Asthma pathophysiology
Asthma is one of the most common and prevalent health problems worldwide affecting over 300 million individuals. Globally asthma affects 1 to 18 % of the population and it is evident that in both developing and developed countries prevalence of asthma increases as communities adopt modern or ‘Western’ lifestyles, becoming urbanised( Reference Bousquet, Bousquet and Godard 1 ). It has been projected that with urbanisation continuing to increase worldwide, there is likely to be a marked increase in the number of individuals with asthma living within the next two decades( 2 ) and it has been estimated that an additional 100 million individuals worldwide could be potentially affected with asthma by 2025( Reference Koshy, Delpisheh and Brabin 3 , Reference Ronchetti, Villa and Barreto 4 ). Asthma is partly characterised by transient narrowing of the airways( Reference McFadden and Gilbert 5 , Reference Anderson and Holzer 6 ). Occasionally this transient narrowing of airways can occur during exercise, resulting in the identification of exercise-induced bronchoconstriction (EIB)( Reference Rundell, Spiering and Judelson 7 , Reference Suman, Beck and Babcock 8 ). For patients with EIB a brief period of exercise or increase in ventilation triggers airflow obstruction which typically lasts for 30–90 min in the absence of treatment( Reference Anderson and Kippelen 9 , Reference Hallstrand 10 ). Clinical focus for asthma therapy has understandably been on the severe disease state; however, a large number of asthmatics have mild to moderate symptoms( Reference Hallstrand 10 ).
Asthma is a heterogeneous disease with respect to immunopathology, clinical phenotype, response to therapy and natural history. Symptoms can be triggered by a variety of environmental factors and are further exacerbated by the poor adherence to prescribed medication schedules and suboptimal treatment regimens( Reference Barnes 11 , Reference Brightling, Gupta and Gonem 12 ). Thus, the clinical spectrum of asthma is highly variable with airway inflammation being a consistent feature. The pattern of inflammation in asthma is associated with airway hyper-responsiveness (AHR) (clinically measured by histamine or methacholine challenge) which leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night or early morning. These episodes are generally associated with airflow obstruction (bronchoconstriction) within the lungs that is often reversible either spontaneously or with treatment. In most asthmatics, inflammation is largely restricted to the conducting airways but with an increase in disease severity, the inflammatory infiltrate spreads to the small airways and in some cases adjacent alveoli( Reference Kraft 13 ).
Another feature of asthma is the response to triggers such as exercise and allergic sensitisation; the airways recognise common triggers and in turn generate a Th2-type cytokine response to them. Asthma is also found to involve local epithelial, mesenchymal, vascular and neurological events, which direct the Th2 lymphocytes to the lung. Repeated bouts of increased inflammation in asthma may lead to damage to the airway epithelium and subsequent abnormal repair leads to structural changes in the airway walls of asthmatic subjects (collectively referred to as airway remodelling)( Reference Hallstrand, Moody and Wurfel 14 , Reference Shifren, Witt and Christie 15 ). Fig. 1 illustrates the role of different cells and mediators involved in the asthmatic inflammatory response to a trigger. There is recruitment and activation of leucocytes in response to the trigger. Following activation, the cells work actively to neutralise the antigens, subsequently they repair any damage; finally, the cells are removed with resolution of the inflammatory process( Reference Rasmussen, Reinert and Paludan 16 , Reference Widgerow 17 ).
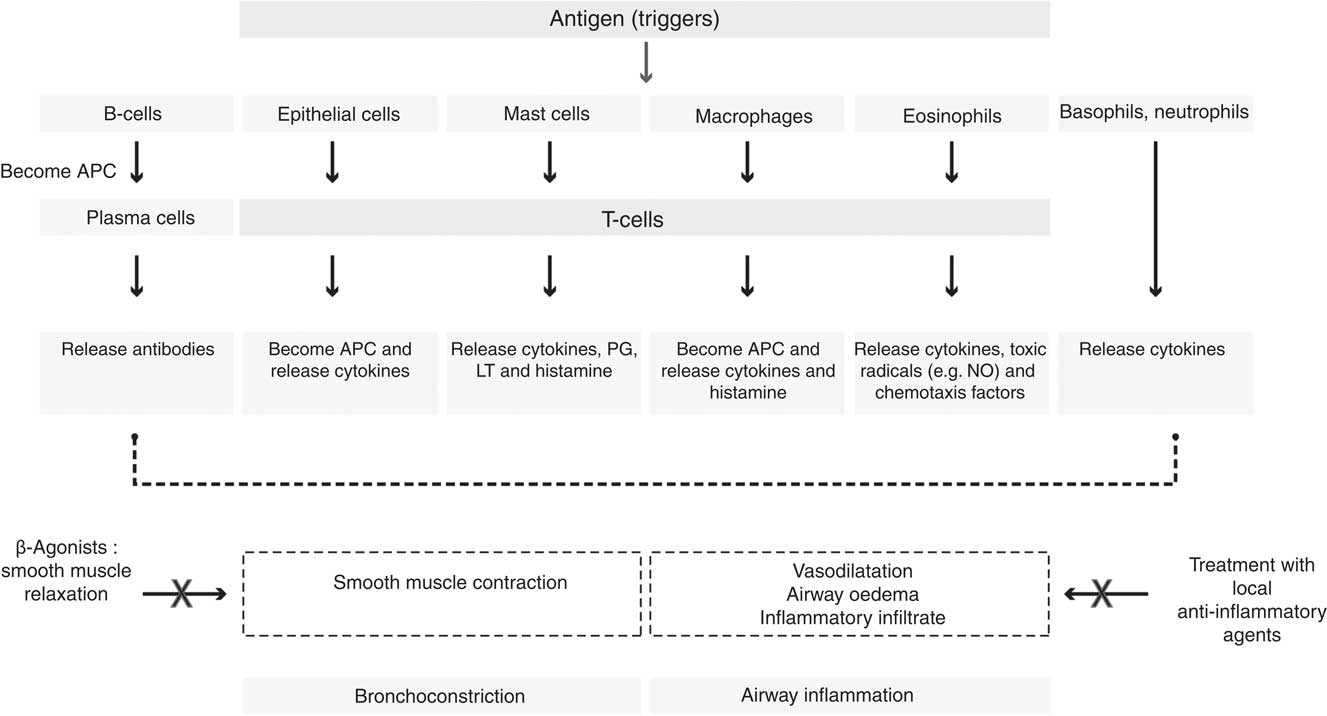
Fig. 1 Cells and mediators involved in the asthmatic inflammatory response. APC, antigen-presenting cells; LT, leukotriene.
