Kidney stone is a chronic disease, with a high prevalence of about 10 % around the world, and is correlated with high cost and morbidity(Reference Scales, Smith and Hanley1,Reference Saigal, Joyce and Timilsina2) . The mechanisms of stone formation can only be identified in a few cases, where congenital abnormalities of the urinary tract or defined disorders of Ca and oxalate metabolism are discovered(Reference Gambaro, Croppi and Coe3). Otherwise, the main risk factors associated with kidney calculi are represented by poor fluid intake(Reference Ticinesi, Nouvenne and Borghi4) and dietary imbalances, including excessive salt(Reference Ticinesi, Nouvenne and Maalouf5) and animal protein intake(Reference Turney, Appleby and Reynard6).
Diet patterns could be characterised by pro-inflammation or anti-inflammation. Different dietary interventions may impact the risk of kidney stone formation and recurrence. Dietary Inflammatory Index (DII) was developed as a standardised scoring system to evaluate the effects of diet on inflammation(Reference Shivappa, Steck and Hurley7). Currently, the DII approach to estimate the pro-inflammatory status of individual dietary intakes has been shown to link high DII scores with adverse health outcomes, such as general obesity(Reference Ruiz-Canela, Zazpe and Shivappa8), cancer(Reference Fowler and Akinyemiju9) and CVD(Reference Namazi, Larijani and Azadbakht10) in various general populations.
The role of nutrition in nephrolithiasis formation has been identified recently. Increased high-oxalate diet intake may significantly accelerate oxalate secretion(Reference Siener, Bade and Hesse11), and high consumption of animal protein (like egg white) reduces urinary pH and elevates urinary uric acid(Reference Borghi, Schianchi and Meschi12), which are harmful elements for the development of Ca and uric acid stones. However, the mechanisms of how pro-inflammatory diet affects the immune system and inflammatory response in kidney stone formation are not fully understood. Some clinical researches noted that a high DII score has also been positively associated with increased levels of inflammatory markers such as TNF, IL-6 and C-reactive protein (CRP)(Reference Tabung, Steck and Zhang13). Besides, experimental evidence in rats showed that some inflammatory cells like macrophages have been reported to facilitate kidney stones formation(Reference de Water, Noordermeer and Houtsmuller14).
To our knowledge, relatively few researches have investigated a potentially inflammatory dietary pattern and kidney stones development. Therefore, our study aimed to evaluate the effect of DII on kidney stones using data from the US National Health and Nutrition Examination Survey (NHANES). We hypothesised that increasing inflammatory potential of dietary intake (i.e. higher DII scores) is associated with higher risk of kidney stones.
Materials and methods
Data source and study population
We performed an analysis using data from the NHANES, a periodic cross-sectional survey to monitor trends in the health and nutritional status of the non-institutionalised US civilian population conducted by the Centers for Disease Control and Prevention. The NHANES provides prevalence estimates for an array of common diseases by performing a complex, multistage, probability sampling design. For the present analysis, five survey cycles (i.e. 2007–2008, 2009–2010, 2011–2012, 2013–2014 and 2015–2016) were combined to produce estimates with greater precision and smaller sampling error. We only include non-pregnant participants aged 18–80 years (n 50 588). Then, we excluded participants without complete information on kidney stones (n 21 467) and dietary intake (n 3137). In total, 25 984 eligible individuals of the NHANES were included. The NCHS Research Ethics Review Board approved the study protocol of 2007–2010 (protocol 2005–2006) and 2011–2016 (protocol 2011–2017) NHANES, and all participants provided written informed consents(15).
