For some years, blockade of N-methyl-D-aspartate receptors (NMDAR) has been associated with the pathogenesis of psychosis.Reference Farber 1 Inhibiting antibodies to NMDAR (anti-NMDAR antibodies) in humans were first described in a group of females with teratoma and severe limbic encephalitis with a psychiatric prodrome.Reference Dalmau, Tuzun, Wu, Masjuan, Rossi and Voloschin 2 Anti-NMDAR antibodies have subsequently been found in a broader population of patients with encephalitis, including males and children with and without demonstrable malignancy and with a variety of clinical features, including psychosis as a sole presenting feature. Many of these people present to mental health services initially, before the autoimmune nature of their illness becomes manifest, with numerous case reports and small case series identifying anti-NMDAR encephalitis in those presenting with an initial diagnosis of psychosis.Reference Moura, Silva-Dos-Santos, Afonso and Talina 3 – Reference Bergink, Armangue, Titulaer, Markx, Dalmau and Kushner 10 Although early detection and initiation of immunotherapy is important to improve their long-term outcome,Reference Irani and Vincent 11 , Reference Wandinger, Saschenbrecker, Stoecker and Dalmau 12 presentation to mental health services potentially delays diagnosis and appropriate treatment. In contrast, detection of classical anti-NMDAR encephalitis is rarely described in established schizophrenia and related psychotic disorders.Reference Masdeu, Gonzalez-Pinto, Matute, Ruiz De Azua, Palomino and De Leon 13 – Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon 15 However, IgA and IgM antibodies against NMDAR, atypically found in anti-NMDAR encephalitis, have been detected in people diagnosed with schizophrenia.Reference Dalmau, Lancaster, Martinez-Hernandez, Rosenfeld and Balice-Gordon 15 , Reference Steiner, Walter, Glanz, Sarnyai, Bernstein and Vielhaber 16 Less commonly, cases have been reported of individuals with voltage-gated potassium channel antibodies (anti-VGKCReference Pruss and Lennox 17 ). Despite the above reports, the clinical significance of detecting anti-NMDA receptor and VGKC antibodies in patients presenting with first episode of psychosis (FEP) remains unclear, and it is uncertain if providing immunomodulatory treatment to patients presenting with psychosis and antineuronal antibodies improves outcomes. Lennox and colleagues reported nine percent of patients with FEP had serum antineuronal antibodies.Reference Lennox, Palmer-Cooper, Pollak, Hainsworth, Marks and Jacobson 18 None of these were diagnosed with encephalitis and the presence of antibodies was not associated with increased need for care over 6 months as defined by admissions, out-patient contacts or crisis service interventions. Immunological interventions were not utilised. We performed a prospective study of all people presenting to three inpatient psychiatry services in southeast Queensland, Australia, with FEP. The aim was to prospectively determine the prevalence of serum antineuronal antibodies, including anti-NMDAR antibodies, in this clinical population and describe antibody-positive patient response to immunotherapy.
Method
Study sites
The study was undertaken in six acute psychiatry units located at three hospitals in Brisbane, Australia (The Royal Brisbane and Women's Hospital, The Logan Hospital Adolescent Unit and the Lady Cilento Children's Hospital). Recruitment commenced in July 2013 and participants were recruited from two 26 bed general adult psychiatry units, one 26 bed unit specialising in the care for female patients aged 18–25 years, two 12 bed adolescent units (ages 14–17 years) and one 12 bed acute psychiatry unit for children up to and including 13 years. Recruitment at all sites ended in May 2015.
Participants
Those aged between 12 and 50 years admitted to one of the three hospitals for the first time for management of their FEP were approached to participate in the study. Patients were included in the study if they were definitively suffering from a psychotic disorder (including substance-induced psychotic disorder) as diagnosed by a multidisciplinary team lead by a consultant psychiatrist for which no overt medical condition could be identified. Those who had capacity consented to a blood test for antineuronal antibodies and the recording of clinical and demographic information for the purposes of the study as early as possible after admission. Where the individual lacked capacity, consent was obtained from an ‘alternative decision maker’, who was usually a family member or partner of the participant. When the participant recovered, they were again approached to explain the study and obtain informed consent. If they declined to participate, their results were not included. Ethics approval was obtained to collect basic demographic (age and gender) and clinical information (diagnosis, duration of untreated psychosis and recruitment site) of patients who refused, so as to compare those who did and did not participate. The study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (approval number HREC/12/QPAH/598).
