Environmental exposure to Hg is a recognised public health hazard. Hg is a well-known neurotoxicant and exposure can cause adverse effects to the nervous system, the developing brain being particularly vulnerable( Reference Myers, Davidson and Shamlaye 1 – Reference Clarkson and Magos 3 ). Hence, fetuses, infants and young children are especially susceptible to the toxic effects. Hg is formed mainly by bacterial methylation of inorganic mercury (IHg) into the organic form methylmercury (MeHg)( Reference Francesconi 4 ). This transformation occurs in aquatic sediments with bioaccumulation in the food web. Fish and other seafood contain the highest concentrations of Hg, with only small amounts in other food groups( Reference Rose, Baxter and Brereton 5 , Reference Clemens, Monperrus and Donard 6 ). Total mercury (THg) concentration in whole blood reflects exposure mainly to Hg in fish-eating populations( Reference Sanzo, Dorronsoro and Amiano 7 ) and has been shown to be directly correlated to the intake of fish and seafood( Reference Mahaffey, Clickner and Jeffries 8 ). The Hg concentration in fish and shellfish species ranges from <0·1 μg/kg for shellfish, such as oysters and mussels, to several mg/kg reported in high-end predatory fish. These include ocean species like tuna, shark, swordfish and halibut, and freshwater species like pike, perch and brown trout( Reference Storelli and Marcotrigiano 9 ). Consequently, Hg exposure depends on the species of fish consumed as well as the frequency and quantity eaten. Hg in blood has an average half-life of 70 d in man, implicating that changing the diet could reduce the body burden of Hg within some months( 10 , Reference Ballatori and Clarkson 11 ). Seafood intake during pregnancy is of special interest owing to the observed positive impact on birth weight and neurodevelopment of the child( Reference Hibbeln, Davis and Steer 12 – Reference Helland, Smith and Saarem 14 ). Dietary seafood is a rich source of protein, vitamins and the essential trace elements iodine and Se, and a predominant source of n-3 fatty acids which may play a role in optimum neurodevelopment of infants( Reference Campoy, Escolano-Margarit and Anjos 15 , Reference Makrides, Smithers and Gibson 16 ). Birth weight is considered a marker of intra-uterine development, is a strong predictor of infant development and also is an indicator of adult health( Reference Barker 17 ). Children born with low birth weight and children born preterm account for most of all neonatal mortality, as well as short-term and long-term morbidity( Reference Savitz, Hertz-Picciotto and Poole 18 ). The prevalence of small for gestational age (SGA) in the Norwegian Mother and Child Cohort Study (MoBa) is 6·6 % and the prevalence of low birth weight (<2500 g) is about 4·6 % in the MoBa cohort and 0·7 % in children born at term (>37 weeks of gestation)( Reference Magnus, Irgens and Haug 19 , 20 ). Positive associations between seafood consumption during pregnancy, and in particular lean seafood, and birth weight and SGA have been found in several studies, including the present cohort, while a few studies report inverse associations( Reference Brantsaeter, Birgisdottir and Meltzer 21 , Reference Olsen, Grandjean and Weihe 22 ). These inverse associations are often connected to the presence of environmental toxicants like polychlorinated biphenyls (PCB) and Hg( Reference Halldorsson, Meltzer and Thorsdottir 23 , Reference Ramon, Ballester and Aguinagalde 24 ). While most studies on prenatal Hg exposure and birth weight did not find any associations( Reference Bjerregaard and Hansen 25 – Reference Drouillet-Pinard, Huel and Slama 27 ), some recent studies suggest a negative association between Hg exposure and birth size( Reference Ramon, Ballester and Aguinagalde 24 , Reference Lee, Hong and Park 26 ). In 2004 the US Federal government issued advice for pregnant women and women of childbearing age to restrict their consumption of seafood to 340 g/week to reduce fetal exposure to Hg, while still providing enough n-3 fatty acids( 28 , Reference Oken and Bellinger 29 ). The relationship between the benefits and risks associated with fish and seafood consumption is an important issue.
