Introduction
The significance of food choices for human health is irrefutable, and recent strides in sensory research have unveiled the intricate interplay between taste perception and the oral microbiota.(Reference Schwartz, Gotz and Jeanneret1,Reference Cattaneo, Riso and Laureati2) While much attention has been devoted to understanding the role of gut microbiota in health and disease, the potential influence of oral bacteria on conditions such as obesity has emerged as a promising area of investigation.(Reference Schamarek, Schmöcker and Rohde3–Reference Lin and Li5) Obesity, a multifaceted condition influenced by genetic, environmental and behavioural factors, including dietary habits, is intricately linked with taste sensitivity, which is shaped by genetic predispositions.(Reference Lee, Cardel and Donahoo6,Reference Riaz, Brown and Smith7) Emerging evidence hints at variations in salivary bacterial profiles between individuals with divergent adiposity levels, suggesting a potential link between certain microbial taxa and obesity markers.(Reference Sharara, Alkebsi and Holmes8) Recent studies have shown a relationship between taste sensitivity and body mass index (BMI), with higher BMI being correlated with reduced taste sensitivity.(Reference Ponnusamy, Subramanian and Vasanthakumar9,Reference Shanmugamprema, Muthuswamy and Ponnusamy10) Given the well-established connection between obesity and chronic inflammation or metainflammation, there is a growing interest in elucidating the contribution of oral microbiota in this inflammatory milieu.(Reference Kaufman, Choo and Roberts11–Reference Alessandrini, Maglia and Pastorino13)
The sensory experience of food consumption, shaped by taste perception, profoundly influences food acceptability and consumption patterns. Recent investigations have unveiled the role of enzymatic degradation of taste compounds in modulating taste perception, underscoring the impact of salivary disorders on food pleasure and intake.(Reference Martin, Gutierrez and Torregrossa14–Reference Aji, Warren and Roura16) Furthermore, research exploring the influence of oral microbiota on taste perception and food choices has shed light on how specific microorganisms generate fragrant molecules in the mouth, thereby shaping food perception.(Reference Schwartz, Gotz and Jeanneret1,Reference Li, Zhu and Zhou17,Reference Rud, Gjerdet and Johansen18) These findings suggest that variations in the microbial composition of the oral cavity may contribute to variations in perception, offering novel insights into the intricate relationship between oral microbiota and sensory experiences. While research has explored differences in taste sensitivity, particularly in the perception of bitterness, between obese and non-obese individuals, investigations into other taste qualities and their relationship to obesity, especially in children, remain scarce and inconclusive. Moreover, food neophobia, characterised by a reluctance to try new foods, may contribute to obesity by limiting dietary diversity to energy-dense options.(Reference Hopkins, Ponnampalam and Dunshea19) Additionally, emerging evidence suggests a potential role of oral microbiota in both obesity development and taste perception, highlighting the need for holistic approaches to address these interconnected factors. This growing field not only promises insights into the mechanisms underlying food preferences, but it also has the potential to update strategies for supporting healthier dietary behaviours.(Reference Cattaneo, Riso and Laureati2,Reference Schamarek, Schmöcker and Rohde3,Reference Huang, Chen and Li20)
The current review aims to delineate the existing literature on the relationships between oral microbiota and taste perception, and to identify and discuss shared metabolic pathways between food processing strains and oral bacteria. Essentially, we are revealing the significant effects of these elements on human health and wellness as we investigate the molecular mechanisms behind taste perception and the function of oral bacteria in taste modulation. Furthermore, we attempt to address important gaps in our understanding by offering a concise summary of the most recent research on oral microbiome and taste perception.
Understanding taste perception: a multifaceted process
Taste perception serves as a cornerstone in shaping food preferences and aversions. This complex process involves the interaction of several modalities, including vision, audition, kinesthesis and taste perception. Among these, taste stands out as a fundamental driver, with well-understood mechanisms governing basic taste perception.(Reference Garcia-Bailo, Toguri and Eny21–Reference Fu, Minokoshi and Nakajima24) Food-derived compounds activate taste receptors located on the tongue, soft palate, pharynx and gut, stimulating neuronal fibres that transmit signals to the brain. These signals are then processed into sensory experiences encompassing taste quality, intensity and hedonics.(Reference Lee and Owyang25,Reference Doyle, Kawano and Margolskee26)
Taste receptors serve as crucial chemosensory receptors found in taste buds and various extra-gustatory tissues.(Reference Fu, Minokoshi and Nakajima24,Reference Behrens and Lang27) These receptors play critical role in distinguishing five principal tastes: sweet, salt, sour, umami and bitter. Recent studies suggest the existence of potential new basic tastes, such as kokumi and fat (oleogustus).(Reference Running, Craig and Mattes28–Reference Ozdener, Contreras and Yee31) Taste receptors, which belong to the G protein-coupled receptor (GPCR) superfamily and some ionic channels, exhibit different specificities to taste stimuli. Bitter, sweet and umami taste signals are thought to converge on a common intracellular signalling transduction pathway in type II cells.(Reference Roper and Chaudhari32,Reference Ahmad and Dalziel33) These tastes are activated by GPCR: taste receptor family 1 member (T1R) for sweet and umami stimuli, and taste receptor family 2 member (T2R) for bitter compounds. Upon activation by corresponding stimuli, the G protein coupled to these taste receptors is resolved into Gβγ subunit and Gα subunit, which includes Gα-gustducin, Gα14 and Gαi.(Reference Li, Staszewski and Xu34,Reference Iwata, Yoshida and Ninomiya35)