Detection of antineuronal antibodies and assessment for autoimmune encephalitis
All samples were analysed in the Autoimmune Section of Pathology Queensland, which is a referral diagnostic laboratory, offering specialist neuroimmunology testing for the east coast of Australia. All consenting participants had their plasma tested for intracellular antineuronal antibodies (including purkinje cell cytoplasmic antibody type 1 (PCA-1)/anti-Yo, PCA-2, type 1 antineuronal nuclear antibody (ANNA-1)/anti-Hu, ANNA-2/anti-Ri and anti-Ma) by indirect immunofluorescence on a composite slide of primate cerebellum/cerebrum and murine gastric tissues (Inova Diagnostics, USA). Anti-NMDAR IgG antibodies were detected in diluted serum (1:10) as per manufacturer's instructions by a commercial assay containing four biochips of primate hippocampus, primate cerebellum, NMDAR-transfected human embryonic kidney cells 293 (HEK293) and nontransfected control HEK293 cells (Euroimmun, Germany). At Pathology Queensland, anti-NMDAR antibody titres are not routinely performed as the commercial NMDAR NR1 subunit-transfected cell lines used by all Australian diagnostic laboratories are not readily amenable for end-point titration.Reference Newman, Blum, Wong, Scott, Prain and Wilson 19 Low positive results for NMDAR antibodies are detected by weak immunofluorescence staining. Anti-VGKC were detected by a commercial radioimmunoassay (RSR, UK). This quantitative assay utilizes detergent solubilized VGKCs extracted from rabbit brain tissue and complexed with 125I-labelled α-dendrotoxin. All positive sera on the radioimmunoassay were then tested by indirect immunofluorescence on biochips of leucine-rich glioma inactivated 1 (LG1) and contactin-associated protein 2 (CASPR2)-transfected HEK293 cells (Euroimmun). Anti-glutamic acid decarboxylase (GAD) antibodies were detected by a commercial quantitative immunoassay (RSR). The assay utilizes GAD65 coated onto the wells of an enzyme-linked immunosorbent assay plate. Sensitivity is increased by a second step that includes the addition of GAD-biotin and a streptavidin detection step.
Where participants were found to be positive for any of the above antineuronal antibodies, consultation was sought from neurologists and immunologists with extensive experience in the treatment of patients with autoimmune encephalitis. Participants were transferred to medical units, where a medical history was obtained, and appropriate investigations were undertaken to confirm or exclude the diagnosis of encephalitis, investigate for a potential paraneoplastic cause and initiate immunotherapy. After diagnosis, antibody-positive patients were followed up by both neurological and psychiatry services.
Results
Comparison of those who participated and those who refused
During the period of data collection, 154 patients were admitted to the three hospitals for treatment of their FEP and 113 (73.3%) consented to the study. Those participating in the study consisted of 66 (58.4%) males, with a mean age of 26.2 years (s.d. 9.2; range 13–50). The median duration of untreated psychosis was 30 days (range 1–2340), with 57 (50.4%) participants having a duration of untreated psychosis estimated by clinicians to be less than 30 days. The most common diagnoses were schizophrenia, substance-induced psychotic disorder and schizophreniform disorder, which accounted for greater than 50% of all patients (Table 1). Of those who refused (n = 41), 29 (70.7%) were male, with a mean age of 28.5 years (s.d. 9.6) and a median duration of untreated psychosis of 60 days. A comparison of those included with those who refused to participate revealed no significant differences in age and gender, although nonparticipants had a longer median duration of untreated psychosis.
Table 1 Initial psychiatric diagnoses of the 113 participants with first episode of psychosis

NOS, not otherwise specified.