Norway has one of the highest average intakes of fish and seafood in Europe( Reference Welch, Lund and Amiano 30 ). However, consumption of seafood varies considerably and the seafood intake of pregnant women is lower than that in the general population( 31 ). Pregnant women with no or a low consumption are therefore encouraged to eat fish during pregnancy. In MoBa, a large, prospective pregnancy cohort, women answered a detailed FFQ in mid-pregnancy. The dietary data provide a unique opportunity to explore dietary exposures to a wide range of foods, nutrients and contaminants and to examine associations between maternal diet and health outcomes in the children( Reference Meltzer, Brantsaeter and Ydersbond 32 ). The aims of the present study were: (i) to estimate the dietary exposure to Hg in MoBa; and (ii) to examine if maternal dietary Hg exposure was associated with infant birth weight.
Materials and methods
Population and study design
The data set used in the present study is part of MoBa, initiated by and maintained at the Norwegian Institute of Public Health( 20 ). In brief, MoBa is a nationwide pregnancy cohort that in the years from 1999 to 2009 included 108 000 pregnancies. Women were recruited to the study through a postal invitation in connection with their first routine ultrasound examination. The participation rate of invited pregnant women was approximately 38·5 %. Written informed consent was obtained from each participant. Pregnancy and birth records from the Medical Birth Registry of Norway (MBRN) are linked to the MoBa database( Reference Irgens 33 ). The study was approved by the Regional Committee for Ethics in Medical Research and the Data Inspectorate in Norway. The present study uses the quality-assured data files released for research in 2010 (version 5). The questionnaires used in the current study were completed in gestational weeks 15 (Q1) and gestational weeks 17–22 (Q2), and when the child was 6 months old (Q4)( 34 ). Q2 is an FFQ, while Q1 and Q4 are general questionnaires covering mother and child health, lifestyles and background factors. In this data file 78 744 women had answered questionnaires Q1, Q2 and Q4, and had singleton births recorded in MBRN. We excluded participants who had not recorded their weight prior to pregnancy (n 1722) and woman having an improbable energy intake, i.e. energy intake <4·5 MJ or >20 MJ (n 1238). All births with gestational age ≤37 weeks and >43 weeks were excluded (n 3730). Data on newborns with reported birth weight lower than 600 g (n 39) were examined and were considered obvious data transfer errors, i.e. the children were recorded with normal birth length and head circumference. Finally we excluded participants with multiple participation in MoBa (n 9074), resulting in a final sample of 62 941 participants for the present study. For the fully adjusted analyses 5953 women were excluded due to missing information on gestational weight gain, leaving 56 988 mother–child pairs.
Dietary information
The MoBa FFQ (downloadable at http://www.fhi.no) was completed in weeks 17–22 of gestation. The dietary data used in the present study were collected from February 2002 to November 2009. The FFQ is a semi-quantitative questionnaire designed to capture dietary habits and intake of dietary supplements during the first four to five months of pregnancy( Reference Meltzer, Brantsaeter and Ydersbond 32 ). The FFQ included questions about intake of 255 food items with special emphasis on various fish and seafood items. There were ten questions about cold cuts and spreads made from fish and shellfish, and sixteen questions about fish or shellfish eaten for dinner. FoodCalc( Reference Lauritsen 35 ) and the Norwegian food composition table( 36 ) were used to calculate food and nutrient intakes. The FFQ has been extensively validated in a MoBa sub-population (n 119) using a 4 d weighed food diary and biological markers in blood and urine as reference measures( Reference Brantsaeter, Haugen and Alexander 37 ). The validation study showed that the MoBa FFQ is a valid instrument for assessing habitual diet during the first four to five months of pregnancy. Median seafood intake by the FFQ and the food diary were 39·2 and 30·4 g/d respectively and the correlation was 0·37 (95 % CI 0·20, 0·52). Furthermore, seafood intake by the two methods was reflected by blood concentrations of THg, arsenic, Se and erythrocyte DHA( Reference Brantsaeter, Haugen and Thomassen 38 ).
