necessary. Anthropometric and morbidity data and blood samples were collected in a clinic near each cluster. The 24 h dietary recall was conducted in the household.
Anthropometry
Body weight was measured in duplicate using a digital scale accurate to ±100 g and standing height was measured in duplicate using a wooden measuring board accurate to ±0·1 cm (Shorr Productions, Olney, MD, USA). Age was determined from the health card or caregiver's memory, aided by recall of local events.
Nutrition and health
Caregivers were probed about the child's attendance at recent Child Health Days, receipt of vitamin A supplements and antihelmintics, and symptoms of illness during the previous 2 weeks. When available, information was extracted from health cards.
Blood sampling
Venous blood samples were obtained by an experienced nurse. Samples were collected into evacuated tubes containing EDTA; the tubes were placed in a cooler box under refrigerated temperatures and then transported the same day to a local facility where they were centrifuged at 3000 g for 10 min. Separated plasma was aliquoted into cryotubes and deposited in a liquid nitrogen tank until shipment to TDRC for storage at −80°C. Biochemical analyses were performed at TDRC.
Modified retinol dose-response test
Children were given an oral dose of 5·3 μmol 3,4-didehydroretinol dissolved in corn oil(Reference Tanumihardjo, Permaesih and Dahro13), followed by 1 ml of corn oil and one tablespoon of groundnut paste on bread. Children remained at the centre for 4 h, after which a blood sample was obtained.
24 h Dietary recall
Dietary intake was assessed using an interactive, multiple-pass 24 h recall method, incorporating several tools to aid women's recall of the foods and beverages consumed(Reference Gibson and Ferguson14). Women attended an informational session on the interview process and the importance of providing accurate information without altering their dietary habits. Picture charts were used to help track foods consumed during the period of recall. Real foods, scaled photographs, measuring spoons and cylinders, and calibrated modelling clay were used to estimate food portion sizes. Prior to the survey, standard recipe data for common dishes were collected. If recipes consumed did not match the ingredients of a standard recipe, complete recipe information was obtained.
A food composition table was compiled using two main sources(15, 16). Vitamin A retinol activity equivalents (RAE) were used, where the retinol equivalency is assumed to be 12:1 for β-carotene and 24:1 for α-carotene and β-cryptoxanthin(17). However, it has been suggested that the retinol equivalency of provitamin A carotenoids from green and yellow vegetables is as high as 26–28:1, while from fruits it is 12:1(Reference De Pee, West and Permaesih18–Reference Tang, Gu and Hu20). We thus calculated ‘alternative vitamin A RAE’ intake data assuming that provitamin A from green and yellow vegetables had a retinol equivalency of 26:1 and that from fruits, orange vegetables and orange and yellow roots had a retinol equivalency of 12:1.
Vitamin A content of sugar
In households reporting to have sugar available during the interview, samples were obtained. Samples were labelled, wrapped in black plastic and stored in a cool box before shipment to the Food and Drug Control Laboratory, Lusaka, for determination of vitamin A content.
Laboratory methods
Plasma retinol and dehydroretinol
Plasma retinol concentration was determined by HPLC (Pye Unicam Philips system with PU4015 pump, PU4025 UV detector, PU4810 integrator and Waters 717 plus autosampler)(Reference Thurnham, Smith and Flora21). Dehydroretinol was determined using the same system, as previously described(Reference Valentine and Tanumihardjo22). External standards were used to calibrate concentrations of retinol and dehydroretinol. A standard reference material for serum retinol (SRM #968d; National Institute of Standards and Technology, Gaithersburg, MD, USA) was used to calibrate internal retinol standards of pooled serum. Three pooled serum samples were analysed in each run; if the intra-run CV was >5 %, the run was repeated. For the MRDR test, the plasma dehydroretinol:retinol molar ratio was calculated.
α-1-Acid glycoprotein and C-reactive protein
Plasma concentrations of α-1-acid glycoprotein (AGP) and C-reactive protein (CRP) were determined by radial immunodiffusion kits following manufacturer's instructions (Kent Laboratories Inc., Bellingham, WA, USA) and measuring ring diameter to ±0·1 mm using a radial immunodiffusion plate reader (Calibration Viewer, Nidek 2743; Transdyne General Corporation, Austin, TX, USA).
Vitamin A content of sugar
Vitamin A content of sugar was determined by a standard UV-light spectrophotometric method modified from Arroyave and Funes(Reference Arroyave and Funes23), with absorbance at 326 nm.