Description of antineuronal antibody-positive patients
Of the 113 participants, six tested positive for antineuronal antibodies in serum (Table 2). Of these, four (patients 1–4) had anti-NMDAR antibodies, one (patient 5) had antibodies to VGKC with a titre of 203 pMol/L (by radioimmunoassay alone, with negative anti-LGI1 and anti-CASPR2 on the transfected HEK293 cells) and a final patient (patient 6) had antineuronal antibodies of uncertain clinical relevance to an uncharacterised antigen. Testing of sera from patient 6 showed weak immunofluorescent, fine-speckled staining throughout the cerebellar molecular and granular layers. There was non-specific Purkinje cell staining but no staining of white matter or hippocampal tissue. Other testing revealed the patient was negative for GAD, NMDA, LGI1, CASPR2, AMPA and GABA.
Table 2 Overview of clinical and paraclinical characteristics of antibody-positive participants
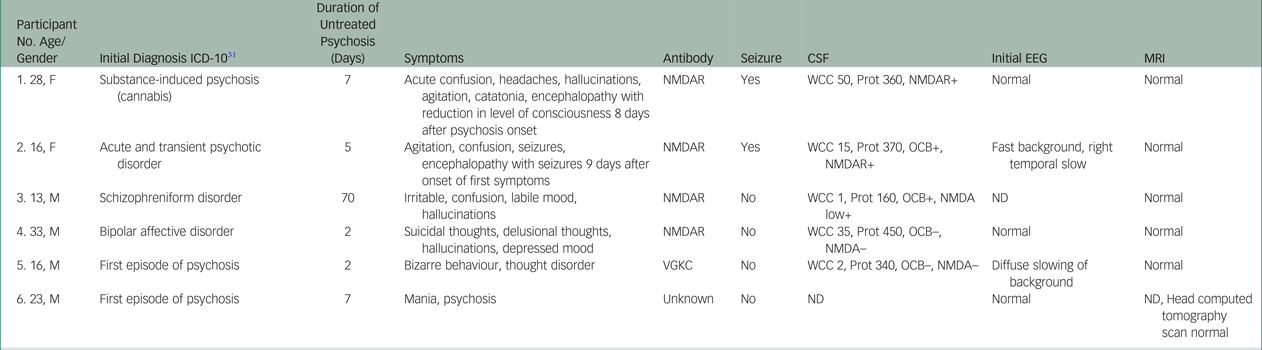
CSF, cerebrospinal fluid; EEG, electroencephalogram; F, female; M, male; MRI, magnetic resonance imaging; ND, not done; NMDAR, N-methyl-D-aspartate receptor antibody; OCB, oligoclonal bands; Prot, protein; VGKC, voltage-gated potassium channel antibody; WCC, white cell count.
Five of the six patients (patients 1–5) lacked capacity to provide informed consent at time of serum collection and an alternative decision maker was needed to initially enrol these patients into the study as per the protocol. Informed consent was obtained when the patients had later recovered.
All six participants initially presented with acute onset of psychosis. The delay in diagnosis and treatment of patient 3 was because of initial attempts at community management. The progressive escalation in symptoms eventually necessitated admission to hospital despite out-patient psychiatric care. Patients 1–4 had NMDAR antibodies detected within 1 week of commencing psychotropic medication, an inadequate length of treatment to determine response. Patient 5 received risperidone (2 mg each night, for 22 days) before immunotherapy was commenced. There was no clinical improvement with this antipsychotic (Table 3).