Calculation of total Hg exposure
A database on THg concentrations in Norwegian food, including fish and other seafood items, was established and has been described in detail by Jenssen et al. ( Reference Jenssen, Brantsaeter and Haugen 39 ). In their study in a non-pregnant population, the estimated Hg exposure from diet was strongly reflected by blood THg levels. The database consists of THg concentrations in marine and freshwater fish and shellfish compiled mainly from Norwegian samples. Samples expected to be impacted by local pollution sources (i.e. from areas with known point sources) were excluded. For each species of fish or other seafood, the mean THg concentration was estimated by averaging the means. When the mean value was not available, the reported median concentrations were used. THg values in other food items were collected from other Scandinavian countries when Norwegian analyses were not available. Lower-bound concentrations (values less than levels of quantification are set to zero) in this database were used to calculate the dietary exposure to Hg at the individual level for the MoBa participants. Comparison of the estimated dietary Hg exposure against blood levels of THg in the MoBa validation study sample (Spearman correlation r = 0·24; 95 % CI 0·06, 0·40) and in a national population sample of men and women with a large range of seafood consumption (r = 0·46; 95 % CI 0·35, 0·57) showed that the dietary Hg estimate will correctly distinguish between individuals with high and low Hg exposure( Reference Jenssen, Brantsaeter and Haugen 39 ). Contribution of Hg from different types of food groups and subgroups of seafood is presented as micrograms per day (μg/d), and the estimated dietary intake of Hg is presented as micrograms per kilogram of body weight (BW) per week (μg/kg BW per week) ranked into quintiles. The Hg exposure was categorized into quintiles to ensure power for the regression analysis.
Definitions of fish and seafood variables
Total seafood describes the combined reported intake of all fish and other seafood items. Fish intake was also examined according to lean and oily species. Lean fish comprised reported intakes of fillets of cod, saithe, haddock, pollock, halibut, plaice, flounder, tuna, perch, pike, catfish and fish roe, all items of lean fish eaten as bread spread, and lean fish in mixed dishes as fish fingers or fish au gratin. Oily fish included intake of salmon, trout, mackerel and herring, both as dinner fillets and as bread spreads. Shellfish comprised intake of shrimps, crab and mussels. In an attempt to disentangle the risk–benefit of fish and seafood intake v. Hg exposure, we stratified the total seafood intake by quartiles. The quartiles for fish intake were corresponding to approximate weekly consumption categories: the first quartile (0–19·0 g/d) corresponds to <1 portion/week, the second quartile (19·1–30·9 g/d) to ∼1·5 portions/week, the third quartile (31·0–44·9 g/d) to ∼1·5–2 portions/week and the fourth quartile (≥45·0 g/d) corresponds to >2 portions/week.
Birth weight
Birth weight was measured by a midwife at birth and reported to MBRN( Reference Irgens 33 ). Data from MBRN also included information on pregnancy duration. Pregnancy duration based on ultrasound was available for 98·2 % of the women, and if ultrasound information was missing, gestational age was based on the last menstrual period. Only pregnancies with duration of 37–42 weeks were included in the analysis. SGA is defined as infants with birth weight below the 10th percentile, at the attained gestational age, in the general population( Reference Skjaerven, Gjessing and Bakketeig 40 ).
Other variables
Potential confounding variables were chosen on the basis of previous knowledge and included maternal age, height, BMI, parity, education, smoking, gestational weight gain, pregnancy duration in weeks, total energy intake, and long-chain n-3 fatty acids from the diet and food supplements. Self-reported pre-pregnancy height and weight were used to calculate BMI (kg/m2). BMI was divided into the WHO categories (<18·5, 18·5–24·9, 25·0–29·9, 30·0–34·9 and ≥35·0 kg/m2). Maternal educational attainment was divided into three categories (≤12, 13–16 and ≥17 years) and first-trimester smoking into three categories (non-smoker, occasional smoker and daily smoker). Maternal age at delivery was retrieved from MBRN and used as a continuous variable except for descriptive purpose. Parity was based on data from both MoBa and MBRN, and categorised into the numbers of previous pregnancies of >22 weeks’ duration. Missing values for pre-pregnant BMI and maternal education were grouped as a ‘missing data’ category. For calculations of long-chain n-3 fatty acids intake from food we used data from the Norwegian food composition table, and for intake from supplements we used data from a database including more than 1000 different dietary supplements with nutrient content per portion( Reference Haugen, Brantsæter and Trogstad 41 ).