Interpretation of biochemical results
VAD was defined as plasma retinol <0·70 μmol/l(Reference De Pee and Dary24) or dehydroretinol:retinol ratio >0·06(Reference Tanumihardjo, Permaesih and Dahro13). Plasma CRP ≥ 5·0 mg/l and AGP ≥ 1·0 g/l were taken to indicate the presence of subclinical infection(Reference Thurnham, McCabe and Northrop-Clewes7). Children were classified by stage of infection based on the latter two indicators (no infection, incubation, early convalescence or late convalescence) and plasma retinol was adjusted for infection by applying previously derived correction factors(Reference Thurnham, McCabe and Northrop-Clewes7).
Data processing
Data were captured with CSPro (Serpro Inc., Santiago, Chile). Anthropometric Z-scores were calculated using the ENA software (SMART, version October 2007; http://www.smartmethodology.org/) with the 2006 WHO reference data. Dietary data were captured with CSDietary (Serpro Inc.). All entered dietary data were verified by a supervisor.
Data analysis
We used the complex samples module of the SPSS Statistics 18·0 statistical software package (IBM SPSS, Armonk, NY, USA) accounting for the cluster design and stratification. Descriptive data are presented as means or prevalence (95 % CI). The prevalence of VAD estimated from plasma retinol and the MRDR test were compared by the McNemar test. By combining dietary intake data from the two survey rounds, the usual vitamin A intakes and prevalence of inadequate vitamin A intakes were estimated using the SAS statistical software package version 9·2 (SAS Institute, Cary, NC, USA) after adjusting for within-person variability(Reference Tooze, Kipnis and Buckman25) and assessing against the Estimated Average Requirement(26).
Pearson correlations and regression analyses were used to ascertain associations of vitamin A intake and infection with vitamin A status. General linear models used plasma retinol as the dependent variable with vitamin A intakes from the harvest/early post-harvest season as the independent variable. Covariates were selected a priori based on previous knowledge of their association with vitamin A status; age, sex, weight-for-height Z-score <−2, elevated CRP and AGP, recent fever (reported fever in the last 2 weeks) and receipt of a vitamin A supplement and/or antihelmintic in the last 6 months were considered.
Results
The participation rate was 85 % (664/780) overall, where 106 refused to participate and ten met the exclusion criteria. Results are presented for children with complete data for the relevant survey components, representing 78 % of the intended sample for blood samples and 84 % of the sub-sample for the MRDR test. For the sub-sample selected for dietary assessment, participation rates were 89 % and 78 % in the harvest/early post-harvest and late post-harvest seasons, respectively.
Households in the survey area were characterized by high rates of land ownership, dependence on agriculture for income, low levels of attained education among heads of households and traditional housing without electricity or piped-in water (Table 1). Children were characterized by a high prevalence of linear growth stunting, moderate prevalence of underweight and low rates of wasting (Table 1). Reported rates of symptoms of illness were high, with nearly two-thirds reporting cough and over half reporting fever in the last 2 weeks. Vitamin A supplement and antihelmintic use in the previous 6 months was reported to be very high.
Table 1 Characteristics of households and pre-school children aged 2–5 years in Central and Eastern Provinces, Zambia, 2009

Vitamin A and infection status
The mean plasma retinol concentration was low, and more than half of all children had plasma retinol concentration <0·70 μmol/l before considering adjustments for infection (Table 2). Overall, 61 % of children were in some stage of infection, the majority of whom had elevated AGP. Malaria parasites were detected in 16 % of children.
Table 2 Vitamin A and infection status among pre-school children aged 2–5 years in Central and Eastern Provinces, Zambia, 2009

CRP, C-reactive protein; AGP, α-1-acid glycoprotein; MRDR, modified relative-dose response.
*No infection, AGP < 1·0 g/l and CRP < 5·0 mg/l; incubation, AGP < 1·0 g/l and CRP ≥ 5·0 mg/l; early convalescence, AGP ≥ 1·0 g/l and CRP ≥ 5·0 mg/l; late convalescence, AGP ≥ 1·0 g/l and CRP < 5·0 mg/l.
After applying correction factors for infection, mean plasma retinol was increased by 8·7 % and the prevalence of plasma retinol <0·70 μmol/l was decreased by 8·8 percentage points (Table 2). Based on the MRDR test, the prevalence of VAD was approximately half that predicted by the infection-adjusted plasma retinol, and the difference was significant (P < 0·001).
Dietary intakes
Macronutrient intakes were balanced, with protein providing 12 % and fat providing 22 % of energy intakes. Protein and fat intakes, but not energy intake, were significantly greater in the harvest/early post-harvest season (P < 0·001; Table 3), likely attributed to greater availability of groundnuts. Vitamin A intakes were also significantly greater during the harvest/early post-harvest season (P < 0·001). This was attributed to plant sources of β-carotene and not to a greater intake of retinol from animal-source foods or fortified foods. Alternative vitamin A RAE intakes were somewhat lower than the standard vitamin A RAE intakes.