Table 3 Overview of antipsychotic and immunotherapy and treatment response
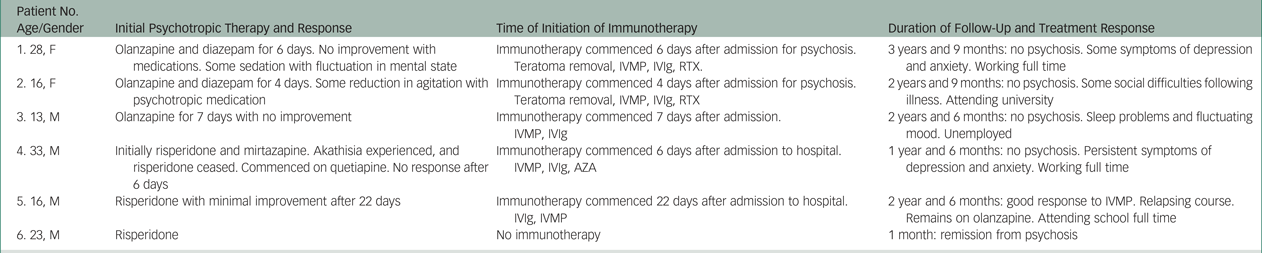
AZA, azathioprine; IVIg, intravenous immunoglobulins; IVMP, intravenous methylprednisolone; RTX, rituximab.
Two participants (patients 1 and 2) developed a diffuse encephalopathy with seizures consistent with classical limbic encephalitis after the testing of serum for antibodies. Three participants (patients 3–5) had isolated psychiatric syndromes without other features of limbic encephalitis, such as seizures or other neurological signs. These patients may have potentially remained in psychiatric services with undiagnosed autoimmune illnesses. One participant (patient 6) was seen on several occasions in the month after discharge having been treated with risperidone. During this time, there were no mood or psychosis symptoms and he was subsequently lost to follow-up.
Two of four anti-NMDAR antibody-positive patients (patients 1 and 2) had an ovarian teratoma detected and removed; these patients quickly deteriorated clinically in the initial stages of their illness. Anti-NMDAR antibodies as well as oligoclonal bands were detected in the cerebrospinal fluid (CSF) of one participant (patient 3), who responded well to immunotherapy. Another participant (patient 4) had no detectable anti-NMDAR antibodies in CSF, but an increased CSF white cell count was identified. One antibody-positive participant (patient 2) required intubation and therapy in an intensive care setting. Three participants (patients 1, 3 and 4) were treated on a neurology ward for the duration of their acute illness. For the patient who tested positive for VGKC antibodies (patient 5), presence of antibodies to VGKCs was confirmed by repeat testing in a quaternary laboratory (Oxford Neuroimmunology Laboratory, UK). He subsequently had relapsing presentations of psychosis with a good response to immunotherapy utilizing intravenous methylprednisolone and intravenous immunoglobulin.
At last follow-up, participants 1 and 4 had ongoing symptoms of depression and anxiety but were functioning well, engaged in full-time employment. Participant 2 had some difficulties socially after her illness, although this had improved over time and at last follow-up she was successfully attending tertiary education. Participant 5 had experienced a brief relapse of psychosis when antipsychotic medication had been withdrawn. Participant 3 had no further psychosis but continued to experience sleep disturbance and some mood instability (Table 3).
In one participant (patient 6), a previously uncharacterised staining pattern was detected by indirect immunofluorescence on primate cerebellum. This person left hospital soon after recruitment and could not be followed up; the medical work-up was incomplete and no immunotherapy was instituted.
Discussion
This real-world clinical study prospectively assessed the prevalence of antineuronal antibodies in patients admitted to hospital with FEP and reports their response to immunomodulatory therapy. We recruited 113 people admitted to hospital with FEP in Queensland, Australia, and detected the presence of antineuronal antibodies in six individuals (5.3%), including four (3.5%) participants with anti-NMDAR antibodies, one (0.8%) with anti-VGKC antibodies and one (0.8%) with a previously uncharacterised staining pattern on primate cerebellum with indirect immunofluorescence.