Exposure and outcome definitions
Dietary intake information was assessed using the first 24-h dietary recall interviews. NHANES processed the dietary data to acquire micro- and macronutrient contents by using the USDA’s Food and Nutrient Database for Dietary Studies that is specific for the years during which each of the 2-year cycles of NHANES was conducted. Shivappa et al. reported the development of the calculation of DII. ‘Inflammatory effect scores’ were evaluated from the peer-reviewed publications for forty-five DII food parameters which include nutrients, foods and bioactive compounds that were assessed based on their relation to six inflammatory cytokines (IL-1β, IL-4, IL-6 and IL-10) in addition to CRP and TNF-α (Reference Shivappa, Steck and Hurley7). In our analysis, twenty-seven of the forty-five food parameters were available to calculate DII, including energy, carbohydrate, fibre, protein, cholesterol, fat, SFA, MUFA, PUFA, niacin, vitamin B6, vitamin B12, Mg, Se, Zn, Fe, thiamin, riboflavin, vitamin A, vitamin C, vitamin D, vitamin E, beta carotene, folic acid, n-3, n-6 and alcohol. Previous studies have reported a stable predictive ability when only using twenty-eight food parameters(Reference Shivappa, Steck and Hurley16). The process of DII score calculation is presented in Supplementary material 1. Finally, all scores were summed from all food parameters to calculate the overall DII score. Higher numerical DII scores indicate a greater pro-inflammatory state of the diet, while lower numerical scores are consistent with anti-inflammatory diets(Reference Shivappa, Steck and Hurley7). The DII score was analysed as a continuous variable, and then we categorised into quartiles (Q1, Q2, Q3, Q4) from the total sample size.
The Kidney Conditions questionnaire was directed at adults aged 20 years and older, which includes questions about a history of nephrolithiasis from 2007 to 2016. The accuracy of self-reported kidney stones has been reported elsewhere; Curhan et al. have confirmed the validity of self-reported stones in the Health Professionals Follow-up Study by analysing medical records from a random sample of sixty men in the cohort. The chart review confirmed that 97 % of the cases reported kidney stone(Reference Curhan, Willett and Rimm17). A similar study in the Nurses’ Health Study I examined medical records from a random sample of ninety women who reported kidney stone. The records confirmed the diagnosis for all except 1 participant (98 %)(Reference Curhan, Willett and Speizer18). Survey participants who answered yes to ‘Have you/Has sample person (SP) ever had a kidney stone?’ were considered to have a history of nephrolithiasis. A follow-up question was then asked: ‘How many times have you/has SP passed a kidney stone?’ We divided the participants into two groups, passed a kidney stone <2 times as well as ≥2 times. We interpret the latter to mean a recurrence of passing kidney stones
Covariates
Potential covariates were identified a priori based on a review of the literature(Reference Fantus, Packiam and Wang19,Reference Wirth, Sevoyan and Hofseth20) . The following confounders were summarised in multivariable-adjusted models: continuous variables consisted of age, poverty:income ratio, BMI, energy, categorical variables included gender (male/female), marital status (married or living with partner/single), race/ethnicity, insurance, education, smoking, alcohol intake per week, physical activity and co-morbidity index. Co-morbid conditions consisted of diabetes mellitus, congestive heart failure, coronary artery disease, chronic obstructive pulmonary disease (chronic bronchitis and/or emphysema), hypertension and cancer. The number of subject reported conditions was then combined to generate an ordinal co-morbidity index(Reference Fantus, Packiam and Wang19).
Statistical analysis
Statistical analysis was performed in accordance with CDC analytical reporting guidelines for complex NHANES data analysis (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). A sample weight was assigned to each person participating in the NHANES. Hence, we considered masked variance and used the recommended weighting methodology. Data are expressed as mean ± sd or proportions. To calculate for differences among different DII score groups (quartiles), statistical differences were determined using a weighted t test for continuous variables, while a weighted χ 2 test was used for categorical variables.
Our statistical analysis consisted of three main strategies to examine whether DII is associated with kidney stones. First, we employed weighted univariate which was simple and easy to interpret, and multivariate logistic regression models were then performed. We estimated the crude model (model 1) as well as model 2 (only gender; age and race were adjusted). In the final model (model 3), we further adjusted for BMI; poverty:income ratio; education level; insurance; marital status; alcohol intake per week; physical activity; co-morbidity index; energy (kcal) and smoking. Second, to account for the non-linear association between DII score and kidney stone, we performed a smooth curve fitting (penalised spline method) and a weighted generalised additive model regression. Third, to further determine the correlation between DII and kidney stones, we used weighted stratified logistic regression models to conduct subgroup analyses. In sensitivity analysis, we further excluded patients with stone disease related to metabolic abnormalities such as Crohn’s disease, primary hyperparathyroidism and non-alcoholic fatty liver disease, as stone disease in this population may not be related to diet habitus.