Taste buds, the sensory organs responsible for transmitting taste sensations, are distributed across various locations in the oral cavity, with the highest concentration on the tongue, particularly within three types of papillae: fungiform, foliate and circumvallate. While taste buds are also present in other areas such as the soft palate, epiglottis, larynx and nasopharynx, research in these regions remains limited.(Reference Witt36,Reference Gravina, Yep and Khan37) Type I cells are predominant and play a supporting role, potentially involved in salt taste perception. Type II cells act as receptor cells, housing receptors for sweet, bitter and umami tastes, while type III cells are considered output cells, responsible for transmitting taste information to the afferent nerve.(Reference Diepeveen, Moerdijk-Poortvliet and van der Leij38,Reference Jang, Kim and Chaudhari39) Despite ongoing research, the exact intercellular signalling within taste buds remains poorly understood. Current models propose that type II cells communicate with type III cells via purinergic signalling mechanisms, and impairment in this signalling leads to diminished gustatory function. The gustatory sensory system, encompassing taste buds, their innervations and the associated papillae, constitutes the taste system. Although resembling somatosensory and pain pathways more closely than olfaction, gustatory afferent neurons share similarities with olfactory sensory cells, including identical transmission processes and turnover of sensory and supporting cells.(Reference Zuccarini, Paleari and Giusti40–Reference Kumari and Mistretta43) Unlike olfactory sensory neurons, which project directly into the brain through nerve fibre bundles called olfactory fila, gustatory sensory neurons first form synapses with taste bud receptor cells before projecting into the brain.
Individual variability in taste perception
Food preferences play a crucial role in determining food intake patterns and are heavily influenced by taste perception and preference. Taste is a fundamental aspect of food choice, as highlighted in the Food Choice Process Model, which identifies taste among the five main values guiding food decisions.(Reference Kourouniotis44,Reference Chen and Antonelli45) Sensory perceptions, including taste sensitivity, vary significantly among individuals and are partly explained by genetic variations in taste perception genes. Genetic factors contribute to individual differences in taste perception and preference, with taste sensitivity and food preferences showing moderate to high heritability estimates.(Reference Ponnusamy46,Reference Feeney47) For example, bitter taste sensitivity, as assessed by compounds such as PROP and quinine hydrochloride, has been found to have high heritability estimates, while sweet taste sensitivity shows moderate heritability. Similarly, food preferences for items such as dessert foods, vegetables, fruits and protein foods also exhibit heritability, indicating the influence of genetic factors on taste-related choices.(Reference Diószegi, Llanaj and Ádány48)
Significant variability exists in taste perception among individuals, attributable in part to differences in taste receptor expression, abundance and salivary flow rate. Genetic predisposition, life stage and eating behaviour further contribute to taste sensitivity disparities. Age, sex and ethnicity contribute to variability in both oral microbiota composition and taste perception. For example, ageing is associated with reduced salivary flow and changes in oral microbiota diversity, which may lead to decreased taste sensitivity.(Reference Duffy and Bartoshuk49,Reference Eriksson50) Women, due to hormonal fluctuations, often exhibit higher sensitivity to certain tastes such as bitterness and sweetness compared with men. Ethnic differences in dietary habits and genetic backgrounds further contribute to the diversity in taste perception and microbial composition.(Reference Eriksson50) Single-nucleotide polymorphisms of taste receptors have been implicated in influencing taste perception and food preferences. Notably, lower sensitivity to a taste often correlates with increased preference for that taste. For instance, individuals categorised as supertasters exhibit heightened responsiveness to bitterness, influencing their dietary choices.(Reference Duffy and Bartoshuk49–Reference Chamoun52) Activation of bitter and umami receptors reduces intracellular cAMP levels by inhibiting protein kinase A through the activity of the Gα subunit. This inhibition of protein kinase A weakens its inhibition on the PLCβ2–IP3 pathway and an increase in Ca2+ release from the endoplasmic reticulum.(Reference Ozeck53–Reference Dong55) Conversely, activation of sweet receptors increases intracellular cAMP levels through the Gα subunit, enhancing protein kinase A activity and inhibiting K+ channels, thereby promoting extracellular Ca2+ influx. Elevated intracellular Ca2+ levels subsequently activate transient receptor potential cation channel subfamily member 5 (TRPM5), an ion channel that induces membrane depolarisation, leading to action potential generation and ATP release.(Reference Welcome, Mastorakis and Pereverzev56,Reference Kunioku57)
