INTRODUCTION
Malaria is a mosquito-borne infectious disease caused by the parasite Plasmodium, of which six species can infect humans; Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale curtisi, Plasmodium ovale wallikeri, Plasmodium malariae and Plasmodium knowlesi. Of these, P. falciparum and P. vivax are the predominant species with an estimated 182·2 million clinical cases of P. falciparum malaria, 15·8 million clinical cases of P. vivax malaria and 584 000 deaths attributable to malaria every year (WHO, 2014). The greatest burden of disease is seen in young children and pregnant women. P. falciparum is responsible for the vast majority of global morbidity and mortality (WHO, 2014). It is estimated that over 125 million pregnancies are at risk of malaria, 32 million are at risk of P. falciparum, 40 million are at risk of P. vivax and 53 million are at risk of both species) (Dellicour et al. Reference Dellicour, Guerra, Kuile, Snow and Tatem2010). Women who acquire a Plasmodium spp. infection during pregnancy commonly experience negative maternal and birth outcomes such as anaemia, low birth weight and preterm birth with an estimated 75 000–200 000 infant deaths annually attributable to malaria in pregnancy (Steketee et al. Reference Steketee, Nahlen, Parise and Menendez2001). Malaria in the period following pregnancy, the postpartum period, is also of public health importance. Malaria was the leading indirect cause of death in postpartum women in a study in Zambia (Vallely et al. Reference Vallely, Ahmed and Murray2005) and the second highest cause of postpartum death in a study in India (Barnett et al. Reference Barnett, Nair, Tripathy, Borghi, Rath and Costello2008).
In malaria endemic areas, individuals develop naturally acquired immunity to both P. falciparum and P. vivax after repeated infections. This immunity does not generally protect against infection per se, but protects against the development of high parasite densities and clinical symptoms (reviewed in Langhorne et al. Reference Langhorne, Ndungu, Sponaas and Marsh2008). Despite acquiring a degree of protective immunity prior to pregnancy, pregnant women are typically more susceptible to P. falciparum and P. vivax. Broad hormonal and immunological changes that occur during pregnancy are likely to play a role, with a general shift from cell-mediated immunity toward humoral immunity (Jamieson et al. Reference Jamieson, Theiler and Rasmussen2006; Robinson and Klein, Reference Robinson and Klein2012). In the case of P. falciparum the increased susceptibility has been largely attributed to the lack of immunity to pregnancy-specific isolates that sequester in the placenta (well documented and extensively reviewed elsewhere, e.g. (Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007; Duffy, Reference Duffy2007; Hviid and Salanti, Reference Hviid and Salanti2007; Rogerson, Reference Rogerson2010; Umbers et al. Reference Umbers, Aitken and Rogerson2011)). The ability of P. vivax to bind and sequester in the placenta, its role in pathogenesis and the role of immunity against this process are debated (Mayor et al. Reference Mayor, Bardaji, Felger, King, Cistero, Dobano, Stanisic, Siba, Wahlgren, del Portillo, Mueller, Menendez, Ordi and Rogerson2012a ). Importantly, P. vivax possesses the ability to form hypnozoites in the liver, a dormant stage which can lead to relapses of blood-stage infections (Krotoski et al. Reference Krotoski, Collins, Bray, Garnham, Cogswell, Gwadz, Killick-Kendrick, Wolf, Sinden, Koontz and Stanfill1982; Krotoski, Reference Krotoski1985). The immunological mechanisms that mitigate P. vivax in pregnancy are unclear, as is the effect of an altered immunological state during pregnancy on the risk of relapse.
The rate at which a woman returns to a normal immunological state after pregnancy, and how this affects malaria risk postpartum has not been well characterized. There is increasing evidence for the altered susceptibility to P. falciparum and P. vivax postpartum (Boel et al. Reference Boel, Rijken, Brabin, Nosten and McGready2012) and a growing literature investigating the immune response to malaria in the postpartum period which may account for observed epidemiological patterns. In this review we highlight the similarities and differences of P. vivax and P. falciparum infection during pregnancy and the postpartum period with respect to epidemiology, clinical pathogenesis and immunology.
Plasmodium falciparum and P. vivax risk in pregnancy
Numerous studies have demonstrated that pregnant women are at increased risk of P. falciparum infection and experience higher parasite densities and rates of clinical malaria when compared to non-pregnant women (reviewed in Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007). We therefore reviewed the P. vivax literature in addition to studies investigating P. vivax and P. falciparum in co-endemic areas. Few studies have investigated the risk of P. vivax infection during pregnancy and available data is conflicting (Table 1). An increased risk of P. vivax infection (Singh et al. Reference Singh, Shukla, Srivastava and Sharma1995, Reference Singh, Shukla and Sharma1999) and increased density of P. vivax infections (Campbell et al. Reference Campbell, Martinez and Collins1980; Singh et al. Reference Singh, Shukla and Sharma1999) have been observed in pregnant compared to non-pregnant women from El Salvador and India (Table 1). An increased multiplicity of P. vivax infections during pregnancy in Thailand has also been observed (Thanapongpichat et al. Reference Thanapongpichat, McGready, Luxemburger, Day, White, Nosten, Snounou and Imwong2013) though no difference was observed in Brazil (Marin-Menendez et al. Reference Marin-Menendez, Bardaji, Martinez-Espinosa, Botto-Menezes, Lacerda, Ortiz, Cistero, Piqueras, Felger, Mueller, Ordi, del Portillo, Menendez, Wahlgren and Mayor2013). Other studies have failed to detect substantial differences in P. vivax risk between pregnant women and non-pregnant women (Campbell et al. Reference Campbell, Martinez and Collins1980; Parekh et al. Reference Parekh, Hernandez, Krogstad, Casapia and Branch2007) (Table 1). Furthermore, a study in Brazil found an increased frequency of P. falciparum relative to P. vivax infections in pregnant compared to non-pregnant women (Martinez-Espinosa et al. Reference Martinez-Espinosa, Daniel-Ribeiro and Alecrim2004). However, another study from the same Brazilian population failed to replicate these findings (Almeida et al. Reference Almeida, Barbosa and Martinez-Espinosa2010) and to further complicate matters, a study in Indonesia found an increased frequency of P. vivax relative to P. falciparum in pregnancy (Barcus et al. Reference Barcus, Basri, Picarima, Manyakori, Sekartuti, Elyazar, Bangs, Maguire and Baird2007) (Table 1). The available evidence is somewhat conflicting but together suggests that there is an increased risk of P. vivax infection during pregnancy compared to non-pregnancy, albeit a smaller increased risk than that observed in regards to pregnancy and P. falciparum infection.
Table 1. Plasmodium vivax risk in pregnancy compared to non-pregnant women and comparisons with P. falciparum risk in co-endemic areas
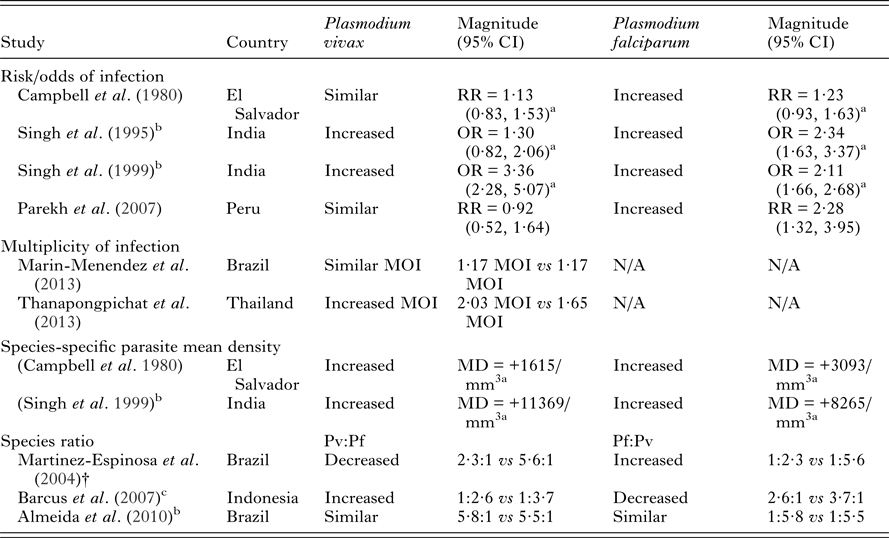
NB – ratios within 0·2 of 1 were considered similar to 1. Abbreviations: MD, mean difference; OR, odds ratio; RR, risk ratio; N/A, not available; MOI, multiplicity of infections. All measures of association are unadjusted unless otherwise specified.
a Calculated from data in paper.
b Women in study restricted to those with history of fever.
c Women with slide-confirmed diagnoses of malaria.