Table 3 Daily dietary intakes of energy and macronutrients, and sources of vitamin A, by survey round among pre-school children aged 2–5 years, Central and Eastern Provinces, Zambia, 2009
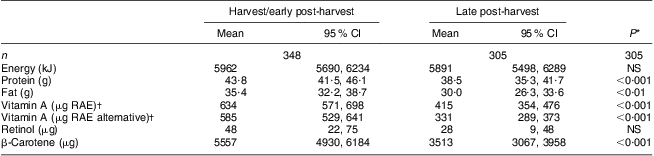
RAE, retinol activity equivalents.
*Median intakes between survey rounds were compared by the Mann–Whitney U test.
†Vitamin A RAE were calculated based on the Food and Nutrition Board, Institute of Medicine recommendations(17); alternative vitamin A RAE were also calculated whereby the provitamin A carotenoids in dark green leafy vegetables and yellow/green vegetables were assumed to have a retinol equivalency of 26:1.
After combining dietary intake data from both seasons, the estimated prevalence of inadequate vitamin A RAE intake for children aged 2–3 years (n 238) was 0·2 % and for children aged 4–5 years (n 150) was 0·8 %.
The dietary sources of vitamin A varied by season (Fig. 1). In the harvest/early post-harvest season (Fig. 1a), vegetables (42 %) and roots and tubers (37 %) provided the majority of vitamin A followed by sugar and sweets (12 %) and meats (5 %). The major food items in these groups were pumpkin and rape leaves, yellow sweet potato, vitamin A-fortified sugar and liver, respectively. The main differences in the late post-harvest season (Fig. 1b) were the much lower contribution from roots and tubers (6 %) and that fruits (primarily mangos) became a major source (34 %) of vitamin A.

Fig. 1 Contribution of food groups to total vitamin A RAE (μg/d) intake in the harvest/early post-harvest (a) and late post-harvest (b) seasons among pre-school children aged 2–5 years, Central and Eastern Provinces, Zambia, 2009 (RAE, retinol activity equivalents)
Sugar vitamin A content
Sugar samples were obtained from 14·2 % of households. The median vitamin A content was 8·8 mg/kg (range: 0·5–54·9 mg/kg) and 59 % of samples contained at least the minimum recommended amount of 10 mg/kg. Only 5 % (g/g) of sugar consumed was reported to be a non-fortified, foreign brand. Applying the median vitamin A content of 8·8 mg/kg, fortified sugar was estimated to contribute 19 % of the RDA.
Associations between vitamin A status, vitamin A intakes and infection
Plasma retinol was inversely correlated with plasma AGP (r = −0·253, P < 0·001) and CRP (r = −0·164, P < 0·001), and positively correlated with alternative vitamin A RAE intake (r = 0·137; P < 0·05); plasma retinol was not significantly correlated with child's age (r = 0·010, P ≥ 0·05), weight-for-height Z-score (r = −0·019, P ≥ 0·05) or standard vitamin A RAE intake (r = 0·101, P ≥ 0·05). No significant correlations were found between plasma dehydroretinol:retinol and AGP, CRP and either the standard or alternative vitamin A RAE intake (P ≥ 0·05). Plasma retinol did not differ significantly by sex (P = 0·658) or reported presence of recent fever (P = 0·738), but varied significantly by quartile of alternative vitamin A RAE intake (P = 0·032).
In the regression analyses, plasma AGP ≥ 1·0 g/l (P < 0·001), weight-for-height Z-score <−2·0 and alternative vitamin A RAE intake (P < 0·05) were significantly associated with plasma retinol, whereas no other covariates were (Table 4). Vitamin A supplement and antihelmintic use and weight-for-height Z-score <−2·0 were excluded from the model as very few children were reported to not have received this prophylaxis and few children were wasted (Table 1). When standard vitamin A RAE intake was substituted for the alternative vitamin A RAE intake in this model, it was not significant. When non-significant covariates were removed from the model, both plasma AGP and alternative vitamin A intake remained significant (data not shown). In a second regression model using alternative vitamin A RAE from the four major food group sources of vitamin A (Table 4), the only food group significantly associated with plasma retinol was roots and tubers (P = 0·035).
Table 4 Regression modelsFootnote * with determinants of plasma retinol concentration among pre-school children aged 2–5 years (n 316), Central and Eastern Provinces, Zambia, 2009

RAE, retinol activity equivalents; CRP, C-reactive protein; AGP, α-1-acid glycoprotein.