Food preferences develop during fetal development and evolve over time, shaped by interactions between genetic predispositions and environmental factors. Early experiences, parental feeding practices, family dynamics and broader environmental contexts all play a role in shaping taste preferences and dietary habits.(Reference Scaglioni58,Reference Ventura and Worobey59) The sensory properties of food, including taste, are key determinants of dietary patterns, with taste receptors responding to specific compounds in food. These receptors, which comprise G-protein-coupled receptors for sweet, umami and bitter tastes and ion channels for salt and sour tastes, mediate taste perception.(Reference Kumar and Behrens60,Reference Pallante61) The finding that the fatty acid transporter CD36 is associated with the fat taste modality has expanded our understanding of taste perception.(Reference Shanmugamprema62) Understanding the genetic basis of taste perception and its association with dietary patterns and health outcomes can inform public health strategies aimed at preventing diet-related non-communicable diseases (NCD). Evolutionary mechanisms may have shaped human taste abilities to avoid the consumption of plant-based toxins, which are often associated with bitter tastes. Moreover, single-nucleotide polymorphisms (SNP) in genes such as TAS2R38 have been shown to significantly influence the perception of bitter taste. These genetic variations not only alter taste receptor expression but also modulate oral microbiota composition, particularly the abundance of Actinobacteria and Bacteroidetes, which interact with taste receptors.(Reference Ponnusamy46,Reference Franzago63)
Exploring the role of oral microbiota
The oral microbiota has received interest in healthy individuals, especially with regard to sensory perception, despite being traditionally investigated in the context of oral diseases. The oral microbiome encompasses various niches within the oral cavity, each characterised by unique environmental factors that shape microbial colonisation and community structure.(Reference Deo and Deshmukh64–Reference Girija and Ganesh66) Saliva, in particular, serves as a major reservoir of oral bacteria, reflecting microbial composition on the tongue dorsum. While certain genera, such as Streptococcus, Actinomyces and Veillonella, are frequently identified in the core oral microbiome, the overall composition and diversity of the oral microbiome exhibit considerable variability across individuals and geographic locations.(Reference Hou65–Reference Wilbert, Mark Welch and Borisy68) Host-related factors, including personal hygiene, diet, genetics and obesity, further influence oral microbiome composition and dynamics.(Reference Ahrens69)
The mouth is home to a bustling community of over 700 different microorganisms, including bacteria, fungi, parasites and viruses. Together, they form what is known as the oral microbiota.(Reference Caselli70) These tiny residents settle in various spots within the mouth, such as the tongue, gums and teeth. Some thrive in areas with little oxygen, such as below the gum line. Teeth, in particular, become host to complex biofilms made up of multiple microbial species. These biofilms foster interactions between different types of microorganisms. It is worth noting that fungi, such as Candida and others, also play a role in this ecosystem, although our understanding of their impact is still evolving. Within this microbial melting pot, bacteria and fungi interact, and the overall balance can shift with changes in diet and oral hygiene habits. Despite these fluctuations, there is a certain level of consistency in the oral microbiota over time. As we age, external factors continue to shape the composition of our oral microbiota.(Reference Berezow and Darveau71,Reference Santonocito72) In infancy, species such as Streptococcus and Lactobacillus dominate, with limited biofilm formation due to the absence of teeth. As teeth emerge and dietary habits change, the microbiota diversifies, influenced by factors such as breastfeeding and early antibiotic use, which can have long-lasting effects on its development.(Reference Azevedo73)
Recent studies have highlighted significant differences in oral microbiome composition between individuals of normal weight and those with obesity. Specifically, obese individuals exhibit microbial signatures in the oral cavity that closely resemble those associated with obesity in the gut.(Reference Rahman74,Reference Gasmi Benahmed75) These findings underscore the potential involvement of oral bacteria in pathways leading to obesity and metabolic dysfunction. Evidence suggests that inflammation plays a crucial role in the metabolic disturbances associated with obesity. Dysbiosis of the gut microbiome has been implicated in inflammation and obesity, prompting investigations into the role of the oral microbiome in similar pathways.(Reference Kang76,Reference Singh77) Studies have highlighted a potential link between oral bacteria and adipose tissue inflammation. For instance, periodontitis-associated systemic inflammation was found to up-regulate inflammatory markers in adipose tissue and liver, exacerbating obesity-related inflammation.(Reference Cecoro78,Reference Yamazaki and Kamada79) Experimental models have further demonstrated that oral bacteria, particularly Porphyromonas gingivalis, can induce local white adipose tissue inflammation and systemic insulin resistance, potentially contributing to metabolic disease progression.(Reference Kang80) Oral microbiota composition may also affect food cravings by modulating taste sensitivity and the hedonic value of foods. Specific strains of Lactobacillus and Bifidobacterium have been associated with reduced cravings for sugary foods, while an imbalance in microbial diversity may lead to heightened cravings for unhealthy options (Table 1). Furthermore, microbial metabolites, short-chain fatty acids (SCFA), are produced during the fermentation of dietary fibres by gut bacteria and can modulate the release of appetite-regulating hormones such as ghrelin and leptin. This interaction creates a feedback loop where the presence of certain microbial communities may increase cravings for specific food types, particularly those high in sugar and fat, which can perpetuate unhealthy eating habits.