The clinical consequences of Plasmodium infection occur during the blood-stage of infection and are exacerbated by high densities of the blood-stage parasite. P. falciparum invades all erythrocytes, whereas P. vivax selectively invades young erythrocytes (reticulocytes), and thus P. vivax parasitaemia is typically lower than P. falciparum parasitaemia (Collins and Jeffery, Reference Collins and Jeffery1999a , Reference Collins and Jeffery b ; Simpson et al. Reference Simpson, Silamut, Chotivanich, Pukrittayakamee and White1999; Collins et al. Reference Collins, Jeffery and Roberts2004). P. vivax has a lower pyrogenic threshold compared to P. falciparum, provoking a stronger inflammatory response for a given level of parasitaemia (Ross and Thomson, Reference Ross and Thomson1910; Luxemburger et al. Reference Luxemburger, Thwai, White, Webster, Kyle, Maelankirri, Chongsuphajaisiddhi and Nosten1996; Hemmer et al. Reference Hemmer, Holst, Kern, Chiwakata, Dietrich and Reisinger2006; Yeo et al. Reference Yeo, Lampah, Tjitra, Piera, Gitawati, Kenangalem, Price and Anstey2010). However, P. vivax infections less frequently progress to severe disease compared to P. falciparum infections, which can result in cerebral malaria, metabolic acidosis, respiratory distress and severe anaemia. P. vivax can lead to severe clinical symptoms such as severe anaemia, respiratory distress and thrombocytopenia (reviewed in Anstey et al. Reference Anstey, Douglas, Poespoprodjo and Price2012).
Few studies have investigated the relative severity of P. falciparum compared to P. vivax during pregnancy on maternal outcomes in co-endemic populations. Most studies show that P. falciparum is associated with more severe maternal and birth outcomes (Tables 2 and 3). Studies in Thailand, India and Indonesia have demonstrated that pregnant women infected with P. falciparum have increased severity and odds of anaemia compared to those infected with P. vivax (Nair and Nair, Reference Nair and Nair1993; Nosten et al. Reference Nosten, McGready, Simpson, Thwai, Balkan, Cho, Hkirijaroen, Looareesuwan and White1999; Singh et al. Reference Singh, Shukla and Sharma1999; Poespoprodjo et al. Reference Poespoprodjo, Fobia, Kenangalem, Lampah, Warikar, Seal, McGready, Sugiarto, Tjitra, Anstey and Price2008). Interestingly, a study in Thailand has indicated a potential interaction in disease severity between the two species demonstrating a protective effect of P. vivax infection against severity and number of P. falciparum episodes during pregnancy (Luxemburger et al. Reference Luxemburger, Ricci, Nosten, Raimond, Bathet and White1997; Nosten et al. Reference Nosten, McGready, Simpson, Thwai, Balkan, Cho, Hkirijaroen, Looareesuwan and White1999). Both P. falciparum and P. vivax infections during pregnancy are associated with detrimental birth outcomes such as low birth weight, preterm delivery and miscarriage (Table 3). Studies conducted in Thailand, India, Colombia and Indonesia have tended to find a greater reduction in birth weight and greater increase in the risk of preterm delivery amongst pregnant women with P. falciparum infections compared to P. vivax infections in pregnancy (Nair and Nair, Reference Nair and Nair1993; Nosten et al. Reference Nosten, McGready, Simpson, Thwai, Balkan, Cho, Hkirijaroen, Looareesuwan and White1999; Singh et al. Reference Singh, Shukla and Sharma1999; McGready et al. Reference McGready, Davison, Stepniewska, Cho, Shee, Brockman, Udomsangpetch, Looareesuwan, White, Meshnick and Nosten2004; Poespoprodjo et al. Reference Poespoprodjo, Fobia, Kenangalem, Lampah, Warikar, Seal, McGready, Sugiarto, Tjitra, Anstey and Price2008; Tobon-Castano et al. Reference Tobon-Castano, Solano, Sanchez and Trujillo2011) (Table 3). A study in India showed reduced odds of foetal loss in P. vivax compared to P. falciparum infections (Nair and Nair, Reference Nair and Nair1993), whereas studies in Thailand which have specifically examined miscarriage found similar odds in P. falciparum and P. vivax infections (McGready et al. Reference McGready, Lee, Wiladphaingern, Ashley, Rijken, Boel, Simpson, Paw, Pimanpanarak, Mu, Singhasivanon, White and Nosten2012). Taken together the above findings suggest that some of the underlying mechanisms by which the two species mediate negative birth outcomes are independent.
Table 2. Adverse maternal outcomes due to P. vivax infection in pregnancy compared to non-infected pregnant women and comparisons with P. falciparum risk in co-endemic areas
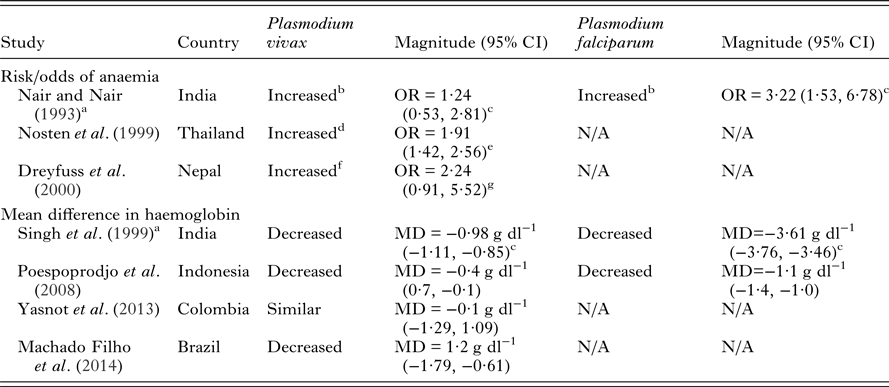
NB – ratios within 0.2 of 1 and mean differences of less than 0.2 g dl−1 were considered similar. All measures of association are unadjusted unless otherwise specified. Abbreviations: MD, mean difference; OR, odds ratio; N/A, not available.
a Women in study restricted to those with history of fever.
b Anaemia defined as <8 hb g dl−1.
c Calculated from data in paper.
d Anaemia defined clinically or by haematocrit <30%.
e Adjusted for age, location, gestational age at first visit, compliance to attendance at the antenatal clinic.
f Anaemia defined as <11 hb g dl−1.
g Adjusted for hookworm infection, vitamin A deficiency and trimester of pregnancy.
Table 3. Adverse birth outcomes due to P. vivax infection in pregnancy compared to non-infected pregnant women and comparisons with P. falciparum risk in co-endemic areas
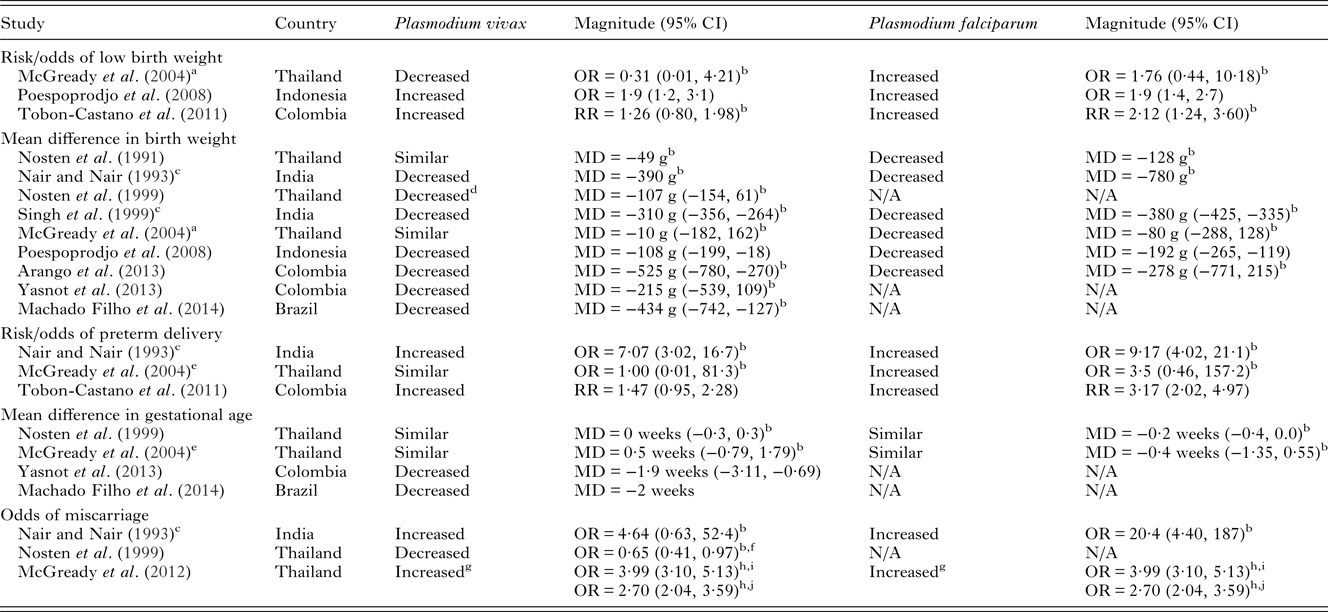
NB – ratios within 0·2 of 1 were considered similar to 1. Birth weight MDs <50 g were considered similar. Gestational age MDs <1 week were considered similar. Low birth weight defined as <2500 g. All measures of association are unadjusted unless otherwise specified. Abbreviations: MD, mean difference; OR, odds ratio; RR, Risk Ratio.