In asthmatics, there is an increased production of a series of cytokines and chemokines such as TNF, IL-4, IL-5, IL-6, IL-8, IL-12 and IL-13( Reference Barnes 18 – Reference Canöz, Erdenen and Uzun 20 ). There is also a release of arachidonic acid (AA)-derived eicosanoids including prostaglandins and leukotrienes (LT; such as LTC4, LTD4 and LTE4) that are found to be potent vasoconstrictors of human airways; these mediators affect microvascular and bronchial dilation, increase AHR, and have been implicated in the pathogenesis of asthma( Reference Broughton, Johnson and Pace 21 , Reference De Caterina and Basta 22 ). Furthermore the prostaglandins exert strong effects on airway function and there is increased expression of the inducible form of cyclo-oxygenase (COX-2) in asthmatic airways; however, the inhibition of their synthesis with COX inhibitors, such as aspirin or ibuprofen, may demonstrate some effect in reducing symptoms in some but not all asthmatics( Reference Barnes 23 ). PGD2 is a bronchoconstrictor produced predominantly by mast cells. Deletion of the PGD2 receptors in mice significantly inhibits inflammatory responses to allergens and inhibits AHR, suggesting that this mediator may be important in asthma( Reference Bloemen, Verstraelen and van den Heuvel 24 , Reference Swedin, Neimert-Andersson and Hjoberg 25 ).
NO is an endogenous regulatory molecule involved in the pathogenesis of asthma. The synthesis of NO in the airways is mediated by a family of enzymes that are collectively called NO synthases (NOS)( Reference Deykin and Kharitonov 26 , Reference Palmer, Ferrige and Moncada 27 ). The NOS can exist as constitutive isoforms (cNOSs) including endothelial NOS (eNOS) and neural NOS (nNOS) or as an inducible isoform (iNOS)( Reference Puckett and George 28 , Reference Ricciardolo 29 ). The inducible isoform (iNOS) is found in the epithelium of the bronchial wall, which is the key source for elevated levels of fractional exhaled NO (FeNO) seen in asthmatics. Alveolar concentration of FeNO is usually low except in diseases such as alveolititis( Reference Lehtimaki, Kankaanranta and Saarelainen 30 ). During an exhalation process, the air from alveolar compartments moves to the bronchial compartment; thus, the NO from the bronchial wall diffuses inside the airway lumen leading to an increase in NO levels in the expired air. Increased concentration of NO is observed when exhalation is slow, as this allows a longer time for the NO to diffuse in the airways( Reference Deykin and Kharitonov 26 , Reference Kharitonov, Chung and Evans 31 ). Patients with asthma usually exhibit 2- to 3-fold higher levels of NO in expired air compared with healthy adults( Reference Saleh, Ernst and Lira 32 ). Healthy adults can exhibit values between 5–35 parts per billion at the standard flow rate of 50 ml/s( Reference Saleh, Ernst and Lira 32 , Reference Zitt 33 ). The elevated levels of NO in expired air in asthmatics are indicative of eisonophilic inflammation; however, a direct pathogenic role of this gas in asthma is yet to be fully established( Reference Jatakanon, Lim and Kharitonov 34 – Reference Sandrini, Taylor and Thomas 36 ).
Diagnosis and classification
Diagnosis of asthma is predominantly determined by measuring symptoms (episodic breathlessness, wheezing, cough and chest tightness), peak expiratory flow (PEF) and other parameters of spirometry( Reference Miller, Hankinson and Brusasco 37 , Reference Miller, Crapo and Hankinson 38 ). Episodic symptoms after allergen exposures, seasonal variability and a positive family history and atopy are useful diagnostic guidelines( Reference Levy, De Sanctis and Devchand 39 ). The presentation of asthma can vary from person to person and asthma may be intermittent with mild to severe episodes requiring treatment( Reference Chung and Adcock 40 , Reference Reddel, Taylor and Bateman 41 ). Asthmatics may experience intermittent symptoms for a period of a few minutes and in some cases this may be life threatening. Asthma subgroups have been characterised to address the complexities of the disease and for better understanding of the symptoms. A phenotype or subgroup identifies the clinically relevant properties of the disease, but does not show the direct relationship with disease aetiology and pathophysiology( Reference Agache, Akdis and Jutel 42 , Reference Wenzel 43 ). Table 1 shows a classification of asthma into main subgroups including early-onset allergic, eosinophilic, aspirin triggered, exercise induced, obesity related and asthma related to airflow obstruction.
Table 1 Asthma subgroups

FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; AHR, airway hyper-responsiveness.
The main diagnostic technique for asthma is an assessment of pulmonary function to identify airflow limitation; this method has been used to demonstrate the reversibility of lung function abnormalities. The measurement of pulmonary function is combined with an assessment of symptoms such as dyspnoea and wheezing; together they provide reliable information about the different aspects of asthma control( Reference Miller, Hankinson and Brusasco 37 , Reference Miller, Crapo and Hankinson 38 , Reference Kerstjens, Brand and de Jong 44 ). Spirometry is the primary method for pulmonary function testing. The most important aspects of spirometry include forced vital capacity (FVC), which is the maximum volume of air an individual can expel from the lungs, during expiration made as forcefully and completely starting from full inspiration. The forced expiratory volume in 1 s (FEV1) is the maximum volume of expired air volume in the first 1 s of a FVC manoeuvre. PEF is the maximum expiratory flow achieved from a maximum forced expiration, starting from the point of maximal lung inflation, and is recorded using a PEF meter.
EIB is a subgroup of asthma that affects up to 90 % of individuals with asthma( Reference Rundell, Spiering and Judelson 7 , Reference Anderson and Kippelen 9 , 45 ). EIB has also been reported in non-asthmatics including schoolchildren, armed force recruits, and athletes and approximately 10 % of the healthy population show symptoms of EIB at some point during their lives( 45 ). Based on the wide prevalence of EIB in both asthmatic and healthy populations, EIB is considered a limiting factor for physical activity for a large number of individuals. The observed symptoms of EIB include coughing, wheezing, chest tightness, shortness of breath or excess mucus production following exercise. However; self-reported symptoms are neither reliable nor specific for EIB. Approximately 50 % of elite athletes report symptoms related to EIB with exercise. However, they do not have EIB, while 50 % of those who report no symptoms for EIB will test positive on the exercise challenge test for EIB( Reference Parsons, Kaeding and Phillips 46 , Reference Rundell, Im and Mayers 47 ). Thus, it is essential to support the diagnosis of EIB by performing a relevant exercise challenge test( Reference Rundell and Sue-Chu 48 ). An exercise challenge test involves exercising at increasing intensities until a heart rate response of 85–90 % of estimated maximal is achieved. The exercise challenge test primarily involves recording a post-exercise reduction in FEV1 of 10 to 15 % of the pre-exercise value. The value for FEV1 may start falling during exercise, however; the lowest value will usually be measured 5–12 min after the end of the exercise challenge test. The reduction in FEV1, if severe, is linked to a decrease in oxygen saturation with hyperinflation of the lungs( Reference Anderson and Kippelen 9 ). In adults, a ≥10 % decline in FEV1 at any time point within 30 min of ceasing exercise is considered a diagnostic of EIB. The decline in FEV1 is usually maintained over two time points and any unsustained decline may be due to respiratory muscle fatigue and does not indicate EIB( 49 , Reference Crapo, Casaburi and Coates 50 ).