Clinical and paraclinical findings support the notion that a central nervous system autoimmune process was present in five of these six participants (patients 1–5). Importantly, neuroimaging with brain magnetic resonance imaging and electroencephalogram did not render abnormal results in any of the antibody-positive patients. Two participants (patients 1 and 2) had a paraneoplastic process in the context of an ovarian teratoma. In patient 3, anti-NMDAR antibodies were detected in CSF, together with oligoclonal bands. In patient 4, anti-NMDAR antibodies were not detected in CSF; however, a lymphocytic pleocytosis was indicative of an inflammatory process. One participant (patient 5) presented with a relapsing-remitting course in the presence of VGKC positivity in the absence of antibodies to LGI1 and CASPR2. Recent consensus suggests this is unlikely to be a clinically significant autoimmune process;Reference van Sonderen, Schreurs, de Bruijn, Boukhrissi, Nagtzaam and Hulsenboom 20 , Reference van Sonderen, Petit-Pedrol, Dalmau and Titulaer 21 however, this participant did respond well to intravenous immunoglobulins and methylprednisolone after having no response to 22 days of low-dose risperidone. Immunotherapy was not commenced in patient 6 as the participant had recovered by the time the serum antibodies were detected. Participants 1–5 received immunotherapy, using different regimens, leading to resolution of psychosis in four patients (Table 3).
These favourable clinical outcomes in patients who presented with FEP with such high acuity as to require inpatient care were similar to a previously reported case series of nine patients with psychosis who received immunotherapy.Reference Zandi, Deakin, Morris, Buckley, Jacobson and Scoriels 22 Of these nine, six had positive responses. The three nonresponders had long histories (>3 years) of treatment refractory illness. The excellent clinical responses seen in our case series may be a result of the very early provision of immunotherapy.
Relationship of antineuronal antibodies to the pathogenesis of chronic psychotic disorders
The high prevalence of antineuronal antibodies in FEP contrasts with the low prevalence in established schizophrenia.Reference Masdeu, Gonzalez-Pinto, Matute, Ruiz De Azua, Palomino and De Leon 13 , Reference Steiner, Walter, Glanz, Sarnyai, Bernstein and Vielhaber 16 , Reference Tsutsui, Kanbayashi, Tanaka and Boku 23 , Reference Zandi, Irani, Lang, Waters, Jones and McKenna 24 However, other studies have reported similar rates of psychosis for which an autoimmune aetiology has been postulated. Steiner and colleagues examined the plasma collected within 24 h of admission of 121 patients diagnosed with schizophrenia for antibodies to the NR1a or NR1a/NR2b subunits of the NMDA receptors.Reference Steiner, Walter, Glanz, Sarnyai, Bernstein and Vielhaber 16 In those patients in whom antibodies were detected, CSF was also obtained for testing. In four people, antibodies to the NMDA receptors were identified. Of these, two who were initially diagnosed as having catatonic or disorganised schizophrenia had high titres of IgG NR1a antibodies in both sera and CSF, and were subsequently reclassified as having anti-NMDAR encephalitis.
In a series of 96 women with postpartum psychosis whose plasma was retrospectively tested for antineuronal antibodies, Bergink and colleagues reported two (2%) had anti-NMDAR encephalitis as diagnosed by the presence of NMDAR antibodies to the NR1a subunit detected with a combination of immunohistochemistry, immunocytochemistry and cell-based assay.Reference Bergink, Armangue, Titulaer, Markx, Dalmau and Kushner 10 Both patients were sensitive to extra pyramidal side-effects but otherwise did not display clinical features that differentiated them from other women with postpartum psychosis. Both achieved remission without immunotherapy and as a result, CSF was not obtained for testing. In addition, two other patients had antineuronal antibodies to unidentified antigens, suggesting that other autoimmune encephalitides may also be present in a small percentage of patients with postpartum psychosis.
In a large sample (n = 228) of patients with FEP, Lennox and colleagues reported that 20 (9%) had antineuronal antibodies, including 11 (5%) with VGKC antibodies and 7 (3%) with NMDAR antibodies.Reference Lennox, Palmer-Cooper, Pollak, Hainsworth, Marks and Jacobson 18 No patients were diagnosed with encephalitis or developed neurological symptoms as assessed by their treating psychiatrist. Participants in this study were recruited from community and hospital services and the participation rate is unknown. It is possible the most severely ill patients were unable to consent to be included in this study. In our study, the majority of antibody positive patients (patients 1–5) were too unwell to initially consent, requiring an alternative decision maker to enable participation. Without this sampling method, those with encephalitis may not have been able to participate.