a Cases included P. malariae and P. vivax cases.
b Calculated from data in paper.
c Women in study restricted to those with history of fever.
d Adjusted for age, location, gestational age at first visit, compliance to attendance at the antenatal clinic.
e Adjusted for hookworm infection, vitamin A deficiency and trimester of pregnancy.
f Error in the published paper, the reported events in the P. vivax group should read 447, not 44. Confirmed by authors of the paper.
g Single episode of P. vivax or P. falciparum in first trimester.
h Adjusted for age, smoking and estimated gestational age.
i Symptomatic malaria.
j Asymptomatic malaria.
It is well documented that the risk of P. falciparum infection during pregnancy is highest amongst primigravid women (reviewed in Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007). Studies in Thailand, India and Indonesia have also found that primigravidae are more at risk of P. vivax infection than multigravidae (Brabin et al. Reference Brabin, Ginny, Alpers, Brabin, Eggelte and Van der Kaay1990; Singh et al. Reference Singh, Saxena, Chand, Valecha and Sharma1998, Reference Singh, Shukla and Sharma1999; Nosten et al. Reference Nosten, McGready, Simpson, Thwai, Balkan, Cho, Hkirijaroen, Looareesuwan and White1999; Poespoprodjo et al. Reference Poespoprodjo, Fobia, Kenangalem, Lampah, Warikar, Seal, McGready, Sugiarto, Tjitra, Anstey and Price2008; Fowkes et al. Reference Fowkes, McGready, Cross, Hommel, Simpson, Elliott, Richards, Lackovic, Viladpai-Nguen, Narum, Tsuboi, Anders, Nosten and Beeson2012), although this finding is not consistent across all study sites (Singh et al. Reference Singh, Shukla, Srivastava and Sharma1995; Luxemburger et al. Reference Luxemburger, McGready, Kham, Morison, Cho, Chongsuphajaisiddhi, White and Nosten2001; Appleyard et al. Reference Appleyard, Tuni, Cheng, Chen, Bryan and McCarthy2008) (Table 4). Broad immunological and hormonal changes that take place with successive pregnancies could play a role in the decreasing risk of both P. falciparum and P. vivax with gravidity (Vleugels et al. Reference Vleugels, Eling, Rolland and de Graaf1987, Reference Vleugels, Brabin, Eling and de Graaf1989; Riley et al. Reference Riley, Schneider, Sambou and Greenwood1989; Bouyou-Akotet et al. Reference Bouyou-Akotet, Issifou, Meye, Kombila, Ngou-Milama, Luty, Kremsner and Mavoungou2004, Reference Bouyou-Akotet, Adegnika, Agnandji, Ngou-Milama, Kombila, Kremsner and Mavoungou2005). Additionally, a degree of protective immunity is acquired to both species during pregnancy, which may play a stronger role in P. falciparum infections than P. vivax infections (reviewed below).
Table 4. Risk/odds of P. vivax infection in primigravidae compared to multigravidae and comparisons with P. falciparum risk in co-endemic areas
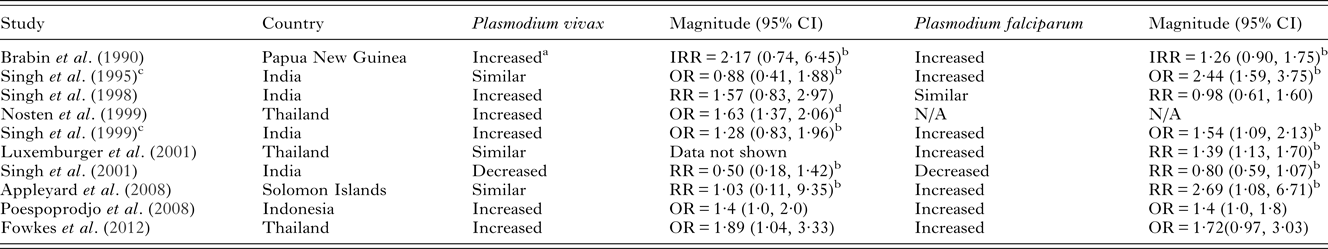
NB – ratios within 0·2 of 1 were considered similar to 1. Abbreviations: OR, odds ratio; RR, risk ratio; IRR, incidence rate ratio; N/A, not available. All measures of association are unadjusted unless otherwise specified.
a Plasmodium vivax and P. malariae.
b Calculated from data in paper.
c Women in study restricted to those with history of fever.
d Adjusted for age, location, gestational age at first visit, compliance to attendance at the antenatal clinic.
A review of the epidemiological data indicates that consolidation of data is challenging due to differences in transmission and clinical criteria. In summary, the data suggest that pregnant women may be at an increased risk of P. vivax during pregnancy, but are relatively more susceptible to P. falciparum than P. vivax compared to their non-pregnant counterparts. Infection with P. falciparum during pregnancy tends to lead to more severe negative maternal and birth outcomes than infection with P. vivax. Evidence suggests that primigravidae are at increased risk of P. falciparum and P. vivax compared to multigravidae. The differential risk, severity and gravidity effects could be attributed to the distinct pathologies of P. falciparum and P. vivax during pregnancy and/or differential immunity to the two species.
Key differences in P. falciparum and P. vivax clinical pathogenesis
During pregnancy, specific P. falciparum variants emerge that can escape pre-existing immunity and sequester in the placenta. Plasmodium falciparum isolates in pregnant women upregulate the expression of PfVAR2CSA, an antigen located on the P. falciparum-infected erythrocyte (Pf-IE) surface. PfVAR2CSA is a specific form of the variant protein PfEMP1 (P. falciparum erythrocyte membrane protein 1) that binds to placental chondroitin-sulphate A (CSA) and helps mediate parasite sequestration in the placenta (reviewed in Khunrae et al. Reference Khunrae, Dahlbäck, Nielsen, Andersen, Ditlev, Resende, Pinto, Theander, Higgins and Salanti2010). The increased burden and detrimental effects of P. falciparum infection observed in pregnant women has been largely attributed to elevated parasite densities and the placental sequestration of Pf-IEs (reviewed in Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007; Hviid and Salanti, Reference Hviid and Salanti2007; Rogerson, Reference Rogerson2010; Umbers et al. Reference Umbers, Aitken and Rogerson2011). Plasmodium falciparum infection during pregnancy is typically associated with a very pronounced sequestration, or selective accumulation, of mature forms of blood-stage parasites in the placenta with a parasitaemia many fold higher than that observed in the peripheral blood (Walter et al. Reference Walter, Garin and Blot1982; Beeson et al. Reference Beeson, Amin, Kanjala and Rogerson2002). The accumulation of large numbers of Pf-IEs at the placenta results in changes to placental histology including inflammation, deposition of pigment in fibrin or inflammatory cells, syncytial knotting and thickening of the trophoblastic basement membrane (Walter et al. Reference Walter, Garin and Blot1982; Bulmer et al. Reference Bulmer, Rasheed, Francis, Morrison and Greenwood1993; Ismail et al. Reference Ismail, Ordi, Menendez, Ventura, Aponte, Kahigwa, Hirt, Cardesa and Alonso2000; Rogerson et al. Reference Rogerson, Pollina, Getachew, Tadesse, Lema and Molyneux2003). P. vivax lacks the PfVAR2CSA protein, or any known PfVAR2CSA orthologues, and P. vivax-IEs (Pv-IEs) are rarely found in the placenta (Singh et al. Reference