Current asthma therapies: challenges and scope for non-pharmacological interventions
Despite effective treatments for asthma, there remain high mortality and morbidity which have implications for public health services. Current asthma treatments target inflammation in one of two ways (acute rescue remedies v. long-term preventatives). The existing pharmacological therapies together with long- and short-acting β-agonists and corticosteroids have proved effective in asthma management in the majority of patients, but still have issues associated with their use. Tachyphylaxis has complicated the use of β-adrenoreceptor agonists while the known systemic and local side effects of inhaled corticosteroids include osteoporosis and glaucoma( 2 , Reference Barnes 51 ). LT modifiers block bronchoconstrictor and pro-inflammatory activity of cysteinyl LT within the asthmatic airway, and IgE monoclonal therapy for reduction in IgE have been found to be effective in asthma treatment( 52 , Reference Holgate 53 ). There is a current focus on identifying specific therapies that target a single inflammatory mediator and are less likely to have major health side effects( Reference Barnes 51 ). These specific therapies have been suggested to be effective for various subgroups of asthma including those with mild–moderate symptoms including EIB. For particularly EIB, inhaled corticosteroids have been identified as the most effective anti-inflammatory treatment available, aiming at reducing AHR and reducing the severity of symptoms. However, inhaled corticosteroids demonstrate both systemic and local health side effects, which affect the sports/physical activity of individuals. Adrenal suppression at high doses, growth retardation in children and adolescents and reduction in bone density are observed with usage of some inhaled corticosteroids( Reference Carlsen, Anderson and Bjermer 54 , Reference Priftis, Papadimitriou and Gatsopoulou 55 ). The other anti-inflammatory treatments for EIB include LT antagonists, disodium cromoglycate, nedocromile sodium, β2-agonists, and ipratropium bromide which have well-established, long-term negative heath side effects providing an impetus for non-pharmacological therapies among researchers and clinical experts( Reference Millward, Tanner and Brown 56 – Reference Spector and Tan 58 ).
In the UK, the National Health Service and Asthma UK have suggested the use of complementary therapies alongside conventional medication in asthma( Reference Asthma 59 , 60 ). Consequently, there are both therapeutic and consumer-derived interests in identifying potential complementary therapies for asthma. The recognition of the role of complementary therapy in asthma is limited because these approaches have been insufficiently researched and their effectiveness is largely unproven( 2 ). A range of non-pharmacological treatments including physical activity (incorporating a warm-up before and a cool-down period following exercise), performing nasal breathing, avoiding cold weather or environmental allergens, using a face mask or other aid to warm and humidify inhaled air, and modifying dietary intake of n-3 fatty acids, salt and antioxidants have been identified( Reference Mickleborough and Lindley 61 ). However, to date the efficacies of each of these therapies have not been well established and further investigations are required to validate these therapies with conventional standards( Reference Mickleborough and Lindley 61 ).
Exploring the potential of non-pharmacological treatments is important due to the comparatively low risk associated with their use. Since physical activity is a limiting factor for EIB-prone individuals, a change in lifestyle and diet could improve the quality of life of these individuals and help them meet the physical activity requirements proposed by the Department of Health. The present review will discuss the mechanisms underlying the effects of n-3 fatty acids and the relationship between n-3 fatty acids, their derived mediators and respiratory health in asthmatics.
n-3 Fatty acids: structure and metabolism
Fatty acids, both non-esterified and as part of complex lipids, play an important role in metabolism, storage and transport of energy, gene regulation( Reference Rustan and Drevon 62 ) and as necessary components of all cell membranes and have been found to be linked to various diseases( Reference Bagby 63 – Reference Woodside, McKinley and Young 66 ). The characteristics of a fatty acid are dependent on the length of carbon chain and the presence, absence and placement of double bonds between carbon atoms. PUFA have more than one double bond present. PUFA are also known by their shorthand nomenclature, which represents the number of carbon atoms in their chain. The n-3 fatty acids are so called because their first carbon double bond is present at carbon number 3 counting the methyl carbon as carbon number 1 while n-6 are so called as their first carbon double bond is present at carbon number 6( Reference Lunn and Theobald 67 , Reference Calder and Yaqoob 68 ).
Not all fatty acids can be synthesised de novo in mammals, as they cannot insert double bonds before carbon 9 in oleic acid (18 : 1n-9). Specifically, mammals cannot convert oleic acid into linoleic acid (LA; 18 : 2n-6) or LA into α-linolenic acid (ALA; 18 : 3n-3). Since ALA and LA cannot be synthesised de novo, their intake from food sources is important and they are classified as essential fatty acids (EFA). Although mammalian cells do not have the ability to synthesise LA and ALA, once these EFA are obtained from the diet they can be metabolised into physiologically active compounds via the introduction of extra double bonds and chain elongation through the processes of desaturation and elongation( Reference Ratnayake and Galli 69 , Reference Wall, Ross and Fitzgerald 70 ) (Fig. 2). Furthermore, LA appears to be an EFA not only because of an immediate cellular function, but because it is the precursor of AA (20 : 4n-6) that has numerous essential functions. Similarly, the importance of dietary ALA is that it is the precursor of EPA and DHA which are found in the phospholipids of cell membranes (Fig. 2). EPA/DHA have a range of biological functions, with EPA demonstrating anti-inflammatory effects while DHA is recognised to be important for visual and neurological functions and vital for the growth and development of premature and newborn infants( Reference Calder and Yaqoob 68 ). Additionally, both EPA and DHA have been found to have roles in the resolution of inflammation( Reference Calder and Yaqoob 68 ). Subsequently, AA, EPA and DHA have been suggested to be termed as ‘conditionally essential’( Reference Burke, Ling and Forse 71 , Reference Kidd 72 ).
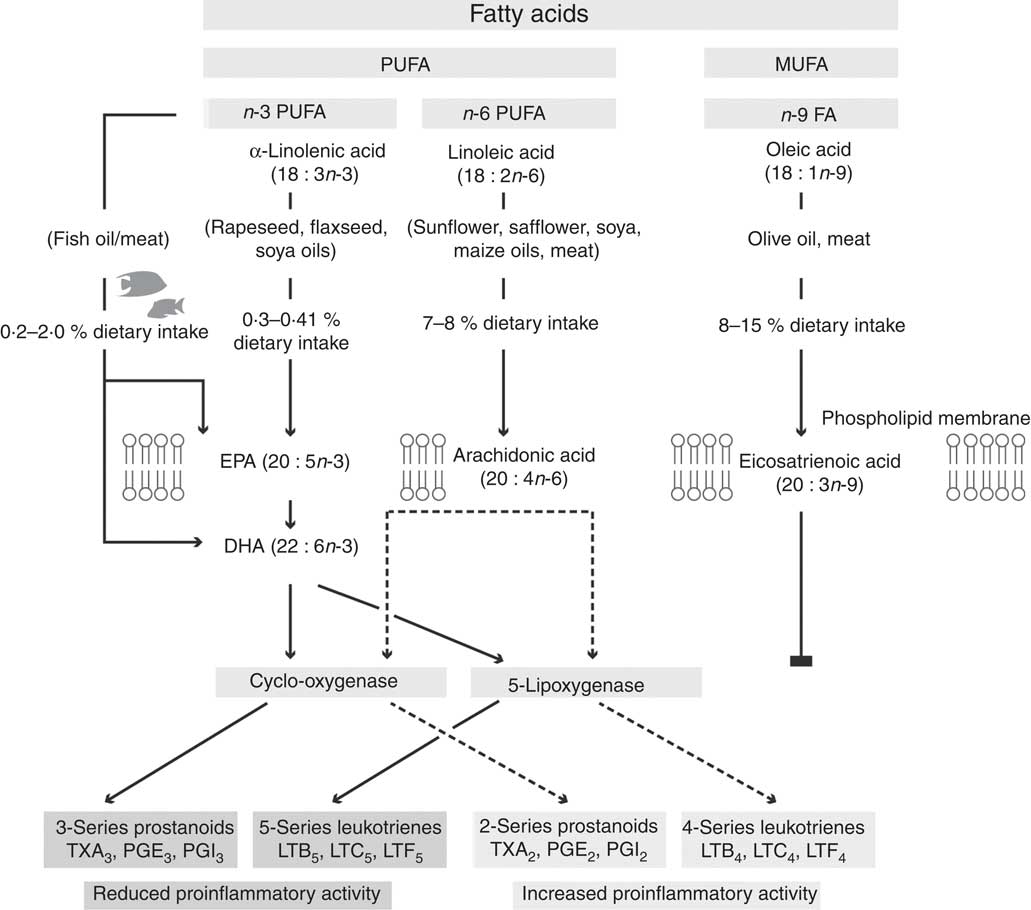
Fig. 2 Proposed pathway for the metabolism of n-6 and n-3 fatty acids, showing the production of n-3 and n-6 fatty acid-derived eicosanoids via the cylo-oxygenase and 5-lipoxygenase enzymes. The n-9 fatty acids do not follow the same pathway, and subsequently are hypothesised to not play a role in inflammation. TX, thromboxane; LT, leukotriene.