Table 1. Microbial impact on taste perception
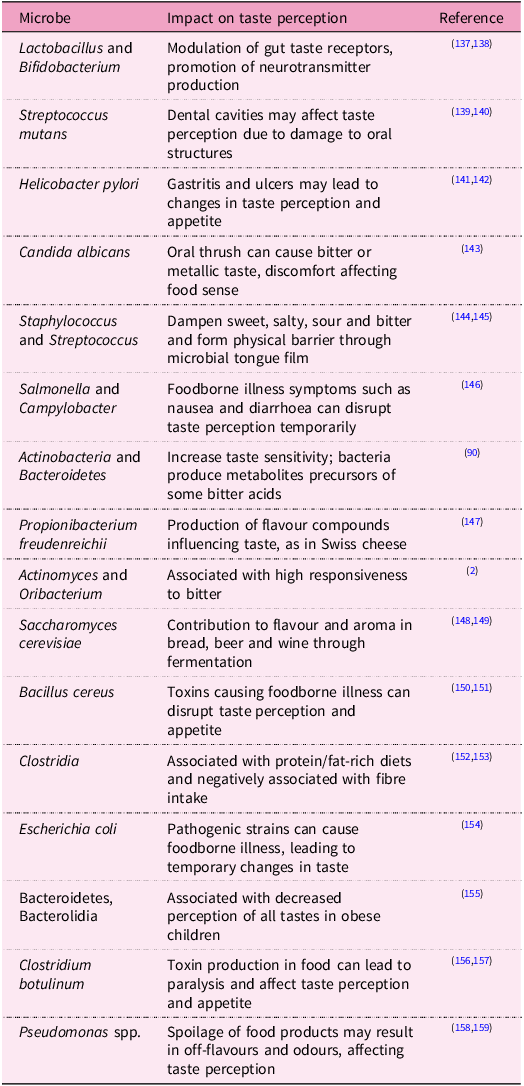
Salivary metabolites, originating from both oral microbiota and host enzymatic activity, influence taste and smell perception through various mechanisms involving the production of flavour-active compounds, which affect the perception threshold and metabolisation of food compounds into sensory-active molecules.(Reference Canon, Neiers and Guichard81,Reference Muñoz-González82) Additionally, the conversion of taste and smell molecules into new compounds without chemosensory properties alters the quantity of flavour compounds. Acetate and propionate, two common salivary metabolites, are SCFA that are primarily produced by oral bacteria.(Reference Gardner83) Their concentrations correlate with bacterial load and can influence the perception threshold of NEFA, which interact with both CD36 and GPR120 receptors, modulating the sensory perception of fat. Amino acids such as glutamate, a key component in umami taste, are also produced by oral bacteria, impacting the T1R1/T1R3 receptor, enhancing umami taste perception.
Microbial enzymes also impact olfactory perception by altering the amplitude and kinetics of odorant responses.(Reference François84) Odorant metabolism by microbial enzymes can generate new odorants in the oral cavity, contributing to flavour perception. Glycoside-derived aroma compounds, mainly produced by bacterial enzymes, highlight the role of the oral microbiome in modulating aroma compound formation and perception.(Reference Mallery85) Microbes can regulate the expression of taste receptor genes, influencing host nutrient detection and taste sensitivity. Germ-free mice exhibit altered expression of taste receptors, leading to increased oral nutrient detection.(Reference Aghili86,Reference Laugerette87) Certain oral bacteria, such as Streptococcus mutans, may impact sweet taste sensitivity, potentially affecting dietary choices and oral microbiota composition. Moreover, bacterial lipopolysaccharides induce cytokine production, reducing taste receptor cell numbers and altering taste perception.(Reference Wang88,Reference Cohn89) Understanding these common pathways sheds light on the intricate relationship between oral microbiota and flavour perception, offering insights into aroma modulation in both fermented products and the oral cavity.
Influence of oral microbiota on dietary preferences
Recent studies suggest a bidirectional relationship between the oral microbiota and dietary preferences, indicating that microbes may influence taste sensitivity and, in turn, dietary choices.(Reference Cattaneo90–Reference Gardner, So and Carpenter92) Investigations in healthy individuals categorised as supertasters or non-tasters revealed intriguing associations between taste perception, microbial composition and dietary habits. Supertasters exhibited lower taste detection thresholds and higher density of certain bacteria on the tongue, suggesting a potential link between microbial abundance and taste sensitivity.(Reference Cattaneo, Riso and Laureati2) Moreover, correlations were observed between bacterial profiles, taste recognition thresholds and dietary intake, shedding light on the intricate interplay between oral microbiota and food preferences.(Reference Li, Zhu and Zhou17,Reference Cattaneo90) For example, individuals with altered taste sensitivity may exhibit preferences for certain types of foods, such as sweets or fatty foods, leading to imbalanced diets and potential health consequences. Understanding these associations can inform strategies for promoting healthier dietary habits and managing conditions such as obesity and dental caries.
The reduction in taste bud numbers has been observed in individuals with obesity, and inflammatory processes are implicated as the underlying cause. Studies have shown that inflammatory stimuli, such as lipopolysaccharides (LPS), can inhibit the proliferation of progenitor cells in taste buds, leading to a decrease in taste cell turnover.(Reference Wang, Zhou, Brand and Huang93,Reference Kumarhia, He and McCluskey94) Elevated levels of tumour necrosis factor alpha (TNFα) associated with low-grade inflammation in obesity have been linked to a lower abundance of taste buds. Interestingly, using TNFα-deficient mice, researchers found no changes in taste bud numbers after obesity induction, suggesting that taste bud loss may be a consequence rather than a cause of obesity in these animals.(Reference Feng95,Reference Feng96) The exact mechanisms by which changes in the oral microbiome affect TNFα levels or contribute to the observed taste bud alterations require further investigation.