* Model 1 uses vitamin A intake (μg revised RAE/d) as the independent variable and Model 2 uses vitamin A intake by major food group sources of vitamin A (μg revised RAE/d) as independent variables.
Discussion
The prevalence of VAD among these rural, Zambian pre-school children was persistently high, exceeding 50 %, despite reported high coverage of vitamin A supplementation, access to vitamin A-fortified sugar and adequate total vitamin A intakes across seasons. However, this population was also characterized by high rates of infection. Both infection and alternative vitamin A RAE dietary intake were associated with vitamin A status, but not standard vitamin A RAE intake.
The high prevalence of elevated acute-phase proteins (61 %) indicative of acute and chronic infection was consistent with the high rates of reported symptoms of illness. It is well established that plasma retinol concentration is depressed in the presence of infection, the magnitude of which is influenced by the stage of infection, and that the prevalence of VAD is overestimated(Reference Thurnham, McCabe and Northrop-Clewes7). However, after applying corrections factors, the prevalence of low plasma retinol was still nearly 50 %, and was 43 % in the non-infected children. Therefore, the majority of VAD is unlikely attributed to the acute-phase response.
The MRDR test is considered a more sensitive indicator of vitamin A status, able to detect subclinical VAD at an earlier stage of depletion than retinol(Reference Tanumihardjo27). When directly compared, MRDR and plasma retinol concentrations have often predicted very different prevalences of VAD among infants and children, with the MRDR sometimes predicting a higher prevalence as one might expect(Reference Idindili, Masanja and Urassa28, Reference Bahl, Bhandari and Wahed29) and sometimes predicting a lower prevalence(Reference Van Jaarsveld, Faber and Tanumihardjo30–Reference Schemann, Banou and Guindo32), such as observed in the present study. This inconsistency may be partly attributed to the lack of adjustment of retinol for the acute-phase response and partly to the fact that the relationship between retinol and dehydroretinol:retinol is not linear(Reference Verhoef and West10). In the present study, the MRDR test predicted a prevalence of VAD half that predicted by infection-adjusted plasma retinol concentration, a difference not accounted for by the acute-phase response alone. However, this difference is consistent with a previous study indicating that the MRDR underestimates VAD in the presence of infection(Reference Stephensen, Franchi and Hernandez11). The effect of infection and its suppressive effect on retinol-binding protein synthesis in the liver, on which the MRDR test depends, have not been directly studied in relation to the validity of the MRDR.
The cross-sectional design of the present survey is limited in that it cannot determine which indicator more accurately represents the true vitamin A status of the population; it is possible that the true prevalence of VAD falls somewhere in between that predicted by these indicators. Nevertheless, plasma retinol was found to be representative of dietary intake of vitamin A, based on the significant associations between plasma retinol and vitamin A intake – associations that were absent with the MRDR test.
Dietary vitamin A intakes were calculated as vitamin A RAE(17), which assumes that the retinol activity of β-carotene is 12:1 and of β-cryptoxanthin and α-carotene is 24:1, and are considered appropriate for North American diets. However, studies have indicated that the retinol equivalency of provitamin A from green and yellow vegetables is as high as 26–28:1, while that from orange fruits is 12:1(Reference De Pee, West and Permaesih18–Reference Tang, Gu and Hu20). Vegetables, primarily green leafy types, were a major source of vitamin A in our population. The alternative estimate of vitamin A intakes using 26:1 retinol equivalency for provitamin A from green and yellow vegetables was a better predictor of plasma retinol than the standard vitamin A RAE, as was evident by the significant, positive correlation and regression coefficients. These results suggest that the alternative vitamin A RAE provides a more accurate estimate of vitamin A intakes and is consistent with previous findings in Indonesia(Reference De Pee, Bloem and Gorstein33).
Vitamin A fortification of sugar was introduced in Zambia in 1998, but its contribution to total vitamin A intakes had not been previously quantified at the individual level and combined with analysis of vitamin A content in household sugar samples. The programme was originally designed to provide 30 % of the RDA(34) for vitamin A retinol equivalents (RE; i.e. 400 RE × 0·30 = 120 μg RE for children 1–3 years of age). It assumed an average intake of 15 g sugar/d, and hence required a minimum content of 16 mg vitamin A/kg sugar at point of sale(Reference Serlemitsos and Fusco35). However, for cost reasons, this was reduced to 10 mg/kg. We found only 59 % of sugar samples to contain ≥10 mg vitamin A/kg, suggesting a significant improvement from 2003, when only 8 % and 20 % of household sugar samples from Central and Eastern Provinces, respectively, met this level.