Fatty acids are important constituents of the phospholipids of all cell membranes and the characteristic fatty acid composition of different cells and tissues is dependent on the availability of different fatty acids as well as the metabolic properties of the cells and tissues( Reference Calder and Yaqoob 68 , Reference Calder 73 ). Once PUFA are incorporated in cell membrane phospholipids they are proposed to be involved in the inflammatory cell response (Fig. 2)( Reference Calder 74 ). Since tissue and blood fatty acid profiles have been shown to be modified by dietary intake, they have been used as compliance markers for dietary supplementation studies( Reference Garcia-Larsen, Luczynska and Kowalski 75 – Reference Jeppesen, Jørgensen and Bjerregaard 77 ). However, it has yet not explicitly been demonstrated that phospholipid or total lipid content of cells can be used as a marker of dietary intake whilst the differences between different cell populations are confounding variables in the literature. In adults, there are a number of epidemiological and supplementation studies evaluating the effects of dietary n-3 PUFA intake on asthma. In a population-based study (n 13 820; ages 42.2 (sd 11·2) years), individual fatty acid intakes (estimated by FFQ) were analysed and related to symptoms of asthma( Reference McKeever, Lewis and Cassano 78 ). The results demonstrate that a high intake of n-3 fatty acids did not protect against asthma; however, higher consumption of several n-6 fatty acids including LA and AA were found to be associated with a significant reduction in FEV1, particularly in smokers( Reference McKeever, Lewis and Cassano 78 ). In another population-based study of Respiratory Health in Northern Europe, 16 187 subjects aged 23–54 years completed a postal FFQ and it was reported that a minimum level of weekly fish intake (>1 serving per week) in adulthood was associated with protection against asthma( Reference Laerum, Wentzel-Larsen and Gulsvik 79 ). Participants who never had fish were found to have an increased risk for asthma( Reference Laerum, Wentzel-Larsen and Gulsvik 79 ). It should be highlighted that the inconsistencies from the population-based studies are possibly due to the methods used. Most studies that have found associations between fatty acid intake and asthma have used indirect measurements of fatty acid intake including FFQ or other dietary recall methods( Reference Woods, Walters and Raven 80 ).
The presence of n-3 fatty acids in the phospholipids of plasma, erythrocytes and even whole blood has been used as a marker for compliance in various supplementation studies. In addition, incorporation of fatty acids in inflammatory cells/membranes has been studied in inflammatory disease states( Reference Woods, Walters and Raven 80 – Reference Stenius-Aarniala, Aro and Hakulinen 83 ). Thus, it can be argued that studies using a direct marker of fat intake may provide a reliable method for evaluating the relationship between asthma and dietary n-3 PUFA. Some but not all studies show a possible agreement between dietary FFQ and plasma fatty acid levels; in a study by Woods et al., it was demonstrated that n-3 PUFA and the n-6:n-3 ratio in plasma phospholipids were not consistently associated with asthma or atopy( Reference Woods, Raven and Walters 84 ). The only positive association with current asthma was found with dihomo-γ-linolenic acid in plasma phospholipids. In this study, there was a good agreement between the dietary FFQ estimated fatty acid intake and the plasma fatty acid levels( Reference Woods, Raven and Walters 84 ). The Global Allergy and Asthma European Network of Excellence (GA2LEN) has shown that a reasonable association exists between estimates of dietary n-3 PUFA and total plasma phospholipid composition using the GA2LEN FFQ within the European countries that took part in the study( Reference Garcia-Larsen, Luczynska and Kowalski 75 ). In supplementation studies, while dietary estimates provide information about the dietary behaviour of individuals, the total cell or phospholipid content of fatty acids can be used as a reliable marker of incorporation.
n-3 Fatty acids and their lipid derivatives in the inflammatory process
n-3 Fatty acid and arachidonic acid derivatives produced by cyclo-oxygenase and lipoxygenase pathways
Eicosanoids are oxidised derivatives of twenty-carbon fatty acids and include prostaglandins, thromboxanes, LT and lipoxins (LX). The membrane phospholipids are the initial substrates for eicosanoid synthesis and due to the abundance of AA in phospholipids of inflammatory cells, AA is considered the major substrate for eicosanoid synthesis( Reference Calder 85 – Reference Rees, Miles and Banerjee 87 ). One of the mechanisms for the action of n-3 PUFA is the altered pattern of lipid mediator synthesis( Reference Tvrzicka, Kremmyda and Stankova 88 ). The metabolites from EPA and AA form the basis for regulatory signals and the eicosanoid synthesis involves PUFA mobilisation from the cell membrane by various phospholipase enzymes, most notably phospholipase A2. Following mobilisation; the free AA or EPA/DHA acts as a substrate for eicosanoid production via the COX and lipoxygenase (LOX) pathways. Prostaglandins, prostacylins and thromboxanes are formed by the action of COX while LT and hydroxy fatty acids are formed by the action of LOX enzymes. The eicosanoids produced from the two families of fatty acids vary in biological activity. AA is one of the most tightly regulated fatty acids in cell membrane phospholipids as it affects the way cells behave, and its actions have far-ranging effects( Reference Calder 85 , Reference Seeds and Bass 89 ). Diets high in LA or AA could potentially result in overactivity of AA-derived eicosanoids which could lead to an overactive immune system which has been hypothesised to cause damage to host tissues, lead to the formation of thrombi, and facilitate inflammatory disorders( Reference Levick, Loch and Taylor 90 – Reference Bagga, Wang and Farias-Eisner 93 ). Despite the pro-inflammatory effects of AA-derived eicosanoids, it is now recognised that not all the metabolites from AA act in the same manner and some metabolites are shown to promote bronchodilation in normal subjects( Reference Walters and Davies 94 ) but may cause constriction in patients with asthma because of activation of reflex cholinergic bronchoconstriction( Reference Pavord and Tattersfield 95 , Reference Tilley, Coffman and Koller 96 ).