All analyses were performed using the statistical software packages R (http://www.R-project.org; The R Foundation) and EmpowerStats (http://www.empower stats.com, X&Y Solutions, Inc.). All P values <0·05 (two-sided) were considered statistically significant.
Results
Participants’ baseline characteristics
Data on kidney stones as well as DII scores were available on 25 984 NHANES participants older than 20 years. The basic demographic characteristics and other covariates of the included participants in the NHANES 2007–2016 population, according to DII score quartiles, are summarised in Table 1. The participants in this sample averaged 49·41 ± 17·71 years old, with males representing 48·6 %. Mean ± sd DII score was 1·30 ± 2·00, with 9·4 % reporting a history of kidney calculi and 32·5 % of these experiencing recurrent kidney calculi.
Table 1 Characteristics of participants in the 2007–2016 continuous National Health and Nutrition Examination Survey*,†,‡
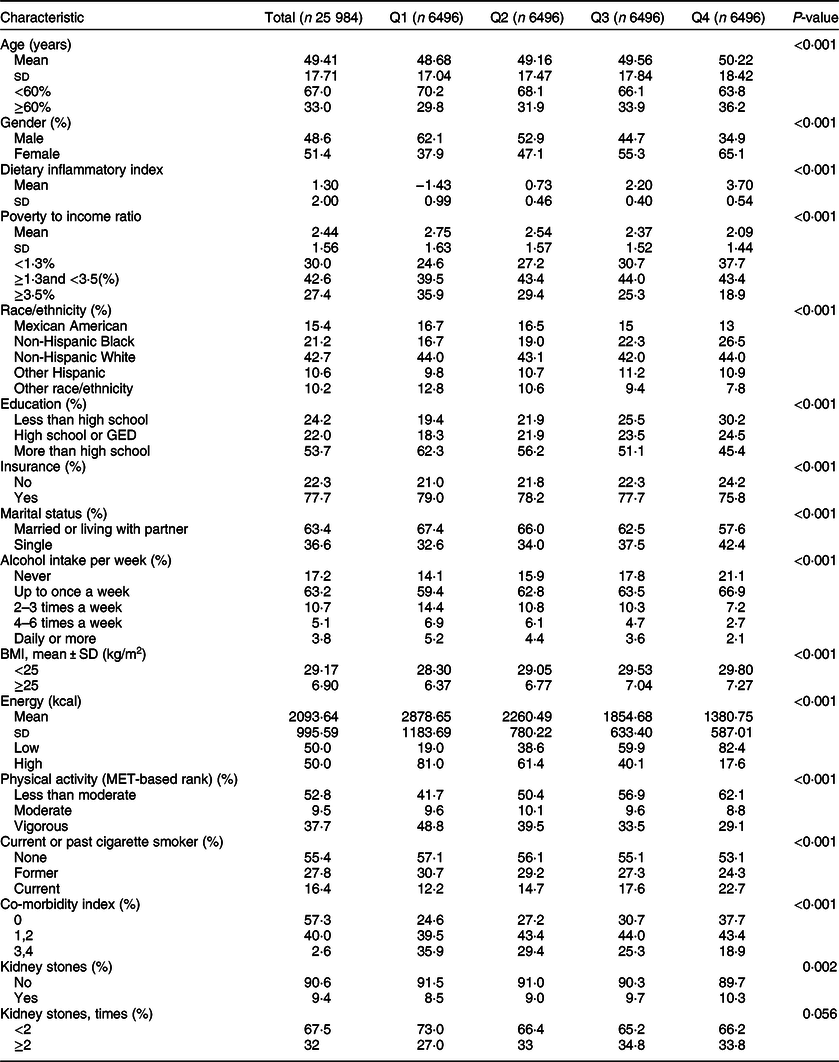
DII, Dietary Inflammatory Index; GED, General educational development.
* Mean and sd for continuous variables: P value was calculated by weighted t test.
† % for Categorical variables: P value was calculated by weighted χ 2 test.