Together, these studies suggest that a small percentage of people presenting to hospital with acute psychosis have an autoimmune illness best detected by testing sera or CSF for neuronal antibodies. Furthermore, it is possible that an initial, untreated insult presenting phenotypically as acute psychosis could lead to long-term changes in brain function phenotypically consistent with chronic schizophrenia, whilst the initial inflammatory cause resolves spontaneously. There are several well-established, postinfectious immune-mediated diseases in which spontaneous normalization of the immune response occurs, with or without detectable autoantibodies, such as in Guillain–Barré syndrome or poststreptococcal chorea. Indeed, although detected and treated cases of anti-NMDAR encephalitis do well in 80% of patients, neurocognitive deficits can persist long after the initial insult.Reference McKeon, Scott, Spooner, Ryan, Blum and Gillis 25 , Reference Finke, Kopp, Pruss, Dalmau, Wandinger and Ploner 26
Strengths and limitations
This study accounted for all patients with a FEP requiring admission to three acute inpatient psychiatric services and engaged a sampling strategy that ensured that the most unwell patients would be included in the study, including accounting for those who refused to participate. It describes the clinical course of the six participants who screened positive for antineuronal antibodies. However, like all research, this clinical study had a number of limitations. There was no healthy control group with whom to compare the prevalence of antineuronal antibodies. However, the current literature suggests that these antibodies are rarely found in nonclinical samples.Reference Irani, Bera, Waters, Zuliani, Maxwell and Zandi 27 , Reference Lang and Pruss 28
Another limitation was that the prevalence of autoimmune encephalitis may have been underestimated as antineuronal antibodies, in particular anti-NMDAR antibodies, were not tested in CSF of all recruited participants, but only in those who had anti-NMDAR and anti-VGKC antibodies detected in the serum. It has recently even been proposed that in patients with psychosis as the only or predominant clinical manifestation of anti-NMDAR encephalitis, the titre of anti-NMDAR antibodies might be too low to be detectable in the serum, and can only be detected in CSF.Reference Masdeu, Dalmau and Berman 29 However, when our methodology was designed, it was decided that a requirement to routinely perform a lumbar puncture for CSF on patients with FEP would have rendered the study unfeasible because of patient refusal. We do recommend testing for antineuronal antibodies in the CSF of people with FEP who have symptoms highly suggestive of an autoimmune encephalitis (acute onset of illness, severe impairment of cognition and confusion, neurological signs), when serum testing for antineuronal antibodies is negative.
Finally, the study was conducted in busy clinical services. Standardised diagnostic instruments were not utilised to obtain the initial diagnosis and there was no use of standardised neuropsychological or functional assessments at follow-up to objectively measure the long-term outcomes.
Clinical significance
This study suggests a small but significant proportion of people unwell enough to require admission to hospital for their FEP have an illness arising from an autoimmune aetiology and respond to immunotherapy. This study supports recent recommendations for routine antineuronal antibody testing as screening for autoimmune encephalitis in patients admitted with FEP.Reference Lennox, Palmer-Cooper, Pollak, Hainsworth, Marks and Jacobson 18 , Reference Galletly, Castle, Dark, Humberstone, Jablensky and Killackey 30 Obviously, these findings require further replication in other FEP populations and should be combined with rigorous assessment of response to immunotherapy, ideally in the context of randomised placebo-controlled trials.
It is important that research is undertaken with people who are presenting initially with psychosis so as to accurately determine the prevalence of autoimmune neuropsychiatric syndromes in this clinical population. This will guide the nature of clinical investigations that may have particular diagnostic utility in this heterogeneous group.
Funding
The study was funded by generous support from the Royal Brisbane and Women's Hospital Foundation and from philanthropic donations (BAP Parking and others). JGS is supported by a National Health and Medical Research Council Practitioner Fellowship grant APP1105807.
Acknowledgements
The authors are grateful to the clinical services and the many clinical staff who supported this study.






eLetters
No eLetters have been published for this article.