Singh, Saxena and Shrivastava2003; Mayor et al. Reference Mayor, Bardaji, Felger, King, Cistero, Dobano, Stanisic, Siba, Wahlgren, del Portillo, Mueller, Menendez, Ordi and Rogerson2012a ; Carmona-Fonseca et al. Reference Carmona-Fonseca, Arango and Maestre2013). Despite this, infections with P. vivax during pregnancy have been associated with some of the same histological changes observed in P. falciparum infections, though these changes are typically less severe (McGready et al. Reference McGready, Davison, Stepniewska, Cho, Shee, Brockman, Udomsangpetch, Looareesuwan, White, Meshnick and Nosten2004; Souza et al. Reference Souza, Ataide, Dombrowski, Ippolito, Aitken, Valle, Alvarez, Epiphanio and Marinho2013) (Table 5). The binding of Pv-IEs to CSA (as well as other endothelial cells) has been described in vitro and may be partly mediated by PvVIR (Variant Interspersed Repeats) proteins expressed on the surface of Pv-IEs. However, the level of cytoadhesion of Pv-IEs to CSA is around ten-fold lower than that displayed by Pf-IEs (Carvalho et al. Reference Carvalho, Lopes, Nogueira, Orlandi, Bargieri, Blanco, Mamoni, Leite, Rodrigues, Soares, Oliveira, Wunderlich, Lacerda, del Portillo, Araujo, Russell, Suwanarusk, Snounou, Renia and Costa2010; Chotivanich et al. Reference Chotivanich, Udomsangpetch, Suwanarusk, Pukrittayakamee, Wilairatana, Beeson, Day and White2012) and cytoadherence to CSA does not differ between P. vivax isolates from pregnant and non-pregnant individuals (Marin-Menendez et al. Reference Marin-Menendez, Bardaji, Martinez-Espinosa, Botto-Menezes, Lacerda, Ortiz, Cistero, Piqueras, Felger, Mueller, Ordi, del Portillo, Menendez, Wahlgren and Mayor2013). The low level of CSA-adherence exhibited by Pv-IEs likely plays a minor role in pathogenesis compared to P. falciparum. The existence of PfVAR2CSA in P. falciparum represents a crucial difference between the two species and explains much of the different infection outcomes experienced by pregnant women. The reduced level of P. vivax cytoadhesion in vitro relative to P. falciparum explains the rarity of clinical observations of P. vivax placental sequestration (Mayor et al. Reference Mayor, Bardaji, Felger, King, Cistero, Dobano, Stanisic, Siba, Wahlgren, del Portillo, Mueller, Menendez, Ordi and Rogerson2012a ; Carmona-Fonseca et al. Reference Carmona-Fonseca, Arango and Maestre2013; Souza et al. Reference Souza, Ataide, Dombrowski, Ippolito, Aitken, Valle, Alvarez, Epiphanio and Marinho2013; Chaikitgosiyakul et al. Reference Chaikitgosiyakul, Rijken, Muehlenbachs, Lee, Chaisri, Viriyavejakul, Turner, Pongponratn, Nosten and McGready2014). Although other ligand/receptor combinations have been proposed as contributing to the sequestration of parasites in the placenta (Table 5) the evidence currently favours PfVAR2CSA/CSA as the principal interaction responsible for placental sequestration of P. falciparum.
Table 5. Key differences in P. falciparum and P. vivax placental and binding pathogenesis
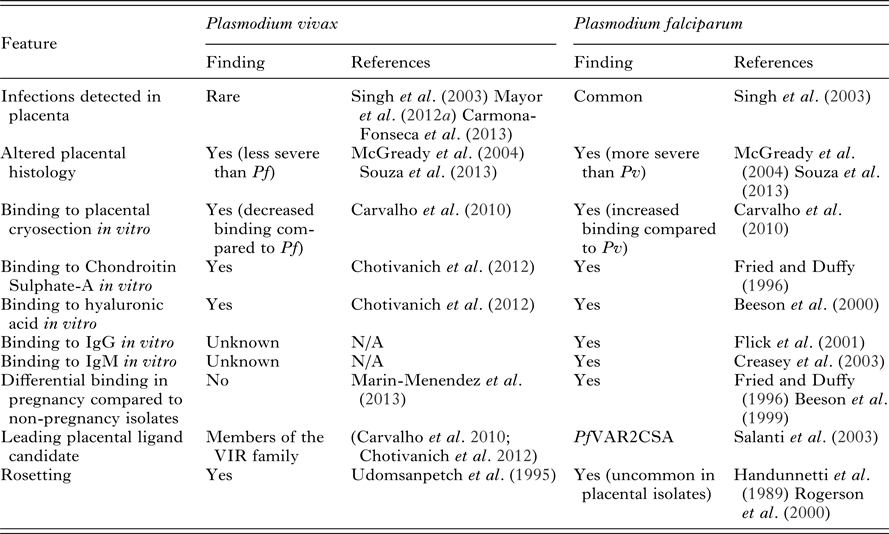
Abbreviations: Pf, Plasmodium falciparum; Pv, Plasmodium vivax; IgG, immunoglobulin G; IgM, immunoglobulin M; N/A, not available.
Another pathophysiological feature mediated by IE surface ligands is rosetting, whereby IEs bind to uninfected erythrocytes. Rosetting is a feature of both P. falciparum and P. vivax isolates from infections in non-pregnant individuals (Udeinya et al. Reference Udeinya, Schmidt, Aikawa, Miller and Green1981; Udomsanpetch et al. Reference Udomsanpetch, Thanikkul, Pukrittayakamee and White1995). Recent evidence suggests that rosetting occurs more frequently in P. vivax isolates than P. falciparum isolates (Lee et al. Reference Lee, Malleret, Lau, Mauduit, Fong, Cho, Suwanarusk, Zhang, Albrecht, Costa, Preiser, McGready, Renia, Nosten and Russell2014) likely due to differential erythrocyte or receptor preferences. Glycophorin C is a ligand for both P. falciparum and P. vivax rosetting (Lee et al. Reference Lee, Malleret, Lau, Mauduit, Fong, Cho, Suwanarusk, Zhang, Albrecht, Costa, Preiser, McGready, Renia, Nosten and Russell2014) whilst numerous other receptors have been identified for P. falciparum rosetting (reviewed in Sherman et al. Reference Sherman, Eda and Winograd2003). Interestingly, rosetting is uncommon in placental P. falciparum isolates (Maubert et al. Reference Maubert, Fievet, Tami, Boudin and Deloron1998; Rogerson et al. Reference Rogerson, Beeson, Mhango, Dzinjalamala and Molyneux2000) and is lacking in isolates that adhere to CSA and upregulate VAR2CSA (Beeson and Brown, Reference Beeson and Brown2004). In the absence of placental sequestration, the rosetting phenotype may contribute more strongly to clinical outcomes in P. vivax infection; rosetting is strongly associated with anaemia and increased parasitaemia in both P. falciparum and P. vivax infection (Rowe et al. Reference Rowe, Obiero, Marsh and Raza2002; Doumbo et al. Reference Doumbo, Thera, Kone, Raza, Tempest, Lyke, Plowe and Rowe2009; Marin-Menendez et al. Reference Marin-Menendez, Bardaji, Martinez-Espinosa, Botto-Menezes, Lacerda, Ortiz, Cistero, Piqueras, Felger, Mueller, Ordi, del Portillo, Menendez, Wahlgren and Mayor2013).
In the absence of considerable interactions between the placenta and Pv-IEs, the altered placental histology associated with P. vivax infection is likely due to broad effects of peripheral infection, such as maternal anaemia, fever or the effect of the cytokine response to infection (Mayor et al. Reference Mayor, Bardaji, Felger, King, Cistero, Dobano, Stanisic, Siba, Wahlgren, del Portillo, Mueller, Menendez, Ordi and Rogerson2012a ; Souza et al. Reference Souza, Ataide, Dombrowski, Ippolito, Aitken, Valle, Alvarez, Epiphanio and Marinho2013). These mechanisms likely also act in P. falciparum peripheral infections in conjunction with the direct effects of placental sequestration. Reticulocytosis occurs in some populations during pregnancy and may contribute to an increased risk of P. vivax (Traill, Reference Traill1975). Taken together, current data show that interactions between P. vivax and placental receptors are rare in contrast to the common interaction of P. falciparum ligands with placental receptors which largely explains the reduced magnitude of negative outcomes in P. vivax infections in pregnancy compared to P. falciparum infections.