‡ DII quartile ranges: Quartile 1 = –5·18 to–0·12; Quartile 2 = –0·12 to 1·50; Quartile 3 = 1·50 to 2·88, Quartile 4 = 2·88 to 5·48
Multivariate regression analysis
For the primary outcome, our multivariate regression analysis noted that DII score positively correlated with nephrolithiasis (OR = 1·07; 95 % CI 1·04, 1·10) (see Table 2). Q3 and Q4 had a significantly higher risk of nephrolithiasis than Q1 in the non-adjusted model (model 1, OR = 1·15; 95 % CI 1·02, 1·30; OR = 1·24; 95 % CI 1·10, 1·40), minimally adjusted model (model 2, OR = 1·24; 95 % CI 1·09, 1·40; OR = 1·40; 95 % CI 1·24, 1·58) and fully adjusted model (model 3, OR = 1·22; 95 % CI 1·06, 1·40; OR = 1·38; 95 % CI 1·19, 1·60), while there is no significant difference between Q1 and Q2. For example, compared Q4 with Q1, a significant 38 % increased likelihood of nephrolithiasis was observed (OR = 1·38; 95 % CI 1·19, 1·60). Furthermore, the risk of nephrolithiasis rose significantly stepwise across DII score quartiles (P for trend < 0·0001). In sensitivity analysis, the positive association still remained significant after excluding patients with stone disease related to metabolic abnormalities (data not shown).
Table 2 Association of Dietary Inflammatory Index with kidney stones
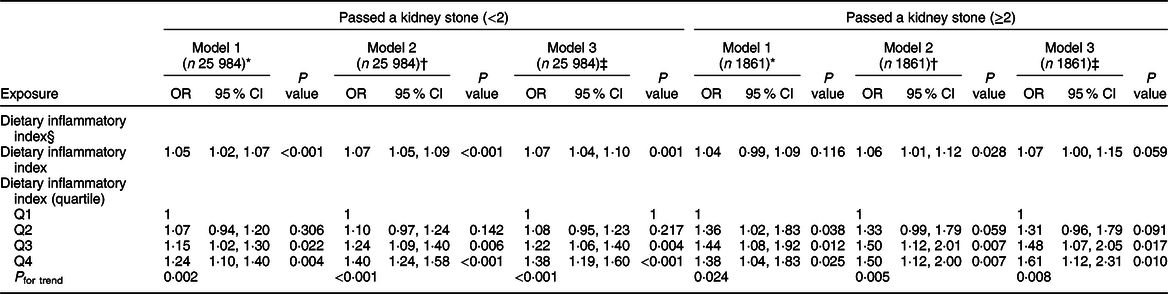
* Model 1: no covariates were adjusted.
† Model 2: adjusted for gender; age; race.
‡ Model 3: adjusted for gender; age; race; poverty:income ratio; education level; insurance: marital status; alcohol intake per week; physical activity; comorbidity index; energy (kcal); BMI; smoking.
§ DII quartile ranges: Quartile 1 = −5·18 to −0·12; Quartile 2 = −0·12 to 1·50; Quartile 3 = 1·50 to 2·88, Quartile 4 = 2·88 to 5·48
For the secondary outcome, multivariate regression analysis showed that DII score also positively correlated with nephrolithiasis recurrence (OR = 1·07; 95 % CI 1·00, 1·15) (see Table 2). After fully multivariate adjustment, the results noted that higher DII scores (Q3 and Q4) are positively associated with a significant 48 % and 61 % increased risk of nephrolithiasis recurrence compared with the reference (OR = 1·48; 95 % CI 1·07, 2·05; OR = 1·61; 95 % CI 1·12, 2·31). Furthermore, the risk of nephrolithiasis recurrence significantly increased stepwise when the DII score was classified as a categorical variable (quartiles) (P for trend = 0·0076).