Statistical methods
The estimated dietary Hg exposure as well as food consumption data were not normally distributed. Medians and percentiles were used for unadjusted analyses. Multivariable linear regression models were used to explore the association between maternal Hg exposure and infant birth weight. Multivariable logistic regression was used to the model association between maternal Hg exposure and the risk of delivering SGA offspring. Relative risks were approximated by odds ratios. The following variables were included in the adjusted models: maternal age at delivery, height, pre-pregnant BMI, parity, education, smoking in pregnancy, gestational weight gain, total energy intake and intake of n-3 fatty acids. Intake of n-3 fatty acids was modelled both as a categorical variable and on a continuous scale in the analysis. Because of the high co-linearity between seafood consumption and dietary Hg exposure, we stratified the results from the multiple regression analyses by quartiles of seafood intake. All models were checked for residual normality. The analyses were performed using the statistical software package PASW Statistics 17 and P < 0·05 was considered significant.
Results
The estimated median (5th–95th percentile) dietary Hg exposure was 1·45 (0·31–3·41) μg/d, corresponding to a daily Hg exposure of 0·02 (0·00–0·05) μg/kg BW and a weekly Hg exposure of 0·15 (0·03–0·38) μg/kg BW. Fish and seafood were the main source of Hg exposure accounting for 88 % of the intake of Hg in diet. The contribution of Hg from different types of seafood and fish products showed that approximately 52 % came from lean fish, 27 % from oily fish and 9 % from shellfish and fish products. Eggs and cereals were the main non-fish sources, contributing 9 % of the total intake of Hg. Other food groups which contained Hg were fruit and vegetables, sweets and chocolate and meat (Table 1). The maternal characteristics of the study population showed that the estimated exposure to Hg from diet increased with increasing maternal age, length of education, energy intake and amount of seafood eaten (Table 2). The estimated Hg exposure was higher among women with a normal and low BMI than among overweight woman, and higher among non-smokers than among smokers. The mean birth weight of the offspring in the present study was 3587 (sd 560) g. In the adjusted multivariable linear regression model with birth weight as outcome, women in the highest quintile of Hg exposure delivered babies with on average 34 g (95 % CI −46 g, −22 g) lower birth weight than women in the lowest quintile of Hg exposure (Table 3). In the logistic regression analysis with SGA as the outcome, no association with Hg exposure was seen for women in the second to fourth quintiles of Hg exposure compared with the lowest quintile, while a significantly increased risk of SGA was observed for women in the highest quintile with an adjusted OR of 1·19 (95 % CI 1·08, 1·30; Table 4).
Table 1 Estimated consumption of food groups contributing to dietary total mercury (THg) exposure, estimated calculated THg exposure (μg/d) and mean (%) contribution to THg exposure in pregnant Norwegian women, the Norwegian Mother and Child Cohort Study, 2002–2009. Total seafood is divided into subgroups

P5, 5th percentile; P95, 95th percentile.
Table 2 Estimated dietary mercury intake during pregnancy according to maternal characteristics, the Norwegian Mother and Child Cohort Study, 2002–2009

BW, body weight; P5, 5th percentile; P95, 95th percentile.
Less than 5 % missing information on all covariates. P value from regression of ranked Hg.
Table 3 Associations between birth weight and estimated dietary mercury exposure in pregnant Norwegian women (n 62 941), the Norwegian Mother and Child Cohort Study, 2002–2009
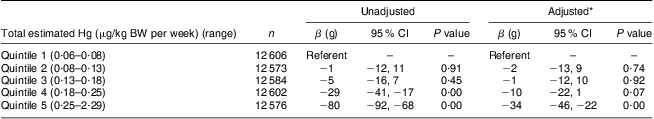
BW, body weight.
n 56 998 in the adjusted model because of missing data on gestational weight gain.
*Adjusted for energy intake, maternal age, pre-pregnancy BMI, parity, smoking during pregnancy, pregnancy duration in weeks, intake of n-3 fatty acids, maternal height, maternal education and gestational weight gain.
Table 4 Associations between maternal dietary mercury exposure and risk of delivering a small-for-gestational-age-baby in pregnant Norwegian women (n 62 941), the Norwegian Mother and Child Cohort Study, 2002–2009

*Adjusted for energy intake, maternal age, pre-pregnancy BMI, parity, smoking during pregnancy, pregnancy duration in weeks, intake of n-3 fatty acids, maternal height, maternal education and gestational weight gain.