EPA-derived eicosanoids produced via cyclo-oxygenase and lipoxygenase pathways
EPA is a competing substrate for COX and LOX enzymes and this competition with AA leads to decreased expression of COX-2 and 5-LOX( Reference Calder 74 , Reference Calder 92 ). This has been suggested as a potential mechanism for the proposed anti-inflammatory benefits of n-3 PUFA. Ordinarily, AA is metabolised by COX into biologically active 2-series prostanoids; however, when EPA is utilised as a COX substrate the resultant prostanoids are of the alternative 3-series which are known to be less pro-inflammatory( Reference Bagga, Wang and Farias-Eisner 93 , Reference Fujitani, Kanaoka and Aritake 97 , Reference Rajakariar, Hilliard and Lawrence 98 ). In an in vitro study by Wada et al. ( Reference Wada, DeLong and Hong 99 ) specificities of prostanoid enzymes and receptors towards EPA-derived and AA-derived prostaglandins were compared. There was a significant decrease in the formation of 2-series prostaglandins via PGHS-2 (prostaglandin endoperoxide H synthase-2) and this was demonstrated to occur only to the extent that AA levels in phospholipids were decreased by EPA replacing AA( Reference Wada, DeLong and Hong 99 ). Approximately two- to three-fold higher activities were observed for AA-derived mediators compared with EPA-derived ones with the different receptors studied. For example, lower potencies were observed for PGE3 compared with PGE2 towards the EP1, EP2, EP3 and EP4 receptors( Reference Wada, DeLong and Hong 99 ). In a separate in vitro study it has been demonstrated that EPA-derived PGD3 antagonises the effect of PGD2-mediated migration of neutrophils across endothelial cells( Reference Tull, Yates and Maskrey 100 ). These studies directly comparing the effect of EPA- and AA-derived lipid mediators provide some evidence to confirm the competition between n-6 and n-3 fatty acids for the production of eicosanoids and go some way towards identifying the possible mechanisms involved. It has also been shown that 5-series LT can be produced in the macrophages of n-3 fatty acid-fed mice( Reference Wall, Ross and Fitzgerald 70 ) and in the neutrophils of human subjects supplemented with n-3 fatty acids for several weeks( Reference Chapkin, Akoh and Lewis 101 – Reference Sperling, Benincaso and Knoell 103 ). LTB5 derived from EPA has been shown to be 10- to 100-fold less potent as a neutrophil chemotactic agent than AA-derived LTB4; thus LTB5 is a much weaker inducer of inflammation( Reference Wall, Ross and Fitzgerald 70 , Reference Lee, Hoover and Williams 102 ). In an n-3 fatty acid supplementation study using EIB participants, it was found that there was a significant reduction in LTB4 and significant increase in LTB5 level in activated polymorphonulcear cells by the end of 3 weeks of supplementation and this reduced inflammatory effect was accompanied by an improvement in post-exercise EIB symptoms( Reference Mickleborough, Lindley and Ionescu 57 ).
The modified cell membrane phospholipid fatty acid content with n-3 PUFA supplementation facilitates the formation of ‘lipid rafts’ which have been studied in T cells. These rafts are formed by the movement of receptors, accessory proteins, and enzymes within the plane of the cell membrane to co-localise into signalling platforms( Reference Katagiri, Kiyokawa and Fujimoto 104 , Reference Yaqoob 105 ). These rafts in turn influence the activity of membrane proteins including receptors, transporters, ion channels and cell signalling enzymes; these result in the transfer of intracellular signals into the cytosol( Reference Miles, Allen and Calder 106 ). Based on the evidence from cell-culture and animal-feeding studies it has been shown that n-3 PUFA supplementation modifies raft formation in T cells, which in turn impairs the signalling mechanism of these cells( Reference Stulnig, Berger and Sigmund 107 , Reference Stulnig, Huber and Leitinger 108 ). Thus, the exposure of T cells to n-3 fatty acid supplementation can alter the chemical structure of rafts which can consequently affect their function( Reference Fan, Ly and Barhoumi 109 , Reference Fan, McMurray and Ly 110 ). Additionally, the supplementation of n-3 PUFA has been reported to affect cell signalling pathways either by altering the expression and activity of membrane receptors or by modifying the expression of genes by the activation of transcription factors such as NF-κB and PPAR-α( Reference Michaud and Renier 111 – Reference Tai, Corella and Demissie 113 ).
Evidence from n-3 intervention trials in asthma
The adult n-3 fatty acid intervention trials in the last two decades have provided a contradictory picture of efficacy with respect to FEV1 or PEF. Table 2 summarises the main intervention trials with primary outcomes. Kirsch et al. ( Reference Kirsch, Payan and Wong 114 ) compared a high-dose n-3 PUFA supplementation (4 g EPA/d; n 6) with a low dose (0·1 g/d; n 6) for 8 weeks on asthmatics in a small study (n 12; aged 42–73 years) and found there was no difference in FEV1 or symptom scores between the two groups( Reference Kirsch, Payan and Wong 114 ). There was also no difference in the lung function determined by PEF between the two groups before the start of supplementation( Reference Kirsch, Payan and Wong 114 ). Hodge et al. reported no change in lung function values in asthmatic children (n 45; aged 8–12 years); however, there was a reduction in TNFα production (by cultured peripheral blood mononuclear cells) compared with baseline; however, the magnitude of change between groups was not significant( Reference Hodge, Salome and Hughes 115 ). PEF has been reported in some studies as a marker for lung function. Emelynov et al. showed a significant increase in morning PEF with 8 weeks of supplementation with a low dose of n-3 fatty acids (50 mg EPA+DHA per d) in mild–moderate atopic asthmatics (n 46; aged 18–56 years)( Reference Emelyanov, Fedoseev and Krasnoschekova 116 ). In addition, Surette et al. reported a significant improvement in quality of life and asthma management scores (including symptoms) assessed by questionnaires after 3 weeks of supplementation (0·5 g EPA+0·75 g DHA per d; n 65, mild–moderate asthmatics)( Reference Surette, Stull and Lindemann 117 ). Conversely, in two cross-over trials there was no significant change in PEF after 10 weeks of n-3 fatty acid supplementation with >2g EPA+DHA per d( Reference Stenius-Aarniala, Aro and Hakulinen 83 , Reference McDonald, Vecchie and Pierce 118 ). Overall, some studies and our knowledge of the physiological action of n-3 fatty acids suggest that we should potentially see effects of supplementation on lung function; the lack of consistent effect of n-3 fatty acid supplementation on FEV1 or PEF could be attributed to the heterogeneity between the studies. These studies have used a range of doses, as low as 50 mg to >3 g of EPA/DHA per d and have studied different subgroups of asthmatics such as mild–moderate, severe and atopic populations.