Nonlinearity analysis and subgroup analyses
We also analysed the non-linear relationship between DII and nephrolithiasis formation and its recurrence (see Fig. 1, Fig. 2). Then, in the multivariable models, no statistical significance was indicated by the interaction terms in the association between DII and kidney stone incidence. In stratified analyses for people with recurrent kidney stones, except for the physical activity, there was no statistically significant interaction effect after adjusting for covariates. With a 1 sd increase in DII score, the odds of recurrent nephrolithiasis among those with less than moderate, moderate and vigorous physical activity increased 10 % (1–20 %), 25 % (1–55 %) and –2 % (–10–8 %), respectively (P for interaction = 0·0375) (see Table 3).
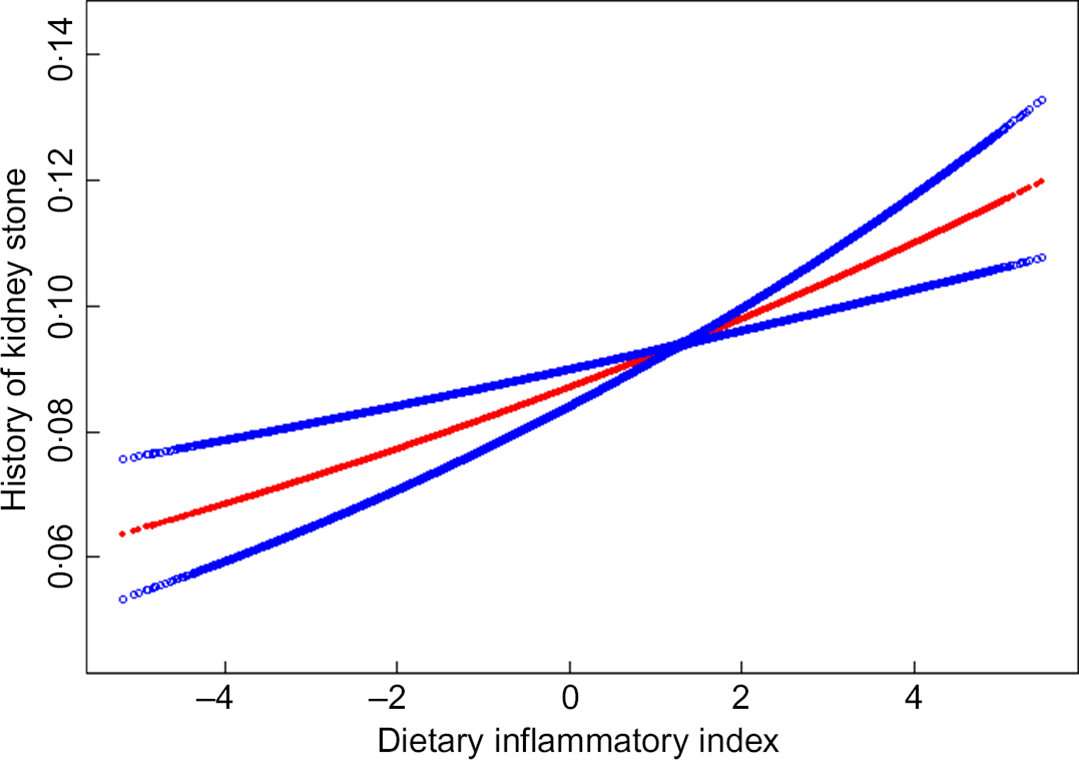
Fig. 1 The non-linear relationship between Dietary Inflammatory Index and history of kidney stone
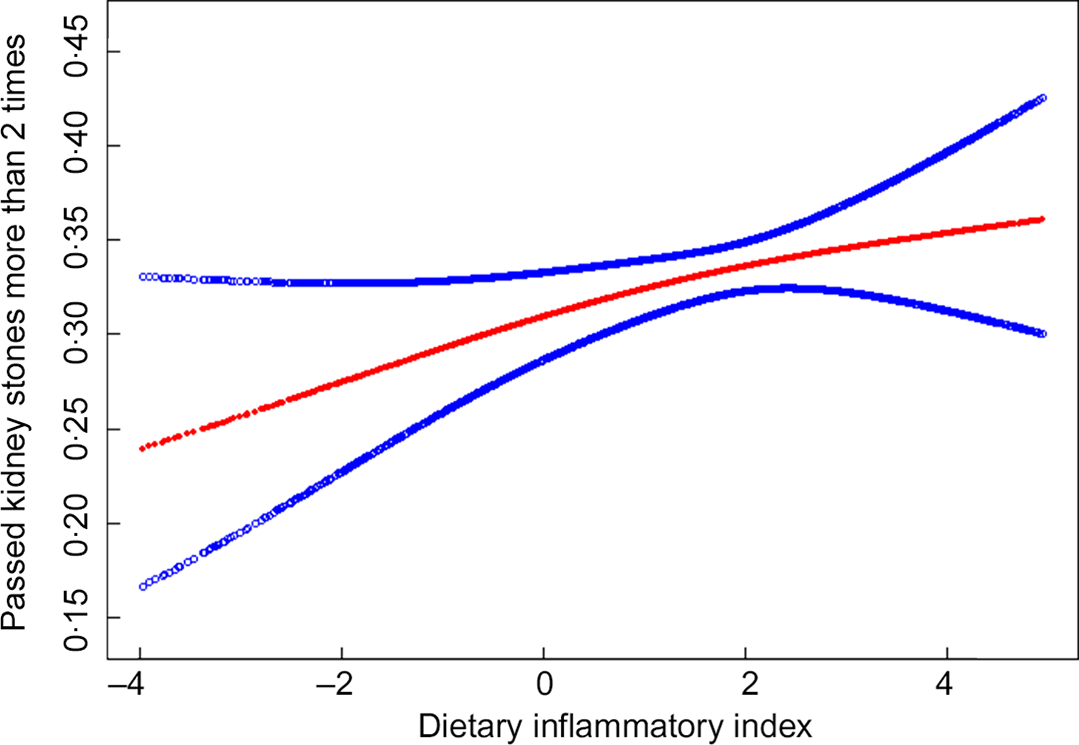
Fig. 2 The non-linear relationship between Dietary Inflammatory Index and recurrent kidney stone
Table 3 Stratified logistic regression analysis to identify variables that modify the correlation between DII and kidney stones*,†
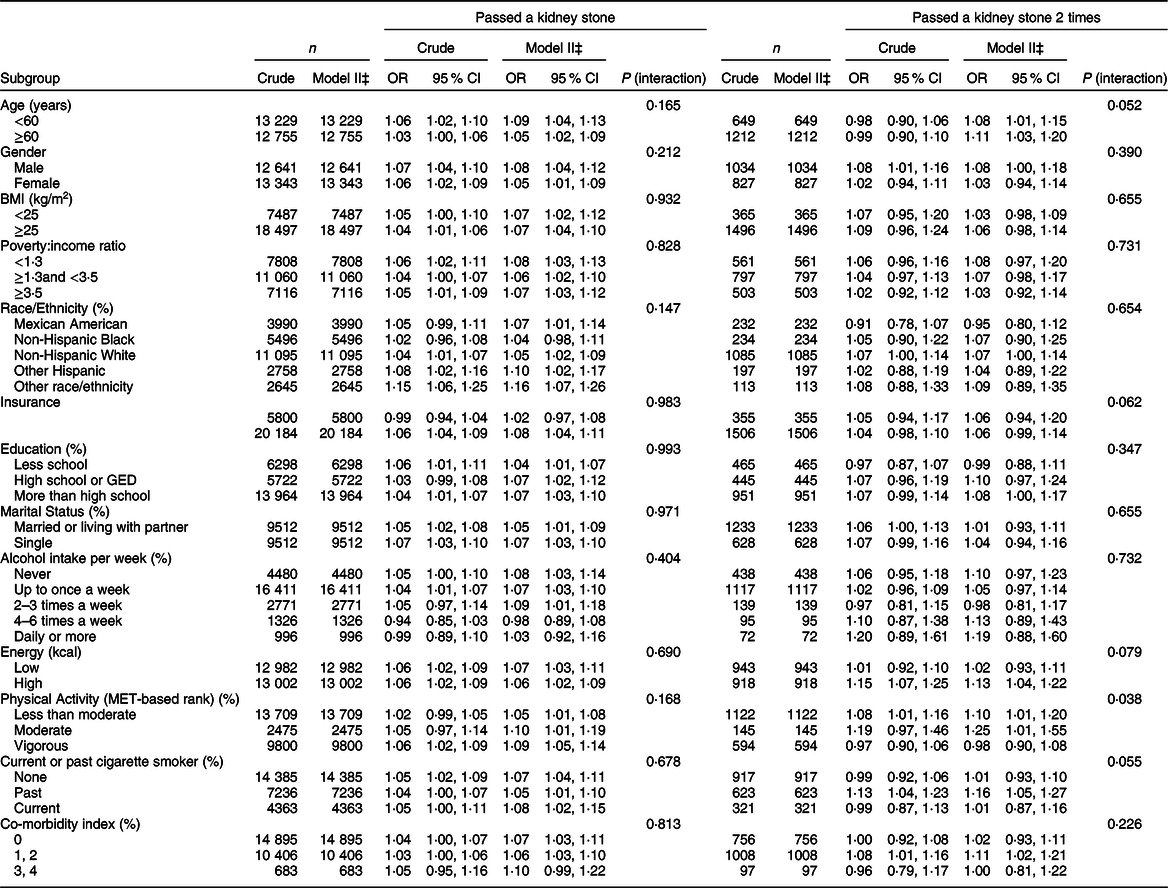
DII, Dietary Inflammatory Index.
* Dependent variable: DII.
† Adjusted for gender; age; race; poverty:income ratio; education level; insurance; marital status; alcohol intake per week; physical activity; co-morbidity index; energy (kcal); BMI; smoking.
‡ In each stratification, the model is not adjusted for the stratification variable.