We used beta values (unstandardized), obtained from analyses stratified by seafood consumption, to plot expected birth weight by increasing Hg exposure (Fig. 1). The plot shows a reduction in birth weight with increasing Hg exposure in all strata of seafood intake. The statistical power in the two groups of highest Hg exposure/lowest seafood intake and lowest Hg exposure/highest seafood intake was low and not presented in Fig. 1. We saw a tendency of a similar pattern for SGA when we stratified by seafood consumption and plotted SGA by increasing Hg exposure (data not shown). In the first quartile of seafood intake there was no linear association found between Hg exposure and SGA. Hg exposure was significantly associated with SGA in the second and third quartile of seafood intake. In the fourth quartile the analysis collapsed due to the low number of SGA cases.

Fig. 1 Associations between maternal dietary mercury exposure (quintiles) and mean birth weight stratified by maternal seafood intake (quartiles*: ![]() , quartile 1, seafood intake 0–19 g/d;
, quartile 1, seafood intake 0–19 g/d; ![]() , quartile 2, seafood intake 19–31 g/d;
, quartile 2, seafood intake 19–31 g/d; ![]() , quartile 3, seafood intake 31–45 g/d;
, quartile 3, seafood intake 31–45 g/d; ![]() , quartile 4, seafood intake 45–350 g/d) in the Norwegian Mother and Child Cohort Study, 2002–2009. *Adjusted for energy intake. P for trend across increasing mercury exposure in all quartiles of seafood intake: <0·001. Data points for the lowest quintile of mercury exposure in the upper quartile of seafood intake (n 10) and for highest quintile of mercury exposure in the lowest quartile of seafood intake (n 39) are not shown due to low statistical power
, quartile 4, seafood intake 45–350 g/d) in the Norwegian Mother and Child Cohort Study, 2002–2009. *Adjusted for energy intake. P for trend across increasing mercury exposure in all quartiles of seafood intake: <0·001. Data points for the lowest quintile of mercury exposure in the upper quartile of seafood intake (n 10) and for highest quintile of mercury exposure in the lowest quartile of seafood intake (n 39) are not shown due to low statistical power
The bivariate correlation coefficient between Hg exposure and long-chain n-3 fatty acid intake was 0·49, which can justify the inclusion of this variable as a confounder in the models. The relatively modest correlation can be explained by the pregnant women's frequent use of long-chain n-3 fatty acid supplements; as many as 67 % of the women reported use of some sort of n-3 supplementation like cod-liver oil. We did the same regression analysis for long-chain n-3 fatty acids as for Hg by dividing the exposure into quartiles, and found no differences in the stratified analysis. We also included long-chain n-3 fatty acids as a continuous variable in the regression model, and no significant impact was found on birth weight.
Discussion
Although the large majority of the participants did not have a calculated dietary exposure exceeding the Provisional Tolerable Weekly Intake (PTWI) of 1·6 μg MeHg/kg BW per week recently established by the Joint FAO/WHO Expert Committee on Food Additives( 42 ), the current population-based study showed that maternal dietary Hg exposure had a significant negative association with the birth weight of offspring, with those in the highest exposure group having an increased risk of giving birth to babies being SGA. These findings are remarkable taking into account that Hg exposure also in the highest quintile is generally below the European Food Safety Authority's Tolerable Weekly Intake (TWI). Only ten women of the cohort exceeded the PTWI of 1·6 μg/kg BW per week. A slightly higher proportion, 0·4 % (n 251) of the women, exceeded the reference dose (RfD) of 0·1 μg MeHg/kg BW per d set by the US Environmental Protection Agency( 43 ). However, as presented before for the present cohort and in agreement with other studies, maternal seafood consumption had an overall positive association with infant birth weight (Fig. 1)( Reference Brantsaeter, Birgisdottir and Meltzer 21 , Reference Olsen, Grandjean and Weihe 22 , 31 ). Hg exposure was strongly related to total seafood consumption with 88 % of the estimated dietary Hg originating from seafood.