Table 2 Relevant trials of n-3 fatty acid supplementation in asthma

RCT, randomized controlled trial; PEF, peak expiratory flow; LT, leukotriene; Tx, thromboxane; PFT, pulmonary function test; AHR, airway hyper-responsiveness; FeNO, fractional exhaled NO; CystLT, cysteinyl LT; ATS, American Thoracic Society; FEV1, forced expiratory volume in 1 s; GLA, γ-linolenic acid; EIB, exercise-induced bronchoconstriction.
In a study by Schubert et al. (n 23; atopic asthma) dietary supplementation with either an n-3 PUFA-enriched fat blend (0·69 g/d, comprising 450 mg EPA and 180 mg DHA per d; twelve participants) or placebo (thirteen participants) for 5 weeks was provided( Reference Schubert, Kitz and Beermann 119 ). After 3 weeks of supplementation, the participants underwent two allergen challenge tests in the remaining 2 weeks of supplementation. FeNO was significantly lower in the n-3 PUFA group (P=0·01); though the levels of FeNO increased during allergen exposure in both groups, the mean values were 5-fold lower in the n-3 PUFA group. No differences were observed between the asthmatic and control groups with regards to asthma symptoms, FEV1 or the allergen dose required to induce deterioration of lung function challenge. Furthermore, compliance was monitored by plasma and erythrocyte cell membrane fatty acid composition and it was found that 2 weeks of supplementation led to a 3-fold higher value of EPA in the n-3 PUFA group compared with placebo and these levels were maintained till the end of supplementation in plasma and erythrocyte cell membranes( Reference Schubert, Kitz and Beermann 119 ).
In a double-blind, placebo-controlled pilot study, a 2-week supplementation with n-3 fatty acids (dose: 0·9 g EPA and 0·65 g DHA/d; n 20) showed no changes in FeNO levels, FEV1 or asthma quality-of-life questionnaires( Reference Moreira, Moreira and Delgado 120 ). However, this study was not well controlled as the participants were on their regular medication of inhaled corticosteroids, which confounds the effect of n-3 PUFA supplementation on pulmonary function and other outcomes. Furthermore, the low dose and duration of supplementation may be a reason why no effect of n-3 fatty acid supplementation was observed. In addition, the participants in this study had stable asthma following their corticosteroid usage and their FeNO levels were not significantly elevated (28 parts per billion) compared with healthy individuals (25 parts per billion)( Reference Moreira, Moreira and Delgado 120 ). Due to these reasons, the exact relationship of n-3 PUFA supplementation and FeNO was difficult to evaluate.
Asthmatics have a reliance on pharmacological medication and despite the significant advancement in asthma medication during the last two decades the treatments are still far from ideal( 2 , Reference Mickleborough and Lindley 61 ). Some of the earlier intervention trials have not shown any significant changes to reliance on medication( Reference McDonald, Vecchie and Pierce 118 , Reference Arm, Horton and Mencia-Huerta 121 ). Hodge et al. ( Reference Hodge, Salome and Hughes 115 ) have shown a significant reduction in medication use in asthmatic children after 9-month supplementation with 1·2 g EPA+DHA per d( Reference Hodge, Salome and Hughes 115 ), while Mickleborough et al. have reported that bronchodilator use was significantly reduced during the last 2 weeks of n-3 fatty acid supplementation (3·2 g EPA+2·2 g DHA per d) in EIB-prone adults( Reference Mickleborough, Lindley and Ionescu 57 ). Furthermore, it has been reported that there are improvements in self-reported asthma status and bronchodilator use in subjects consuming an n-3 emulsion (1 g EPA+1·5 g γ-linolenic acid per d) for 4 weeks compared with a placebo( Reference Surette, Stull and Lindemann 117 ). The authors have also reported results from another trial showing an improvement in the asthma quality of life and asthma control based on non-validated questionnaires (primarily based on bronchodilator usage)( Reference Surette, Stull and Lindemann 117 ).
Chronic inflammation is associated with AHR, which is responsible for recurrent episodes of wheezing, breathlessness, chest tightness and coughing among asthmatics. The majority of intervention studies in children show inconsistencies when reporting effects of n-3 fatty acids on AHR( Reference Thien, De Luca and Woods 122 ). There is a need for further studies in children to understand the effect of n-3 fatty acid supplementation in asthma. Studies( Reference Arm, Horton and Mencia-Huerta 121 , Reference Thien, Mencia-Huerta and Lee 123 ) have reported AHR in terms of the provocation dose of histamine required to produce a 35 % fall in specific conductance and showed no effect of n-3 fatty acid supplementation on AHR. In children, Nagakura et al. ( Reference Nagakura, Matsuda and Shichijyo 124 ) reported AHR as the provocative concentration of acetylcholine causing a 20 % fall in FEV1 for each subject and saw a reduction in acetylcholine responsiveness in the n-3 fatty acid group but not in the control group( Reference Nagakura, Matsuda and Shichijyo 124 ). Schubert et al. ( Reference Schubert, Kitz and Beermann 119 ) reported a reduction in AHR after an allergen challenge with n-3 fatty acid supplementation; however, this change failed to reach significance( Reference Schubert, Kitz and Beermann 119 ).
Overall the evidence from in vitro and in vivo studies shows that n-3 PUFA supplementation has the potential for inhibiting T cell proliferation and production of cytokines. Inhibition of T cell responses has been observed with higher dosage of n-3 fatty acids while this effect is not observed at low n-3 fatty acid levels. These inconsistencies may be related to differences in subject characteristics including age, sex, heath, diet, differences in study design (dose and duration) as well as experimental methods (cell preparation, cell culture, cytokine assays). In conclusion, there is evidence to suggest that n-3 fatty acids may modulate T cell response and functions independently of eicosanoid production( Reference Wallace, Miles and Evans 125 , Reference Mickleborough, Murray and Ionescu 126 ). Furthermore, increasing phospholipid EPA:AA ratios in inflammatory cells with dietary n-3 fatty acid supplementation is likely to be one of the mechanisms that can potentially facilitate the production of weaker eicosanoids that may exhibit anti-inflammatory effects. Thus, there are different mechanisms for the immunomodulatory action of n-3 PUFA, which require further verification in in vivo human studies.
New class of lipid mediators in resolution of inflammation
In the last decade, several lipid mediators have been identified that have a potential role in the resolution of inflammation. Towards the end of an inflammatory process, there is neutralisation and elimination of pathogens, followed by removal of cellular components to prevent excessive tissue damage( Reference Calder 92 , Reference Nathan and Ding 127 ). The mechanism of resolution is continuous with a decrease in the number of inflammatory cells, there is a reduction in the levels of pro-inflammatory cytokines and eicosanoids ‘switch’ from being inflammatory in nature (LT, PG, etc.) to anti-inflammatory or specialised pro-resolving mediators such as LX, resolvins (Rv), protectins (PD) and maresins( Reference Serhan, Chiang and Van Dyke 128 ) (Fig. 3). These mediators have the potential to control the duration and magnitude of inflammation( Reference Serhan, Chiang and Van Dyke 128 , Reference Serhan 129 ). These Rv, PD and maresin mediators are produced from n-3 PUFA (EPA and DHA). The accessibility, affordability and lack of health side effects related to n-3 fatty acid supplementation have generated interest in these potent mediators for research studies in human subjects with or without inflammatory diseases( Reference Calderon Artero, Champagne and Garigen 130 ). Serhan et al. ( Reference Serhan and Petasis 131 – Reference Serhan 133 ) identified, characterised and explained families of pro-resolving lipid metabolites from EPA and DHA using a lipidomics approach( Reference Serhan and Petasis 131 – Reference Serhan 133 ). There are two classes of Rv, the E-series derived from EPA and D-series derived from DHA (Fig. 3). It has been suggested that once the inflammatory process reaches initial resolution phases, there is a ‘switch’ from the inflammatory nature of AA-derived metabolites (LT, PG, etc.) to anti-inflammatory or specialised pro-resolving LX which stop leucocyte recruitment and help promote generation of lipid mediators such as Rv and PD( Reference Serhan and Savill 134 , Reference Levy 135 ).