Recent studies have focused on how taste receptors engage in immune responses and interact with oral microbiota, providing insights into their role in oral diseases. The ability to perceive sweetness is governed by the T1R2/T1R3 heterodimer receptor. Genetic variations in T1R2 and T1R3 genes influence thresholds for sweet taste perception and food preferences.(Reference Ponnusamy, Subramanian and Vasanthakumar9) These genetic differences can lead to varying levels of sugar intake, impacting the risk of metabolic disorders and oral diseases.(Reference Eny97,Reference Melo98) Current studies also have demonstrated that SNP in CD36, a gene implicated in fat taste perception, influence the interaction between oral microbiota and taste receptors, particularly through microbial metabolites such as SCFA. These variations can alter individual sensitivity to dietary fats, which is further modulated by the composition of oral bacteria. Similarly, SNP in T1R2 and T1R3 influence sweet taste perception, with oral microbial composition playing a critical role in mediating taste sensitivity.(Reference Ponnusamy, Subramanian and Vasanthakumar9,Reference Ponnusamy46) (Fig.1).
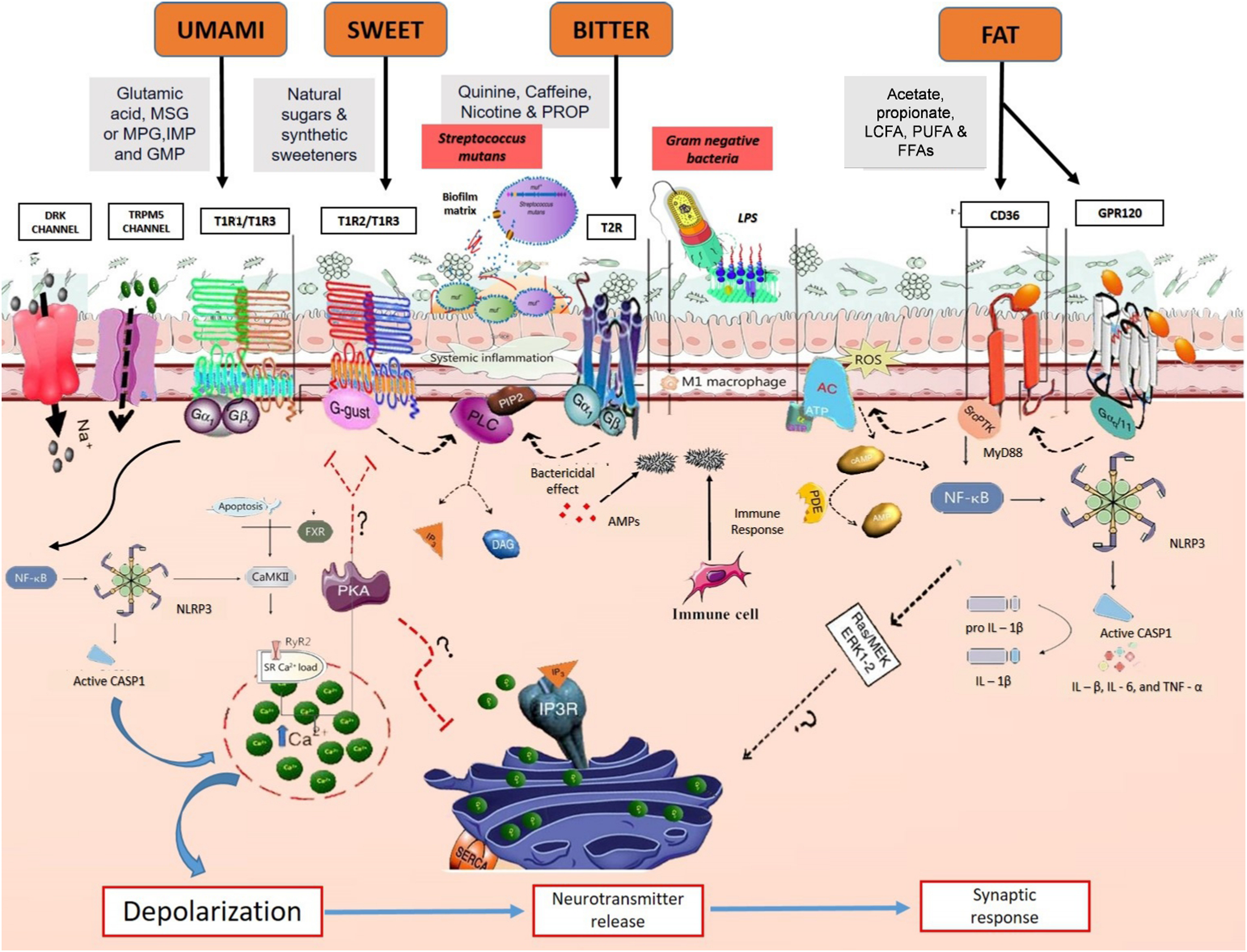
Figure 1. Oral microbiome and metabolite-mediated molecular pathways: interplay with taste receptors, bacterial stimuli and oral immune responses in taste modulation. Taste perception involves the activation of G-protein coupled receptors (GPCR) in taste receptor cells. Bitter taste is detected by T2R receptors, while sweet and umami tastes are detected by T1R1/T1R2/T1R3 receptors. Upon stimulation of these receptors, the downstream Gβγ complex is activated, initiating a cascade of intracellular events. First, phospholipase C isoform β2 (PLCβ2) is activated, leading to the production of inositol 1,4,5-trisphosphate (IP3). IP3 then triggers the IP3 receptors (IP3R) on the endoplasmic reticulum (ER), causing the release of calcium ions (Ca2+) into the cytoplasm. This increase in intracellular calcium activates transient receptor potential cation channel subfamily M member 5 (TRPM5), resulting in sodium ion (Na+) influx and cell depolarisation. Depolarisation activates calcium homeostasis modulator 1 (CALHM1) channels, leading to the release of adenosine triphosphate (ATP) as a neurotransmitter. Furthermore, bacterial stimuli, such as S. mutans competence stimulating peptide-1 (CSP-1) and lipopolysaccharides (LPS), can modulate taste receptor signalling pathways and trigger immune responses. CSP-1 primarily activates the Gβγ–PLCβ pathway, leading to intracellular calcium mobilisation and secretion of cytokines/chemokines, while LPS induces adenylate cyclase (AC) activity, elevating