Our findings of estimated levels of dietary Hg exposure are in agreement with what was found in a study of Swedish women of childbearing age, as well as similar to dietary exposure levels reported worldwide in women of childbearing age( Reference Bjornberg, Vahter and Grawe 44 , Reference Schober, Sinks and Jones 45 ). Studies of maternal Hg exposure and infant birth weight have until recently not reported significant associations( Reference Bjerregaard and Hansen 25 , Reference Grandjean, Bjerve and Weihe 46 , Reference Lucas, Dewailly and Muckle 47 ). Although studies have found associations between exposure to other environmental contaminants accumulating in fish and seafood and fetal growth( Reference Ribas-Fito, Sala and Cardo 48 , Reference Sagiv, Tolbert and Altshul 49 ), it is only recently that reports of a direct adverse association to low-level Hg exposure have been published. A recent meta-analysis on fetal Hg exposure and birth outcome shows mixed results, but with some studies showing a negative association between low Hg exposure and fetal growth( Reference Karagas, Choi and Oken 50 ). Hg easily crosses the placenta and disrupts many steps in brain development, which may indirectly lead to fetal growth restriction. Hg results in a non-optimal intra-uterine environment which could impair fetal growth, but the biological mechanism is not clear. A Korean study from 2010 suggested that interactions of Hg with glutathione S-transferase (GST) play a role in reducing birth weight. That study found that both cord blood Hg and maternal blood Hg were inversely related to birth weight, and specifically examined the association between GST polymorphisms in mothers, blood THg and infant birth weight( Reference Lee, Hong and Park 26 ). Hg excretion rates vary widely among individuals and involve glutathione conjugation by GST( Reference Schlawicke, Stromberg and Lundh 51 ). The results showed that the negative association with birth weight was stronger among women with the GSTM1 null genotype or with both GSTM1 and GSTT1 null genotypes( Reference Lee, Hong and Park 26 ).
Our finding of a negative association between maternal exposure to Hg during pregnancy and birth weight is in accordance with a study from a birth cohort in Spain, where high cord blood THg concentrations were inversely related with birth weight( Reference Ramon, Ballester and Aguinagalde 24 ). In the Spanish study, infants in the highest quartile of cord blood THg weighed on average 143·7 g less (95 % CI −251·8 g, −35·6 g) than those in the first quartile, adjusted for intake of fish and seafood. That study also indicated a possible increasing risk of delivering SGA babies with increasing THg, but the result was not statistically significant( Reference Ramon, Ballester and Aguinagalde 24 ). In most studies where no association between Hg exposure and birth weight is found, lack of adjustment for fish and seafood intake in the analysis may result in underestimation of both the toxicity of Hg and the nutritional benefits of eating fish products( Reference Choi, Cordier and Weihe 52 – Reference Budtz-Jorgensen, Grandjean and Weihe 54 ).
There is a general concern that the benefits of fish and other seafood consumption may be offset by the concomitant presence of environmental contaminants. Contaminants that have been studied include dioxins and PCB and these contaminants are found to be associated with lower birth weight( Reference Guo, Lambert and Hsu 55 ). The effect of prenatal low-level PCB exposure on birth weight is assumed to be low( Reference Sagiv, Tolbert and Altshul 56 ). A Belgian study by Sioen et al.( Reference Sioen, De Henauw and Van Camp 57 ) found that to meet the daily recommendations for PUFA through seafood consumption one would have to consume fatty fish twice weekly or vary between lean and fatty fish minimally three times weekly. At this level of seafood consumption they concluded that Hg exposure is below levels of toxicological concern, while for the dioxin-like compounds the TWI was reached. In Norway, low exposure to dioxins/PCB and Hg have been reported( Reference Jenssen, Brantsaeter and Haugen 39 , Reference Caspersen, Knutsen and Brantsæter 58 ). Whether PCB exposure interacts with the association between lower birth weight and Hg exposure may be addressed in future investigation.
We found that about 12 % of the Hg exposures were from other sources than seafood, with 9 % coming from eggs and cereals. Fishmeal is used as a source of protein for poultry and pigs and may be a source of Hg in addition to fish. Hg can also occur as residues in food (mainly as IHg) due to their presence in the environment, as a result of human activities such as farming, industry or motor traffic. Contamination during food processing and storage are also possibilities.