Fig. 3 Lipid mediators derived from arachidonic acid (AA), EPA and DHA. COX, cylo-oxygenase; LOX, lipoxygenase; RvE1, resolvin E1;RvE2, resolvin E2.
Resolution of inflammation in airway diseases involves the removal of inflammatory cells from injured tissues, which is driven by apoptosis of leucocytes and elimination from the tissues( Reference Rossi, Sawatzky and Walker 136 , Reference Uller, Persson and Erjefält 137 ). The LX demonstrate their anti-inflammatory effects by reducing the formation of reactive oxygen species by leucocytes, decreasing transendothelial migration of leucocytes, and promoting non-phlogistic phagocytosis( Reference Kohli and Levy 138 ). LX also stop neutrophil infiltration and hence stop local inflammatory signals( Reference Serhan 132 , Reference Takano, Clish and Gronert 139 , Reference Takano, Fiore and Maddox 140 ). In asthmatics, there is decreased generation of LX and this is particularly explained by the deregulated expression of LX biosynthetic genes which vary by disease severity and anatomic compartment( Reference Planaguma, Kazani and Marigowda 141 ). The only study assessing the relationship between LX and EIB has been conducted in children (aged 6–17 years; n 12) and it was reported that children with EIB have lower levels of circulating LXA4 than healthy controls( Reference Tahan, Saraymen and Gumus 142 ). Overall, it has been suggested that LX are generated in airways during airway inflammation and any reduction in the generation of LX could lead to persistent inflammation and contribute to the pathogenesis of asthma( Reference Tahan, Saraymen and Gumus 142 , Reference Levy, Vachier and Serhan 143 ).
The DHA-derived D-series Rv are involved in resolution by preventing TNF-α from making pro-inflammatory cytokines which would be responsible for cascading neutrophil infiltration( Reference Serhan and Petasis 131 , Reference Uddin and Levy 144 ). A group of D-series Rv are aspirin triggered after acetylation of the COX-2 enzyme by aspirin and its interaction with DHA. It has been hypothesised that Rv and PD are a part of molecular mechanisms that highlight the role of aspirin in enhancing the conversion of EPA and DHA to Rv of the E- and D-series( Reference Serhan 133 , Reference Aoki, Hisada and Ishizuka 145 ). The E-series Rv are found in two major forms –RvE1 and RvE2. RvE1 has been found to exhibit its activity by responding to neutrophils. RvE1 impedes the migration of polymorphonuclear cells (PMN) to the site of inflammation, stops the PMN response to inflammatory cytokines and promotes the clearance of inflammatory cells via phagocytosis by macrophages( Reference Levy 135 ). Furthermore, RvE1 has been found to block the synthesis of pro-inflammatory cytokines and induce apoptosis and phagocytosis by up-regulation of chemokine receptor type 5( Reference Ariel, Fredman and Sun 146 ). RvE1 has been shown to be involved in the suppression of the production of cytokines such as IL-1, IL-2, IL-6 and TNF-α( Reference Arita, Bianchini and Aliberti 147 , Reference Marcheselli, Hong and Lukiw 148 ), as well as facilitating wound healing( Reference Gronert 149 ). Recent animal models have shown that RvE1 regulates IL-23 and LXA4 to promote resolution of allergic airway inflammation in a mouse model of asthma( Reference Haworth, Cernadas and Levy 150 – Reference Hisada, Ishizuka and Aoki 152 ). RvE1 can act along with LX as resolution-phase mediators to regulate IL-17 while only RV have the ability to regulate IL-23 and IFN-γ levels( Reference Haworth, Cernadas and Yang 151 ). In other animal models, it has been shown that RvE1 is highly potent when supplied intraperitoneally before and during sensitisation and aeroallergen challenge phases( Reference Aoki, Hisada and Ishizuka 153 ). This concept has been further investigated to confirm that administration with RvE1 in allergic asthma (murine models) can prevent the development of AHR, mucous metaplasia, eosinophil accumulation, and Th2 cytokine generation, for example, IL-13( Reference Aoki, Hisada and Ishizuka 145 ). Haworth et al. have reported in murine models that NK cells express CMKLR1 (chemokine-like receptor 1; a receptor for RvE1), and depletion of NK cells leads to a reduction in RvE1-mediated resolution of allergic inflammation( Reference Haworth, Cernadas and Levy 150 ). Subsequently these findings signify novel functions of NK cells in facilitating resolution of adaptive immune responses and emphasise that NK cells are possible targets for specialised resolution-phase lipid mediators for clearance of activated T cells from injured or inflamed lungs( Reference Haworth, Cernadas and Levy 150 ). While the functions of RvE1 have been investigated thoroughly, there is less information available about the specific activity of RvE2. This mediator has been reported to be produced by neutrophils and acts in a similar manner as RvE1( Reference Calderon Artero, Champagne and Garigen 130 , Reference Serhan and Petasis 131 ). The two forms of E-series Rv have been hypothesised to have separate receptors as there is an additive effect when the two types of Rv are administered together( Reference Levy, Vachier and Serhan 143 ).
PD, maresins and D-series Rv are DHA-derived lipid mediators and they function as anti-inflammatory molecules by blocking the activation and migration of neutrophils to sites of inflammation and reduce the production of pro-inflammatory cytokines( Reference Serhan 129 – Reference Serhan 133 , Reference Stables and Gilroy 154 ). In healthy individuals, airways and other mucosal surfaces have been found to be enriched with DHA while those individuals with asthma/cystic fibrosis have low levels of DHA( Reference Freedman, Blanco and Zaman 155 ). There is little evidence related to the effects of D-series Rv, maresins, and other DHA-derived mediators in asthma and only PD1 has been investigated. To date no receptors for PD have been found, although like RvE2 there is a combined effect with RvE1, suggesting distinct receptors for the two mediators( Reference Levy 135 , Reference Freedman, Blanco and Zaman 155 ). PD1 has been reported to facilitate the expression of CCR5 ligands on neutrophils and to inhibit NF-κβ induction, which prevents the migration of neutrophils( Reference Hong, Gronert and Devchand 156 ).