intracellular cyclic adenosine monophosphate (cAMP) levels. This, in turn, activates the NF-κB pathway, promoting the release of pro-inflammatory cytokines. Activation of taste receptors by bacterial metabolites can also stimulate the release of antimicrobial peptides (AMP) from epithelial cells and recruit immune cells to modulate the oral immune response. Additionally, oral flora contributes to M1 macrophage polarisation and modulation of NLRP3 inflammasome activation. SCFA attenuate NLRP3 signalling, preventing disruption of calcium handling and NLRP3 activation.
Research by Kulkarni et al. (2013)(Reference Kulkarni99) and Haznedaroglu et al. (2015)(Reference Haznedaroğlu100) revealed a correlation between the Ile191Val polymorphism and sugar consumption in relation to dental caries risk. Their findings indicate that individuals with this polymorphism tend to consume less sugar and have a reduced likelihood of developing dental caries. Moreover, increased sugar intake associated with T1R2/T1R3 SNP can alter the oral microenvironment, affecting the composition of oral microflora and increasing the risk of caries. Research has demonstrated associations between T2R38 SNP and dental caries risk, with ‘supertasters’ exhibiting a lower risk of caries. Additionally, other bitter taste receptors, such as T2R14 and CA6, have been implicated in interactions with oral flora and the risk of dental caries.(Reference Mathew101,Reference Furquim102) The impact of SNP in other taste receptors, such as ENaC and OTOP1, on taste perception and susceptibility to oral diseases requires further investigation.
Many oral health issues arise from an imbalance in bacterial communities, leading to the buildup of harmful biofilms, commonly referred to as dental plaques.(Reference Bowen103) Dental caries, or tooth decay, stem from excessive sugar consumption, fostering the growth of acid-producing bacteria such as S. mutans that erode tooth enamel.(Reference Lamont, Koo and Hajishengallis104) Halitosis, commonly known as bad breath, results from the proliferation of bacteria on the tongue’s surface, particularly those producing volatile sulphur compounds such as Porphyromonas and Fusobacterium.(Reference Krespi, Shrime and Kacker105) Beyond these oral afflictions, xerostomia, often associated with conditions such as Sjogren’s syndrome, leads to dry mouth sensation due to altered salivary gland function. This condition predisposes individuals to infections from non-oral bacteria, likely due to decreased saliva flow and compromised immune protection.(Reference MacFarlane and Mason106) As saliva plays a crucial role in oral health maintenance, its diminished presence exacerbates these disorders.