Plasmodium falciparum and P. vivax risk in the postpartum period
How the increased burden and risk of P. falciparum and P. vivax malaria during pregnancy relates to the risk of malaria in the postpartum period is the focus of increasing research. The World Health Organization defines the postpartum period as beginning 1 h after the delivery of the placenta and continuing until 6 weeks after the birth of the infant (WHO, 2011). This definition is rarely adhered to in the malaria literature, so for the purpose of this review the postpartum period is defined as the period from delivery to 6 months post-delivery. The majority of postpartum studies have been conducted in Africa and have compared the risk of P. falciparum infection postpartum to the risk during pregnancy; with these studies observing a reduced risk of P. falciparum infection in the postpartum period (Table 6) (Bray and Anderson, Reference Bray and Anderson1979; Watkinson and Rushton, Reference Watkinson and Rushton1983; Steketee et al. Reference Steketee, Wirima, Bloland, Chilima, Mermin, Chitsulo and Breman1996; Fievet et al. Reference Fievet, Cot, Ringwald, Bickii, Dubois, Le Hesran, Migot and Deloron1997; Green et al. Reference Green, van Eijk, van Ter Kuile, Ayisi, Parise, Kager, Nahlen, Steketee and Nettey2007; Menendez et al. Reference Menendez, Bardaji, Sigauque, Romagosa, Sanz, Serra-Casas, Macete, Berenguera, David, Dobano, Naniche, Mayor, Ordi, Mandomando, Aponte, Mabunda and Alonso2008; Serra-Casas et al. Reference Serra-Casas, Menendez, Dobano, Bardaji, Quinto, Ordi, Sigauque, Cistero, Mandomando, Alonso and Mayor2011). Conversely, the single study that investigated P. falciparum clinical malaria found an increased risk during the 60 days postpartum compared to each trimester of pregnancy (Diagne et al. Reference Diagne, Rogier, Sokhna, Tall, Fontenille, Roussilhon, Spiegel and Trape2000). The sole study assessing the risk of P. vivax and P. falciparum infection in postpartum compared to pregnant controls (in Papua New Guinea) found the incidence of P. vivax and P. falciparum parasitaemia increased from delivery until 4 months postpartum (Brabin et al. Reference Brabin, Ginny, Alpers, Brabin, Eggelte and Van der Kaay1990) with a greater relative increase in postpartum P. vivax incidence than P. falciparum incidence. Importantly, chemoprophylaxis was ceased at delivery so this finding may be more reflective of a ‘rebound effect’ than an indication of the natural course of susceptibility during the postpartum period (Brabin et al. Reference Brabin, Ginny, Alpers, Brabin, Eggelte and Van der Kaay1990). Overall, the heightened risk of P. falciparum seen during pregnancy is typically reduced in the postpartum period, whilst the limited evidence of P. vivax risk postpartum compared to pregnancy suggests that the risk is elevated.
Table 6. Risk of P. vivax and P. falciparum in the postpartum period

NB – ratios within 0·2 of 1 were considered similar to 1. All measures of association are unadjusted unless otherwise specified.
Abbreviations: mos., months; LM, light microscopy; OR, odds ratio; IRR, incidence rate ratio; RR, risk ratio; HR, hazard ratio.
a Detected by light microscopy.
b Calculated from data in the paper.
c Study restricted to primigravid women only.
d Asymptomatic infection.
e Detected by PCR.
f Clinical malaria defined as any case of fever or fever-related symptoms associated with a ratio of parasites to leucocytes that exceeds a pyrogenic threshold.
g Adjusted for exposure, parity, duration of residence in village and effects within study subjects.
h Clinical malaria defined as asexual P. falciparum parasitemia with >100 parasites μL−1 of blood, fever (current or within the previous 24 h), or presence of other symptoms associated with malaria.
To truly evaluate whether malaria risk returns to non-pregnant levels immediately after pregnancy, the postpartum risk needs to be compared to non-pregnant controls. Two studies in Africa (Senegal and Gabon) found an increased risk of P. falciparum infection relative to non-pregnant women (Diagne et al. Reference Diagne, Rogier, Sokhna, Tall, Fontenille, Roussilhon, Spiegel and Trape2000; Ramharter et al. Reference Ramharter, Grobusch, Kiessling, Adegnika, Moller, Agnandji, Kramer, Schwarz, Kun, Oyakhirome, Issifou, Borrmann, Lell, Mordmuller and Kremsner2005) (Table 6). Both studies also found a greater increase in the risk of clinical malaria relative to the risk of Plasmodium spp. infection indicating that the postpartum state is more susceptible to clinical malaria than the non-pregnant state independent of an increased risk of infection. Depressed immunity may explain this finding with the Senegal study finding that after 90 days postpartum the risk of clinical P. falciparum malaria returned to the level seen prior to pregnancy, suggesting that the responsible factor for altered postpartum risk returns to normal after 3 months (Diagne et al. Reference Diagne, Rogier, Sokhna, Tall, Fontenille, Roussilhon, Spiegel and Trape2000). In contrast to African findings, a recent study on the Thai–Myanmar border found that postpartum women had decreased risk of P. falciparum episodes than age and location matched non-pregnant controls, whilst there was an increased risk of P. vivax episodes in postpartum women compared to non-pregnant controls (Boel et al. Reference Boel, Rijken, Leenstra, Pyae Phyo, Pimanpanarak, Keereecharoen, Proux, Laochan, Imwong, Singhasivanon, White, McGready and Nosten2013). Further research into postpartum risk of malaria will help address the current conflicting evidence of the risk of malaria in the postpartum period.
It has been suggested that the differential risk of malaria in the postpartum period may be due to immunological changes that occur during pregnancy and gradually return to ‘normal’ in the postpartum period (Diagne et al. Reference Diagne, Rogier, Sokhna, Tall, Fontenille, Roussilhon, Spiegel and Trape2000; Ramharter et al. Reference Ramharter, Grobusch, Kiessling, Adegnika, Moller, Agnandji, Kramer, Schwarz, Kun, Oyakhirome, Issifou, Borrmann, Lell, Mordmuller and Kremsner2005). Immunological changes during pregnancy include changes in cell-mediated and humoral immunity (Jamieson et al. Reference Jamieson, Theiler and Rasmussen2006; Robinson and Klein, Reference Robinson and Klein2012), which would presumably differentially affect susceptibility to P. falciparum and P. vivax due to underlying species differences in immunopathology.
Immunity to P. falciparum and P. vivax in pregnancy
A variety of immunological changes occur during pregnancy, with changes in the nature of cytokine responses, a general suppression of cell-mediated immunity and increased humoral immunity (reviewed in Jamieson et al. Reference Jamieson, Theiler and Rasmussen2006; Robinson and Klein, Reference Robinson and Klein2012). This shift is believed to reduce the chance of foetal rejection and increase the maternal transfer of antibodies to the foetus. These broader immunological changes are also likely to contribute to an altered susceptibility to both Plasmodium spp. during pregnancy in addition to the availability of the placenta as a sequestration site.
The broadly suppressed cell-mediated immunological state that exists during pregnancy should dampen the effectiveness of T cells on both P. falciparum and P. vivax. However, few studies have investigated the impact of an altered cell-mediated response on Plasmodium spp. infection during pregnancy. CD8 T cell levels are higher in the inflammatory infiltrate in chronically P. falciparum infected placentas compared to uninfected placentae, past infections, acute infections and placentae from non-exposed women (Ordi et al. Reference Ordi, Menendez, Ismail, Ventura, Palacin, Kahigwa, Ferrer, Cardesa and Alonso2001). This observation was supported by another study which found greater frequencies of CD8 T cells producing IFN-alpha and TNF-alpha in P. falciparum infected placentae compared to uninfected placentae (Diouf et al. Reference Diouf, Fievet, Doucoure, Ngom, Andrieu, Mathieu, Gaye, Thiaw and Deloron2007). Less is known about the role of T cells at the placenta during P. vivax infections. An increased presence of mononuclear cells in the placenta was detected in instances of P. vivax and P. falciparum infections compared to no infection, with similar numbers of mononuclear cells in P. vivax and P. falciparum infections (Souza et al. Reference Souza, Ataide, Dombrowski, Ippolito, Aitken, Valle, Alvarez, Epiphanio and Marinho2013).
Another important impact an altered cell-mediated immune response may have in pregnancy is an altered ability to control liver stage infection and P. vivax relapse. Though direct human evidence is lacking, cell-mediated immunity is thought to be particularly important for pre-erythrocytic immunity on the basis of animal models (reviewed in Doll and Harty, Reference Doll and Harty2014). However, what constitutes an adequate immune response against clinical relapses of P. vivax is unknown as is the risk of relapses during the altered immunological state of pregnancy.