The biological characteristics of these new anti-inflammatory and pro-resolving mediators and the pathways that drive the formation and actions of these molecules have provided a new concept for treating inflammatory diseases. The majority of studies conducted have been in animal models including mice, rats and rabbits, with limited studies on human subjects. Most recently, in a clinical trial designed by Resolvyx, the phase 2 results show that when RX-10045 (a Rv) is administered as a tropic eye drop for the treatment of patients with chronic dry eye syndrome there was a dose-dependent improvement in both the signs and symptoms of dry eye, and the intervention did not show any health side effects( Reference Brooks 157 ). This first clinical study of the effect of Rv in human subjects will help improve the understanding of the agents that can stimulate the resolution mechanisms and resolve acute inflammation along with chronic inflammation to reduce human diseases where uncontrolled inflammation forms the basis of their pathophysiology( Reference Serhan and Petasis 131 ). Early-phase trials are currently on going using natural and synthetic Rv in various disease conditions such as asthma, inflammatory bowel disease, and other related inflammatory diseases; however, no information about the appropriate dosage of these compounds has been publicised. Based on a renal reperfusion study with 23–28 g mice, intravenous Rv dosage ranged from 0·01 to 0·1 mg/kg( Reference Duffield, Hong and Vaidya 158 ), while in another study investigating the effect of RvE1 in the asthma mouse model, a reduction in airway mucous, AHR and leucocyte bronchoalevolar lavage was achieved with intravenous dosages of 50–200 ng/mouse( Reference Haworth, Cernadas and Yang 151 ). Furthermore, Xu et al. have recently shown that a dose of only 10 ng/mouse of RvE1 and RvD1 is sufficient to reduce inflammation and pain via regulation of the central and peripheral nervous system( Reference Xu, Zhang and Liu 159 ).
A recent study investigated the protective effect of a different form of marine oil (PCSO-524TM; lyprinol®/omega XL®), a stabilised lipid extract from New Zealand green-lipped mussel, Perna canaliculus, in treating airway inflammation and hyperpnoea-induced bronchoconstriction in asthmatic patients( Reference Mickleborough, Vaughn and Shei 160 ). A moderate dose of this lipid extract (containing 400 mg n-3 PUFA; 72 mg EPA and 48 mg DHA) over 3 weeks was shown to significantly reduce airway inflammation and bronchoconstriction after a dry gas airway challenge. Additionally there was reduced bronchodilator usage and improved symptom scores. The levels of EPA/DHA in this recent study are comparable with the dose studied by Emelyanov et al. ( Reference Emelyanov, Fedoseev and Krasnoschekova 116 ), using PCSO-524TM; however, the mechanisms underlying the reduction in airway inflammation and improvement in lung function are not well established. The strong anti-inflammatory effect of PCSO-524TM has been suggested to be due to the nature of this extract comprising of up to ninety-one fatty acid components, including furan acid that is being argued to exhibit more potent anti-inflammatory activity than EPA( Reference Mickleborough and Lindley 61 ).
To summarise, LX, Rv, PD and maresins have been identified to have potent action (in the nanomolar and picomolar range) in a variety of cell types in vitro, as well as in many in vivo models of inflammatory diseases. Human supplementation studies are required to evaluate the action of these novel lipid mediators in other diseases including asthma. Furthermore, dose–response studies are required to elucidate the most appropriate dose for the anti-inflammatory effects. Since Rv, PD and maresins are biological molecules derived from n-3 PUFA which are integral components of cell membrane phospholipids, these molecules are suggested to regulate pleotrophic effects via cell signalling. Furthermore, these characteristics distinguish the lipid mediators from industrially produced drugs and what have been conventionally regarded as primary biological therapeutic agents, which have been shown to exhibit more limited and specific effects( Reference Serhan 132 ). However, the major challenges that have limited the application of these new mediators are the standardisation of appropriate methods for measurement of these in a laboratory setting. Other areas of interest in recent years have been a comparison of the health benefits of EPA and DHA supplementation v. treatment with Rv or PD. The differences, similarity and acceptance of health benefits of dietary n-3 PUFA and/or their mediators will be dependent on factors such as costs, safety and public health implications that are affected as a result of adopting a particular treatment approach( Reference Calderon Artero, Champagne and Garigen 130 ). Thus, well-designed trials are required to understand the efficacy of these novel mediators in inflammation.
Summary
Though the currently available pharmacological therapies for asthma and EIB are effective, long-term usage of these therapies is associated with issues of tachyphylaxis and health side effects. Complementary therapies are becoming gradually more popular among individuals with asthma for management of their symptoms. Increasing evidence from observational and intervention studies has suggested the possible anti-inflammatory effects of n-3 fatty acids on various chronic inflammatory diseases, including asthma and there is, therefore, an impetus towards using n-3 fatty acids as a complementary therapy. There are no major health side effects associated with the dietary supplementation of n-3 fatty acids, thereby making n-3 supplementation an attractive non-pharmacological intervention which may assist with the management of symptoms. The anti-inflammatory effect of n-3 fatty acids has been linked to a change in cell membrane composition, with n-3 fatty acid supplementation (primarily EPA and DHA) modifying lipid mediator generation by producing a less inflammatory series of eicosanoids. A newly identified group of lipid mediators produced from the oxidation of n-3 fatty acids (EPA and DHA) include Rv and PD, which have also been suggested as key players in the resolution of inflammation. Reduced inflammation attenuates the severity of asthma including symptoms (dyspnoea) and thereby exerts a bronchodilatory effect.
The n-3 fatty acid intervention studies on asthmatics have shown that there is a possible beneficial role of n-3 on asthma as well as EIB. There is a consensus within the literature that n-3 PUFA exert a range of anti-inflammatory effects and that they do not demonstrate any major negative side effects. The advantages of using n-3 PUFA supplementation in asthma have been widely reviewed and their effectiveness as a complementary therapy is acknowledged. However, there are some studies which show that there may be subgroups of asthmatics (EIB and allergic asthma) who benefit greatly and others who do not benefit from long-chain n-3 PUFA. Inconsistencies in study outcomes may be as a direct result of different dosages and durations of supplementation whilst the impact of investigating different subclassifications of asthma (each having its own characteristic inflammatory pattern) cannot be underestimated. Further studies differentiating asthma subgroups with specific phenotype/genotype profiles are required where specific physiological and biochemical characteristics of these groups are monitored with n-3 fatty acid supplementation. In addition, a number of studies on inflammation have suggested a threshold for an anti-inflammatory effect exhibited by n-3 fatty acids to be exerted in the range of 1·3–2·7 g EPA per d in both adults and children. Thus, appropriate dose should be considered when designing the studies. Finally, there is paucity of data relating to n-3 fatty acid supplementation and EIB with studies that have been conducted focusing primarily on athletes. There is a need for structured studies in both adults and children to respond to identified gaps in the current literature to move forward the field of asthma research.
Acknowledgements
There are no individuals who contributed to the present review other than the authors as listed. The authors thank Loughborough University for providing research facilities and research funds.
All authors fulfilled conditions of authorship: (1) substantial contributions to conception and design of the manuscript; (2) drafting the manuscript or revising it critically for intellectual content; and (3) final approval for the version to be published.
There were no external funds associated with the present review.
The authors declare no conflicts of interest.