The microbial community in saliva mirrors that of the oral mucosa and tongue,(Reference Bescos107) encompassing over 2000 bacterial proteins across 50 bacterial genera.(Reference Grassl108) Environmental factors wield greater influence over microbiota composition than host genetics, with familial upbringing imprinting a lasting mark on salivary microbiome composition over years.(Reference Shaw109) Some studies advocate specific diets to deter periodontitis by curbing the proliferation of pathogenic anaerobes.(Reference Rowińska110) Consumption of tea-rich diets amplifies oral microbial diversity, favouring genera such as Fusobacteriales and Clostridiales.(Reference Peters111) Conversely, oolong tea consumption diminishes microbial diversity, targeting species such as Streptococcus sp., Prevotella nanceiensis and Fusobacterium periodonticum,(Reference Liu112) owing to antimicrobial flavan-3-ol compounds such as epigallocatechin gallate found in tea.(Reference Stapleton113)
Unravelling the nexus between oral microbiota and taste sensitivity
Among the various niches within the human body, the oral cavity emerges as a focal point, teeming with a bacterial populace nearly equivalent in number to the human cellular constituents, second only to the gastrointestinal tract.(Reference Kitamoto114) While these microbial ensembles typically navigate daily challenges with remarkable resilience, disturbances such as frequent sugar intake or compromised immune defences can precipitate dysbiosis, disrupting their delicate regulatory sway over the host. The build-up of oral bacteria on the tongue forms a film that acts as a physical barrier, impeding tastants from accessing taste receptors.(Reference Madiloggovit, Chotechuang and Trachootham115) Studies reveal that individuals with tongue coating often exhibit diminished taste sensitivity across various taste modalities. Notably, the removal of such coating, whether through brushing or scraping, correlates with enhanced taste perception, underscoring the pivotal role of oral bacteria in taste modulation.(Reference Feng116)
Beyond physical obstruction, oral bacteria contribute to taste perception through the production of metabolites that wield multifaceted effects (Fig.1). These metabolites can modulate taste perception thresholds, directly activate taste receptors or even degrade flavour compounds, thereby influencing their interaction with taste receptors.(Reference Gardner, So and Carpenter92) Consequently, variations in oral bacterial metabolism manifest as differences in taste sensitivity, highlighting the intricate interplay between oral microbiota composition and taste perception.(Reference Cattaneo, Riso and Laureati2) Additionally, taste receptors, beyond their canonical role in sensing chemical stimuli, also serve as sentinels for microbial detection, orchestrating immune responses that contribute to oral microbial homeostasis.(Reference Dong55) Recent investigations into taste perception have spotlighted the PROP phenotype as a pivotal factor influencing individual taste sensitivity and food preferences. Studies have consistently shown that supertasters (ST) rate certain tastes – such as bitterness of caffeine, sweetness of sucrose, saltiness of sodium chloride and sourness of citric acid – as more intense compared with non-tasters (NT). Moreover, our recent report suggests a correlation between the higher density of fungiform papillae (FPD) on ST and their heightened sensitivity.(Reference Ponnusamy117) While initial hypotheses proposed a connection between higher FPD in ST and increased sensitivity, recent research has offered new perspectives, further expanding our understanding of this association.(Reference Melis and Tomassini Barbarossa118)
Despite the expanding focus on tongue physiology, genetics and related phenotypes, studies examining specific oral microbial communities and their relationship with taste perception are scant, often employing limited methodologies for taste perception evaluation or oral microbiota analysis. For instance, Solemdal and colleagues(Reference Solemdal119) found that taste perception, particularly sourness, was diminished in acutely hospitalised elderly individuals with high lactobacilli growth, suggesting a potential role of bacterial organic acids in modulating taste thresholds. Conversely, Besnard et al.(Reference Besnard120) identified specific bacterial and salivary signatures discriminating between NT and ST but solely assessed the detection threshold for linoleic acid. Analysis of tongue microbiomic profiling data revealed no significant differences in intra- and inter-subject ecological diversity between ST and NT groups, consistent with previous observations of limited microbial community variation among healthy adults. Moreover, the presence of Actinobacteria and Bacteroidetes has been associated with increased taste sensitivity, potentially through the production of secondary metabolites that influence bitterness and astringency perception.(Reference Besnard91,Reference Hopwood121,Reference Ley122) Collectively, these studies illustrate how alterations in the microbial community of the tongue can influence taste sensation (Table 1). However, the oro-sensory implications of such changes remain to be fully understood, necessitating further research employing robust analysis techniques on predicted metagenomics data to explore microbial metabolic pathways.
Mechanistic insights into microbiota-mediated taste sensitivity
Understanding the intricate relationship between oral bacteria and taste perception sheds light on how the oral microbiome influences dietary preferences, food intake and potentially weight regulation. While the mechanistic underpinnings of oral microbiota’s role in taste sensitivity are still emerging, two predominant mechanisms have been proposed. Firstly, the microbial biofilm development may generate a physical barrier that restricts the contact of tastants to their receptors, thereby modulating taste perception.(Reference Ohno123) Secondly, the metabolic activity of oral microbiota could influence taste sensitivity through the consumption or production of bioactive metabolites derived from food or saliva. For instance, saccharolytic bacteria can degrade carbohydrates into organic acids, potentially altering taste perception, while proteolytic bacteria may produce metabolites that influence taste sensitivity.(Reference Takahashi124) Notably, a novel hypothesis implicating differences in methanogenesis activity of oral microbiota in modulating fatty taste perception in obese individuals has recently emerged, suggesting a nuanced interplay between microbial metabolism and taste perception.(Reference Besnard125)