The humoral immune response is a crucial component of naturally acquired immunity and antibody responses to both P. falciparum and P. vivax antigens are important biomarkers of exposure and protective immunity in meta-analyses of non-pregnant populations (Fowkes et al. Reference Fowkes, Richards, Simpson and Beeson2010; Cutts et al. Reference Cutts, Powell, Agius, Beeson, Simpson and Fowkes2014). Numerous studies have demonstrated the important role of anti-PfVAR2CSA humoral immunity in P. falciparum infections during pregnancy (reviewed in Duffy, Reference Duffy2007; Hviid and Salanti, Reference Hviid and Salanti2007; Rogerson, Reference Rogerson2010; Ataide et al. Reference Ataide, Mayor and Rogerson2013). Antibodies reactive against recombinant PfVAR2CSA, and to the surface of erythrocytes infected with P. falciparum placental isolates and P. falciparum lines selected by their adhesion to CSA increase with gravidity (reviewed in Ataide et al. Reference Ataide, Mayor and Rogerson2013), indicating that immunity to PfVAR2CSA is acquired or boosted progressively with successive pregnancies, and is associated with parasite clearance and reduced odds of placental infection (Guitard et al. Reference Guitard, Cottrell, Magnouha, Salanti, Tengfei, Sokhna, Deloron and Ndam2008; Feng et al. Reference Feng, Aitken, Yosaatmadja, Kalilani, Meshnick, Jaworowski, Simpson and Rogerson2009; Tutterrow et al. Reference Tutterrow, Avril, Singh, Long, Leke, Sama, Salanti, Smith, Leke and Taylor2012a , Reference Tutterrow, Salanti, Avril, Smith, Pagano, Ako, Fogako, Leke and Taylor b ). There is currently no complementary evidence for P. vivax. The risk of P. vivax also tends to decline with gravidity (Table 4), rendering the existence of P. vivax antigens that are upregulated in pregnancy and important as immune targets a viable hypothesis. However, in the absence of pregnancy-specific P. vivax isolates or antigens, the gravidity effect may also be explained by a broader acquisition and boosting of immunity towards Pv-IEs and merozoite antigens during exposure to P. vivax during pregnancy or merely a reflection of immune acquisition with age. Additional mechanisms that influence susceptibility to both species during pregnancies are increased cortisol concentrations (Vleugels et al. Reference Vleugels, Brabin, Eling and de Graaf1989; Bouyou-Akotet et al. Reference Bouyou-Akotet, Adegnika, Agnandji, Ngou-Milama, Kombila, Kremsner and Mavoungou2005) and reduced NK cell activity (Bouyou-Akotet et al. Reference Bouyou-Akotet, Issifou, Meye, Kombila, Ngou-Milama, Luty, Kremsner and Mavoungou2004) particularly in primigravid pregnancies.
There is little data available on antibodies to merozoite antigens during pregnancy (including orthologues expressed in both P. falciparum and P. vivax, e.g. AMA1, MSP119). Pregnant women in endemic settings have typically acquired protective immunity to these antigens during childhood; this immunity would likely contribute to a protective effect in pregnancy by reducing parasitaemia, which would have a knock-on protective effect on the burden of placental infection. Evidence to support this is limited, however some studies have found ad hoc associations with particular merozoite antigens (e.g. PfMSP1-19 and PfAMA-1) with improved birth outcomes in women exposed to P. falciparum (Taylor et al. Reference Taylor, Zhou, Marsillio, Thuita, Leke, Branch, Gowda, Long and Leke2004; Mayor et al. Reference Mayor, Kumar, Bardaji, Gupta, Jimenez, Hamad, Sigauque, Singh, Quinto, Kumar, Gupta, Chauhan, Dobano, Alonso, Menendez and Chitnis2013). Results must be interpreted with caution, given the lack of similar associations with other non-pregnancy specific antigens in the same studies (PfCSP, PfLSA1 PfRESA, PfDBLγ, PfDBLα, PfMSP1-19, PfAMA1 PfEBA175). Furthermore, P. falciparum merozoite responses (and P. vivax responses in co-endemic areas) are often highly correlated with PfVAR2CSA (Fowkes et al. Reference Fowkes, McGready, Cross, Hommel, Simpson, Elliott, Richards, Lackovic, Viladpai-Nguen, Narum, Tsuboi, Anders, Nosten and Beeson2012) so associations observed can serve as a proxy for higher levels of other protective responses.
Alternatively, in the absence of P. vivax-specific mechanisms, the gravidity effect could be indirect. It has been hypothesized that relapses of P. vivax infections are triggered by fever, notably by other malaria infections (reviewed in Shanks and White, Reference Shanks and White2013). If this were the case then one would expect P. falciparum erythrocytic immunity (both cell-mediated and humoral), to indirectly protect against P. vivax relapse by protecting against febrile symptoms. This indirect mechanism could explain the decreased risk of P. vivax with increasing gravidity in co-endemic regions in the absence of more direct immunological mechanisms.
Cross-species immunity also provides an alternative explanation for the gravidity effect of P. vivax. There is little reliable human data on cross-species immunity. An experimental infection of a non-pregnant individual with P. vivax showed that antibodies induced by P. vivax, are capable of recognizing P. falciparum schizont extract and may be able to inhibit P. falciparum growth in vitro (Nagao et al. Reference Nagao, Kimura-Sato, Chavalitshewinkoon-Petmitr, Thongrungkiat, Wilairatana, Ishida, Tan-Ariya, de Souza, Krudsood and Looareesuwan2008). How this translates vice versa or in pregnancy is unknown but may explain the interaction in disease severity between the two species in Thailand where P. vivax infection reduced the severity and number of P. falciparum episodes during pregnancy (Luxemburger et al. Reference Luxemburger, Ricci, Nosten, Raimond, Bathet and White1997; Nosten et al. Reference Nosten, McGready, Simpson, Thwai, Balkan, Cho, Hkirijaroen, Looareesuwan and White1999). Evidence also suggests that high-density blood stage infections may be able to inhibit liver stage infections through an increase in hepcidin levels (reviewed in Portugal et al. Reference Portugal, Drakesmith and Mota2011). Mechanisms of Plasmodium species-transcending immunity are poorly defined in humans and require further elucidation to determine their role in pregnancy and postpartum.
There is a relatively scarce amount of literature regarding the role of non-IgG antibodies in P. falciparum and P. vivax infection during pregnancy and postpartum which is not surprising given that IgG is considered to be the key immunoglobulin for naturally acquired immunity against malaria (Doolan et al. Reference Doolan, Dobano and Baird2009). IgM is typically observed in the primary response to infection and numerous P. falciparum and P. vivax antigens elicit IgM responses (Cutts et al. Reference Cutts, Powell, Agius, Beeson, Simpson and Fowkes2014). IgM has been shown to bind non-specifically to PfVAR2CSA a feature which may have evolved as an immune evasion mechanism (Creasey et al. Reference Creasey, Staalsoe, Raza, Arnot and Rowe2003; Elliott et al. Reference Elliott, Brennan, Beeson, Tadesse, Molyneux, Brown and Rogerson2005; Rasti et al. Reference Rasti, Namusoke, Chene, Chen, Staalsoe, Klinkert, Mirembe, Kironde and Wahlgren2006; Semblat et al. Reference Semblat, Raza, Kyes and Rowe2006; Barfod et al. Reference Barfod, Dalgaard, Pleman, Ofori, Pleass and Hviid2011). The binding of IgM to PfVAR2CSA has been shown to interfere with specific IgG recognition and opsonic phagocytosis of IEs infected with pregnancy-specific isolates ((Barfod et al. Reference Barfod, Dalgaard, Pleman, Ofori, Pleass and Hviid2011) but not other non-pregnancy specific PfEMP-1s (Stevenson et al. Reference Stevenson, Huda, Jeppesen, Laursen, Rowe, Craig, Streicher, Barfod and Hviid2014). IgM has also been implicated in rosetting and strengthening Pf-IE erythrocyte interactions (Stevenson et al. Reference Stevenson, Huda, Jeppesen, Laursen, Rowe, Craig, Streicher, Barfod and Hviid2014) however rosetting is rare in P. falciparum placental isolates (Maubert et al. Reference Maubert, Fievet, Tami, Boudin and Deloron1998; Rogerson et al. Reference Rogerson, Beeson, Mhango, Dzinjalamala and Molyneux2000). There is currently no data on the role of IgM in P. vivax rosetting. Further investigation of the role of IgM in P. falciparum and P. vivax infections is warranted.