The strength of the present study was that it is a large population-based study of pregnant women where food intake was reported during the first part of pregnancy. The potential influence of self-selection is a general concern in cohort studies and has been extensively explored in MoBa( Reference Nilsen, Vollset and Gjessing 59 ). Comparison of several exposure–disease associations in MoBa with registry data for all births in Norway showed that the associations were not biased. The exposure to Hg is based on calculations using national data on THg concentrations in food data( Reference Brantsaeter, Haugen and Alexander 37 ). Calculations of the amount of seafood consumed were performed with use of a validated FFQ designed to capture the consumption of a range of seafood items( Reference Brantsaeter, Haugen and Thomassen 38 ). Most other studies which have investigated neonatal Hg exposure have been performed in communities with very high fish consumption, while the participants in the present study have a more moderate fish intake. The weakness of our study is the lack of biological markers. Analyses done by measuring biomarkers, such as the concentrations of THg in blood or hair, are regarded as more accurate measures of exposure than assessments based on dietary data. Estimated Hg intake based on data from an FFQ was shown to be significantly associated with THg concentrations in maternal blood and hair in a Japanese study( Reference Yaginuma-Sakurai, Shimada and Ohba 60 ). Estimated Hg values may involve uncertainties, but the FFQ appears to be a valid tool for assessment of exposure to Hg. The main uncertainty in the data is that Hg concentrations in fish and seafood vary with type of species, the size of the fish and location of catch( Reference Mahaffey, Clickner and Jeffries 8 ). However, in the Norwegian Fish and Game study a comparison of estimated dietary exposure of MeHg calculated by the same FFQ as used in our study did show a strong association with THg measured in blood( Reference Jenssen, Brantsaeter and Haugen 39 ). Furthermore, individual genetic and metabolic variation may influence the absorption and excretion of Hg( Reference Sagiv, Tolbert and Altshul 49 , Reference Karagas, Choi and Oken 50 ).
In an attempt to overcome the dependence of Hg exposure on seafood intake, we analysed the impact of Hg exposure per gram of total seafood intake (see online supplementary material, Supplementary Table 1). A significant effect on birth weight was still seen for Hg exposure although weakened (26 g compared with 34 g lower birth weight between the lowest and the highest quintile of Hg exposure). This finding is in accordance with the results from the analysis of the exposure measured as Hg μg/kg BW per week.
Conclusion
While maternal seafood consumption has an overall positive association with infant birth weight, seafood also contributes to the dietary Hg exposure. The present study suggests that women in the highest range of Hg exposure have an increased risk of giving birth to SGA babies. A negative association between dietary Hg exposure and birth weight was observed within strata of seafood consumption, but the clinical impact of this is not clear. It is important to bear in mind that there is an overall positive association between seafood intake and birth weight in the present cohort. This would in itself suggest that the benefits of fish consumption are outweighing the risks from contaminant exposure at present concentrations in this population; although this would need to be confirmed with biological data. Dietary Hg exposure in women of childbearing age is of public health concern and needs to be monitored on a regular basis. Dietary recommendations need to focus on a balance between promoting fish and seafood as part of a balanced diet, while at the same time contain advice for restricting the intake of species and items with known high Hg concentrations.
Acknowledgements
Sources of funding: The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, and the National Institute of Environmental Health Science (contract no. N01-ES-75558) and the National Institute of Neurological Disorders and Stroke (grant no. 1 UO1 NS 047537-01 and grant no. 2 UO1 NS 047537-06A1) of the US National Institutes of Health (NIH). The Norwegian Ministry of Health and the Ministry of Education and Research and the NIH had no role in the design, analysis or writing of this article. Conflicts of interest: The authors declare they have no actual or competing financial interests. Authors’ contributions: The work presented here was carried out in collaboration between all authors. K.V., M.H., P.M. and A.L.B. defined the design and research theme. K.V., M.H. and A.L.B. analysed the data, interpreted the results and wrote the paper. H.K.K., J.A. and P.M. discussed analyses and interpreted the results. H.K.K. and H.E.K. co-worked on associated data collection and their interpretation. All authors have contributed to, seen and approved the manuscript. Acknowledgements: The authors are grateful to all the participating families in Norway who take part in this ongoing cohort study.