Several mechanisms have also been proposed to elucidate the connection between oral bacteria and obesity-related metabolic disorders. These mechanisms comprise local influences of oral bacteria on taste perception and food preferences, as well as systemic effects on adipose tissue function, gut microbiome composition and systemic inflammation.(Reference Rohde, Schamarek and Blüher12,Reference Gasmi Benahmed75,Reference Goodson126,Reference Endo127) Notably, changes in inflammatory tone, modulation of gut microbiome composition, and alterations in taste perception may contribute to the development and maintenance of obesity and metabolic disease.(Reference Rohde, Schamarek and Blüher12,128) Two proposed mechanisms connect oral bacteria with inflammatory and metabolic effects in distant organs: the oral–gut axis(Reference Lee129–Reference Kleinstein, Nelson and Freire131) and the oral–blood axis.(Reference Konkel, O’Boyle and Krishnan132–Reference Ogrendik134) The oral–gut axis suggests that oral bacteria can translocate to the gut, influencing gut microbiome composition and metabolic processes. Recent studies support this notion, demonstrating extensive transmission of oral bacteria to the intestine and their potential impact on gut health and metabolism.(Reference Lee129) Meanwhile, the oral–blood axis posits that oral bacteria and inflammatory molecules can enter the bloodstream, leading to systemic inflammation and local inflammatory responses in distant organs. This pathway has been implicated in various inflammatory diseases, including rheumatoid arthritis and cardiovascular diseases.(Reference Han and Wang133,Reference Ogrendik134)
Chemical perception arises from the activation of chemoreceptors by a diverse range of compounds across different chemical families. Recent studies indicate that metabolic processes in the mouth can alter both the quality and quantity of compounds activating these receptors.(Reference Muñoz-González82,Reference Ijichi135) Three mechanisms through which the oral microbiota can modulate host chemosensory perception can be elucidated. Firstly, microbial enzymes generate metabolites that activate or modulate host chemoreceptors.(Reference Parker136) Secondly, bacterial metabolism of exogenous molecules contributes to terminating their perception.(Reference François84) Thirdly, the microbiota can manipulate the host’s chemical senses by altering receptor density.(Reference Wang88,Reference Cohn89) Understanding these intricate interplay between the oral microbiome and obesity-related metabolic disturbances holds promise for the development of novel therapeutic approaches.
Mapping the current landscape and identifying future directions
As our understanding of how taste perception intertwines with the oral microbiota progresses, it is evident that there are significant gaps in our knowledge, prompting a call for further investigation. While some studies have hinted at links between oral bacteria and taste sensitivity, there are inconsistencies that need addressing, emphasising the necessity for thorough longitudinal studies. Future research should prioritise uncovering fundamental relationships, decoding natural fluctuations and investigating the effects of perturbations. Collaborative efforts across disciplines are vital for fully comprehending the intricate dynamics of human host–microbiome interactions and for exploring the potential manipulation of taste perception through oral microbiota modulation. The association between the oral microbiome and obesity is gaining recognition, with oral bacteria implicated in various mechanisms underlying metabolic diseases. These bacteria contribute to metabolic inflammation in adipose tissue and target different metabolic organs in various pathological conditions. However, further investigations are necessary to fully understand the pathways connecting oral bacteria with distant metabolic tissues and the potential metabolic disruptions they may cause. While both the oral–blood axis and the oral–gut axis show promise as routes of bacterial translocation, their exploration has been uneven. The oral–blood axis has been primarily explored in dental medicine, focusing largely on dental pathogens, while the oral-gut axis, despite its implication in several metabolic disorders, requires deeper investigation, especially concerning its role in obesity and the potential impact of oral bacteria on gut microbiome composition.
Furthermore, investigating the interaction between oral bacteria and taste cells and receptors, as well as potential central effects regulating food preference akin to the gut-brain axis, deserves attention in forthcoming studies. These insights suggest that the oral microbiome may wield a more significant influence on obesity and metabolic diseases than previously acknowledged, emphasising the urgency of understanding these mechanisms and their health implications. Disruptions in taste receptors can interrupt oral microbial balance and exacerbate diseases like periodontitis, suggesting potential alternative therapeutic avenues for oral diseases caused by specific pathogens. Delving into genetic variations in taste receptors could aid in predicting susceptibility to oral diseases and tailoring personalised treatment strategies. In addition to summarising the key points of the review, future research should focus on addressing specific questions such as understanding the molecular mechanisms by which microbial metabolites interact with taste receptors and how these interactions influence long-term dietary behaviour. Methodologies for future studies could include longitudinal assessments and intervention-based research, particularly using probiotics or prebiotics to modulate the oral microbiota. Clinically, these findings pave the way for developing personalised dietary recommendations tailored to an individual’s taste perception and oral microbiota profile, offering potential for more effective dietary interventions, especially in the context of obesity and metabolic disorders.
Concluding remarks
In conclusion, our review sheds light on the intricate interplay between oral microbiota and taste sensitivity, expanding our understanding of human taste perception. While previous studies have predominantly focused on genetic and physiological determinants, this review underscores the potential role of oral bacteria in modulating taste perception. Our findings suggest that the composition of oral microbiota may contribute to individual differences in taste sensitivity, with certain bacterial species potentially enhancing or dampening taste perception through their metabolic activities. However, future investigations leveraging advanced analytical techniques on metagenomic data hold promise for uncovering microbial metabolic pathways that may serve as biomarkers for taste sensitivity phenotypes. Ultimately, a deeper understanding of how oral microbiota influence taste perception could have implications for personalised nutrition interventions and the development of novel strategies to promote healthy eating behaviours. As we continue to unravel the complexities of taste perception, incorporating the role of oral microbiota promises to unveil new insights with far-reaching implications for human health and wellbeing.