The functional roles IgG antibodies require for protection against P. falciparum and P. vivax infection are fairly poorly defined. The predominant isotypes found against P. falciparum placental isolates are IgG1 and IgG3, the dominant isotypes against most malarial antigens (Elliott et al. Reference Elliott, Brennan, Beeson, Tadesse, Molyneux, Brown and Rogerson2005; Megnekou et al. Reference Megnekou, Staalsoe, Taylor, Leke and Hviid2005; Stanisic et al. Reference Stanisic, Richards, McCallum, Michon, King, Schoepflin, Gilson, Murphy, Anders, Mueller and Beeson2009; Richards et al. Reference Richards, Stanisic, Fowkes, Tavul, Dabod, Thompson, Kumar, Chitnis, Narum, Michon, Siba, Cowman, Mueller and Beeson2010), which can function through adhesion-inhibition/invasion-inhibition, phagocytosis, antibody-dependent cell-mediated cytotoxicity and/or complement fixation. Anti-PfVAR2CSA IgG can inhibit adhesion by interfering with the binding of Pf-IEs and CSA or recombinant PfVAR2CSA to CSA (Ricke et al. Reference Ricke, Staalsoe, Koram, Akanmori, Riley, Theander and Hviid2000; Barfod et al. Reference Barfod, Dobrilovic, Magistrado, Khunrae, Viwami, Bruun, Dahlback, Bernasconi, Fried, John, Duffy, Salanti, Lanzavecchia, Lim, Ndam, Higgins and Hviid2010; Khunrae et al. Reference Khunrae, Dahlbäck, Nielsen, Andersen, Ditlev, Resende, Pinto, Theander, Higgins and Salanti2010). Opsonic phagocytosis against CSA-binding parasite isolates have been identified in sera from pregnant women (Keen et al. Reference Keen, Serghides, Ayi, Patel, Ayisi, van Eijk, Steketee, Udhayakumar and Kain2007; Tippett et al. Reference Tippett, Fernandes, Rogerson and Jaworowski2007; Feng et al. Reference Feng, Aitken, Yosaatmadja, Kalilani, Meshnick, Jaworowski, Simpson and Rogerson2009; Ataide et al. Reference Ataide, Hasang, Wilson, Beeson, Mwapasa, Molyneux, Meshnick and Rogerson2010, Reference Ataide, Mwapasa, Molyneux, Meshnick and Rogerson2011; Barfod et al. Reference Barfod, Dobrilovic, Magistrado, Khunrae, Viwami, Bruun, Dahlback, Bernasconi, Fried, John, Duffy, Salanti, Lanzavecchia, Lim, Ndam, Higgins and Hviid2010). There is little information at present on the contribution of anti-PfVAR2CSA IgG to antibody-mediated complement activity, with some indications that excessive innate complement binding is detrimental (Conroy et al. Reference Conroy, Serghides, Finney, Owino, Kumar, Gowda, Liles, Moore and Kain2009, Reference Conroy, Silver, Zhong, Rennie, Ward, Sarma, Molyneux, Sled, Fletcher, Rogerson and Kain2013; Khattab et al. Reference Khattab, Kremsner and Meri2013). Antibody-mediated immune functions against a range of P. falciparum targets are present during pregnancy (Teo et al. Reference Teo, Hasang, Randall, Feng, Bell, Unger, Langer, Beeson, Siba, Mueller, Molyneux, Brown and Rogerson2014), but whether altered immunology during pregnancy alters their magnitude as compared to non-pregnant individuals is unknown. Studies on Pv-IE are severely hindered by the inability to culture P. vivax long-term in vivo.
In non-pregnant populations, clinical immunity is thought to develop more rapidly to P. vivax than P. falciparum as indicated from parasitological data from syphilis malariotherapy patients (Collins and Jeffery, Reference Collins and Jeffery1999a ; Collins et al. Reference Collins, Jeffery and Roberts2004) and from malaria endemic areas whereby the prevalence of P. vivax infection and clinical episodes peaks at younger ages compared to P. falciparum (Maitland et al. Reference Maitland, Williams, Bennett, Newbold, Peto, Viji, Timothy, Clegg, Weatherall and Bowden1996; Smith et al. Reference Smith, Hii, Genton, Muller, Booth, Gibson, Narara and Alpers2001; Mueller et al. Reference Mueller, Widmer, Michel, Maraga, McNamara, Kiniboro, Sie, Smith and Zimmerman2009; Lin et al. Reference Lin, Kiniboro, Gray, Dobbie, Robinson, Laumaea, Schopflin, Stanisic, Betuela, Blood-Zikursh, Siba, Felger, Schofield, Zimmerman and Mueller2010). It is hypothesized that this is due to a reduced immune threshold required to achieve protection against P. vivax compared to P. falciparum or the ability of P. vivax to relapse giving rise to a higher molecular force of infection (Koepfli et al. Reference Koepfli, Colborn, Kiniboro, Lin, Speed, Siba, Felger and Mueller2013). Species-specific differences in the rate of immune acquisition have yet to be reconciled in pregnancy but longitudinal studies show that antibody responses to both P. falciparum and P. vivax antigens during pregnancy are similarly dynamic in response to species-specific Plasmodium spp. exposure (Aitken et al. Reference Aitken, Mbewe, Luntamo, Maleta, Kulmala, Friso, Fowkes, Beeson, Ashorn and Rogerson2010; Fowkes et al. Reference Fowkes, McGready, Cross, Hommel, Simpson, Elliott, Richards, Lackovic, Viladpai-Nguen, Narum, Tsuboi, Anders, Nosten and Beeson2012; Ampomah et al. Reference Ampomah, Stevenson, Ofori, Barfod and Hviid2014b ), lending support to the notion that regular exposure is required to maintain malarial immunity. Interestingly, a recent longitudinal study of antibodies in pregnancy found that antibodies to P. vivax (PvAMA1) were not boosted with successive infections in pregnancy, in contrast with P. falciparum antibodies which were boosted with each exposure (including the homologue PfAMA1) (Fowkes et al. Reference Fowkes, McGready, Cross, Hommel, Simpson, Elliott, Richards, Lackovic, Viladpai-Nguen, Narum, Tsuboi, Anders, Nosten and Beeson2012). This may indicate a difference in immunological memory or recall response between the two species or the much lower parasitaemia densities in P. vivax infections are less efficient in boosting responses. The implications of this lack of boosting for immunity and increased risk of P. vivax in pregnancy and the postpartum period (observed in the same study area) are unknown and further studies are necessary. Furthermore an understanding of antibody dynamics postpartum would help elucidate how pregnancy-favoured antibodies are maintained in between pregnancies with apparent limited exposure to pregnancy-favoured antigens.
Antibody responses postpartum and between pregnancies
The strong link between gravidity and PfVAR2CSA antibodies suggests that antibody responses and immune memory are maintained between pregnancies and postpartum when exposure to PfVAR2CSA is low. This is at odds with the paradigm that frequent exposure is required to develop a long lasting antibody response to malaria and that, in the absence of repeated exposure, immunity is short lived (i.e. weeks) (Kinyanjui et al. Reference Kinyanjui, Bull, Newbold and Marsh2003; Langhorne et al. Reference Langhorne, Ndungu, Sponaas and Marsh2008).
Antibodies are secreted by plasma cells, which can be either short-lived or long-lived (Manz et al. Reference Manz, Hauser, Hiepe and Radbruch2005). Mathematical modelling has demonstrated that separate populations of long and short lived cells can describe the rapid decay of antibodies observed immediately following exposure and the long-lived maintenance of a lower level of antibodies in African children (White et al. Reference White, Griffin, Akpogheneta, Conway, Koram, Riley and Ghani2014). This is reflected in studies that have investigated antibody longevity. Estimates in individuals’ shortly after a drug treated acute episode of malaria typically find short P. vivax and P. falciparum antibody half-lives (6 to 52 days) (Soares et al. Reference Soares, da Cunha, Silva, Souza, Del Portillo and Rodrigues1999; Kinyanjui et al. Reference Kinyanjui, Conway, Lanar and Marsh2007) whereas studies investigating long-term decay of antibodies in uninfected individuals have estimated longer P. vivax and P. falciparum antibody half-lives in excess of 5 years (Drakeley et al. Reference Drakeley, Corran, Coleman, Tongren, McDonald, Carneiro, Malima, Lusingu, Manjurano, Nkya, Lemnge, Cox, Reyburn and Riley2005; Wipasa et al. Reference Wipasa, Suphavilai, Okell, Cook, Corran, Thaikla, Liewsaree, Riley and Hafalla2010). Additionally, antibodies have been detected in individuals who have not been exposed to either species in over 5 years (Luby et al. Reference Luby, Collins and Kaiser1967; Druilhe et al. Reference Druilhe, Pradier, Marc, Miltgen, Mazier and Parent1986; Braga et al. Reference Braga, Fontes and Krettli1998; Wipasa et al. Reference Wipasa, Suphavilai, Okell, Cook, Corran, Thaikla, Liewsaree, Riley and Hafalla2010; Moncunill et al. Reference Moncunill, Mayor, Jimenez, Nhabomba, Casas-Vila, Puyol, Campo, Manaca, Aguilar, Pinazo, Almirall, Soler, Munoz, Bardaji, Angov, Dutta, Chitnis, Alonso, Gascon and Dobano2013; Ndungu et al. Reference Ndungu, Lundblom, Rono, Illingworth, Eriksson and Farnert2013).