Fortified sugar provided 77 μg RAE/d, or 19 % of the WHO RDA for vitamin A RE(34), falling short of the original target of 30 % of the RDA(34). Vitamin A intake from sugar was not found to be associated with plasma retinol concentration in this population. The lack of association may be partly due to the lower amount of total vitamin A provided by this individual food source compared with roots and tubers. But it may also be partly attributed to the high variability of vitamin A content in sugar samples and hence inaccuracy when estimating vitamin A intake from sugar by individuals.
The observational design of the present survey precludes any conclusions on the direct causes of VAD. Dietary intake data were obtained for only one day per season for each individual and this cannot fully describe the usual intake of vitamin A across the whole year. The adequacy of vitamin A intakes is also dependent on the accuracy of food composition data, which can vary. Nevertheless, several contributors to vitamin A status and intakes were quantified, which may better direct future research on the persistence of VAD in this population.
The only food group source of vitamin A associated with plasma retinol was roots and tubers, the second largest contributor to vitamin A intakes in the harvest/early-post harvest season. This highlights the potential important contribution of orange and yellow plant food sources, including sweet potato, to vitamin A status. The regression analysis suggests that alternative vitamin A RAE intake of 210 μg RAE/d (i.e. equivalent to the Estimated Average Requirement for children 1–3 years of age(17)) was associated with a 0·014 μmol/l greater concentration of plasma retinol. Increased vitamin A intake from available plant food sources may thus reduce the risk of VAD.
Reported coverage with vitamin A supplements was very high (97 %), yet the high prevalence of VAD persisted. However this is consistent with a previous survey where plasma retinol was not observed to increase following vitamin A capsule distribution(2). A subsequent stable isotope tracer study found that the absorption, retention and excretion of vitamin A from a supplement by Zambian children was as expected, but absorption and retention of vitamin A were negatively associated with reported fever(Reference Aklamati, Mulenga and Dueker5). Increased urinary excretion of vitamin A also occurs with acute infections, particularly when febrile(Reference Stephensen, Alvarez and Kohatsu3, Reference Alvarez, Salazar-Lindo and Kohatsu4). Given the high rates of infection and reported fever in this population, it is possible that vitamin A requirements are greatly increased and contribute to the high rates of VAD, despite the abundance of available vitamin A(Reference Miller, Humphrey and Johnson36). This may partly explain the discrepancy between the prevalence of inadequate vitamin A intakes and VAD. Comparison of vitamin A intakes with requirements for healthy reference children may thus be inappropriate.
Conclusions
We observed a persistently high prevalence of VAD in this population of rural Zambian children, despite the high coverage with vitamin A supplements, presence of a sugar vitamin A fortification programme and apparently adequate vitamin A intakes. These high rates of VAD co-occurred with a high rate of infection. Both vitamin A intake and the acute-phase response were significant predictors of plasma retinol. While increasing vitamin A intakes in this population will continue to be important to avert further VAD, another major factor, possibly infection, appears to be contributing importantly to the persistent high rates of VAD.
Acknowledgements
This study was administered by HarvestPlus, a programme of the International Food Policy Research Institute (IFPRI) and the International Center for Tropical Agriculture (CIAT). The authors report no conflict of interest. C.H. conducted data analysis and drafted the manuscript. U.P. managed field data collection, data entry and cleaning. W.S. assisted in the survey design and managed field operations. J.C. supervised field collection of blood samples, summarized and interpreted biochemical results. The late E.K. assisted in the design and management of all aspects of the biochemical component of the survey and drafted the final report of results. All authors contributed to the interpretation of results. The authors wish to acknowledge the support of Freddie Mubanga, the former acting director of the National Food and Nutrition Commission, Zambia and Dr Cassim Masi, its present director. They thank Dr Sherry Tanumihardjo for preparing the didehydroretinol and standards for use in the MRDR test, Rebecca Surles for training field staff in the field and laboratory methods for the MRDR test, and Joanne Arsenault for her assistance in statistical analyses of adequacy of nutrient intakes. They are grateful to Musonda Mofu, Vincent Chowa and Patricia Sakala for field supervision of data collection; and Samson Mwale, Sydney Mwanza, Phidelis Malunga and Eric M. Njunju for assistance in collection and analysis of blood samples. They are also grateful for the support of the Mkushi and Nyimba District Health Offices and Central and Eastern Provincial Health Offices, and thank the children and parents of Nyimba and Mkushi Districts for their participation in the study.