Little is known about antibody longevity in pregnancy and postpartum. A study in a low transmission co-endemic area of Thailand found that P. vivax and P. falciparum merozoite antibody response half-lives calculated during pregnancy were shorter than that calculated for PfVAR2CSA responses and was longer in those who had been exposed (0·8–7·6 years for merozoite antigens vs 57·6–142 years for VAR2CSA (Fowkes et al. Reference Fowkes, McGready, Cross, Hommel, Simpson, Elliott, Richards, Lackovic, Viladpai-Nguen, Narum, Tsuboi, Anders, Nosten and Beeson2012)). While these estimates should not be directly extrapolated from pregnancy into the postpartum period, recent evidence from cohorts of pregnant and postpartum women provide further evidence for long-term antibody maintenance postpartum. A study in Mozambique found that women 1–2 months postpartum had a median level of antibodies against the surface of a placental parasite line (CS2) comparable (3·3% higher) to women at delivery (Mayor et al. Reference Mayor, Serra-Casas, Rovira-Vallbona, Jimenez, Quinto, Sigauque, Dobano, Bardaji, Alonso and Menendez2012b ). A study in Malawi found that at 6 months postpartum 72·3% of women were still seropositive for antibodies to CS2 surface antigens (Aitken et al. Reference Aitken, Mbewe, Luntamo, Maleta, Kulmala, Friso, Fowkes, Beeson, Ashorn and Rogerson2010). More than 40% of women in Ghana who had not been pregnant in 1–6 years remained seropositive to PfVAR2CSA suggesting that there is some level of antibody response maintenance in the relative absence of exposure (Ampomah et al. Reference Ampomah, Stevenson, Ofori, Barfod and Hviid2014a , Reference Ampomah, Stevenson, Ofori, Barfod and Hviid b ). Importantly they also demonstrated that the level of PfVAR2CSA specific IgG-secreting B cells did not depend on time since last pregnancy or number of pregnancies suggesting that PfVAR2CSA B cell memory is stably maintained in the absence of exposure (Ampomah et al. Reference Ampomah, Stevenson, Ofori, Barfod and Hviid2014a ).
Explanations for an apparent increased longevity of PfVAR2CSA responses are unclear, but could relate to a large sequestered parasite load providing a strong and sustained antigenic stimulus or be reflective of boosting as a result of undetected placental infection during pregnancy, or the greater immune longevity that appears to occur in adults. It is thought that there is limited or infrequent exposure to PfVAR2CSA prior to the first pregnancy, in contrast to most malarial antigens, which are generally encountered throughout life. However, studies have shown that antibodies to PfVAR2CSA can be acquired in childhood (Beeson et al. Reference Beeson, Ndungu, Persson, Chesson, Kelly, Uyoga, Hallamore, Williams, Reeder, Brown and Marsh2007). This would influence subsequent response to PfVAR2CSA in pregnancy such that antibody levels may be boosted more rapidly upon re-exposure and be better maintained. Younger individuals tend to have shorter half-lives than older individuals (Taylor et al. Reference Taylor, Egan, McGuinness, Jepson, Adair, Drakely and Riley1996; Akpogheneta et al. Reference Akpogheneta, Duah, Tetteh, Dunyo, Lanar, Pinder and Conway2008) and the age of primary exposure to an antigen may affect the longevity of immune responses to that antigen. The detection of PfVAR2CSA antibodies in women who have not been pregnant in years and the observed persistence of PfVAR2CSA specific IgG-secreting B cells supports the hypothesis that PfVAR2CSA antibodies acquired in earlier pregnancies are maintained to protect subsequent pregnancies against P. falciparum. However, further longitudinal studies of women followed after pregnancy are required to assess this.
Whether there are P. vivax antigens that are specifically upregulated in pregnancy and whether antibodies against P.vivax are maintained postpartum and throughout pregnancies is unknown. Furthermore, the extent to which the immunological changes that occur during pregnancy and postpartum influence the risk of P. vivax relapse is unclear. If cell-mediated immunity is important in controlling liver infection, as it is in mouse models (reviewed in Doll and Harty, Reference Doll and Harty2014), then the dampening of cell-mediated immunity would have a greater impact on P. vivax than P. falciparum due to the former parasites relatively longer period of residence in the liver. More immunological research is needed to further understand how immunity relates to the differential risk of P. falciparum and P. vivax postpartum.
Box I. Research priorities
-
• Further epidemiological studies on the risk of P. vivax by pregnancy status and gravidity in different populations.
-
• Elucidate the mechanisms by which P. vivax infection during pregnancy contributes to negative maternal and infant outcomes.
-
• Quantify the clinical relevance of putative in vivo P. vivax binding to the placenta.
-
• Conduct longitudinal studies in pregnant women that incorporate humoral, cellular and functional immunity against both P. vivax and P. falciparum to quantify their relative contributions towards protection against infection and its course.
-
• Determine the risk of P. vivax and P. falciparum postpartum in different settings, ideally with both pregnant and non-pregnant comparison groups.
-
• Elucidate the immunological mechanisms of altered risk postpartum.
-
• Investigate the modulating effect of pregnancy on cell-mediated immunity in a malaria context.
-
• Discover the mechanisms that underpin the cause of P. vivax relapse.
-
• Identify immune correlates of protection against P. vivax relapse.
-
• Determine the contribution of cross-species immunity in naturally exposed human populations.
FUTURE DIRECTIONS
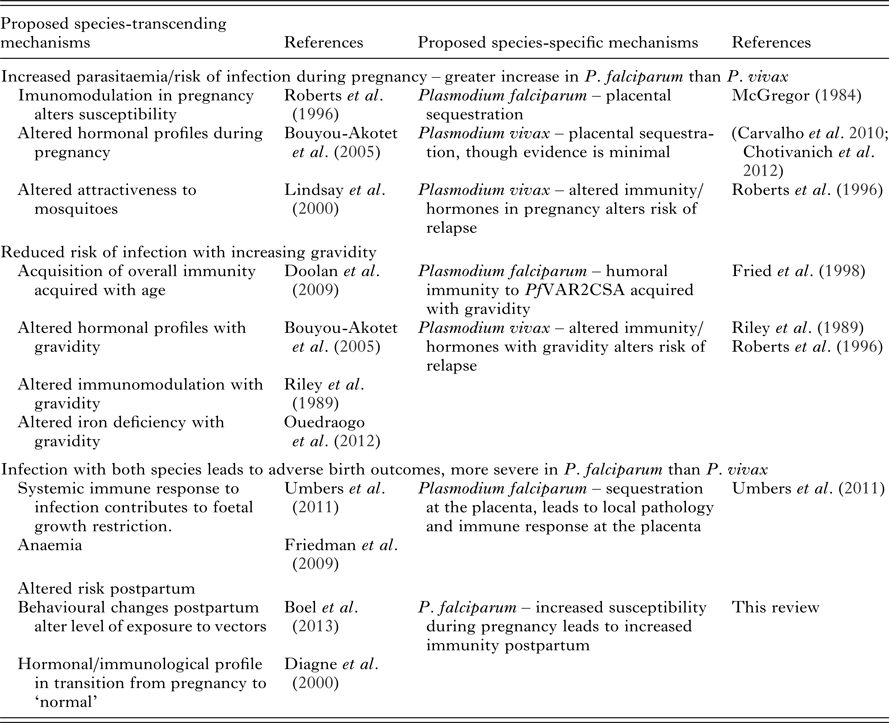
Immunological evidence has helped provide a convincing explanation for the unique epidemiology of P. falciparum in pregnancy. Many questions remain to be answered in relation to P. vivax during pregnancy and the risk of both species postpartum (Table 7 and Box 1). Currently the availability of both immunological and epidemiological evidence pertaining to P. vivax in pregnancy is limited and inconsistent. A more comprehensive understanding of the epidemiology of P. vivax in pregnancy will act as a primer for future studies on the immunology of P. vivax in pregnancy. Ideally, comprehensive longitudinal studies that incorporate measurements of multiple immunological mechanisms would be able to assess the relative contribution of each of these functions towards protection and the observed epidemiology. Whether delivery marks the end of a period of increased risk of malaria is debatable. The epidemiology of the postpartum period remains unclear, with the few studies conducted providing conflicting results. Further epidemiological studies are needed to explore the differential risk of P. falciparum and P. vivax in the postpartum period, preferably in tandem with immunological studies, which may be able to explain the mechanisms underlying the epidemiology.
Table 7. Epidemiological observations of P. falciparum and P. vivax during pregnancy and postpartum and proposed mechanisms
ACKNOWLEDGEMENTS
ARDM is supported by an Australian Postgraduate Award, FJIF is supported by a Future Fellowship from the Australian Research Council, JGB is supported by a NHMRC Senior Research Fellowship. The Burnet Institute is supported by the NHMRC Independent Research Institutes Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support scheme.