Discussion
We performed a correlation study in a large population of American adults, using a food-based pro-inflammatory dietary index to elucidate the relationship between potential inflammation of diet and kidney stones. The study demonstrates two important findings. First, the consumption of diets with greater inflammatory properties was significantly associated with a higher prevalence of kidney stones after adjusting for a variety of potential confounders. Higher dietary inflammation, as assessed by a high DII score, was correlated with a higher risk of developing kidney stones in men and women. Second, the risk of kidney stones recurrence rose as high as 61 % with the increase in dietary inflammation (Q4 v. Q1).
The incidence rates of kidney stones have been rising in many countries in recent years(Reference Scales, Smith and Hanley1). Besides, increased stone recurrence rates coupled with expensive treatments place a considerable burden on healthcare systems(Reference Saigal, Joyce and Timilsina2). The previous studies showed that both genetic and environmental factors had contributions to the pathogenesis of the various types of stones synergistically(Reference Worcester and Coe21). Among environmental factors, dietary habits are associated with a marked influence on the pathogenesis of nephrolithiasis and are likely contributing to the growing prevalence in the last few decades. Meschi et al.(Reference Meschi, Maggiore and Fiaccadori22) suggested that citrus fruits contributed to increase urinary excretion of citrate and decrease calcium oxalate and urate saturation, which could effectively prevent kidney stones. Then, some micronutrients like Zn and Mg were found related to kidney stones, higher dietary Zn consumption may be correlated with an increased risk of kidney stone incidence(Reference Tang, McFann and Chonchol23), whereas increasing Mg consumption may be correlated with a lower risk of kidney stones(Reference Liebman and Costa24). Subsequently, in line with our study, Benjamin et al.(Reference Turney, Appleby and Reynard6) highlighted two important results that vegetarians had a lower risk of being hospitalised for kidney stones compared with those meat eaters. Then, among meat eaters, higher meat intake is correlated with an increased risk of developing kidney stones; on the other hand, increased consumption of high-fibre and fruit diets, and Mg-containing foods (bananas and almonds) may protect against the risk of stone occurrence.
Inflammation is an important biological pathway regulating the interaction between organisms and the environment, and diet intake is a major part of the environment(Reference Tabung, Steck and Zhang13). Various foods are considered to be pro-inflammatory foods, like high sugar foods, refined grains, red and processed meats and fried foods, which can increase levels of inflammation(Reference Bordoni, Danesi and Dardevet25). In contrast, higher consumption of anti-inflammatory foods, such as legumes, unrefined cereals, nuts, fruits and vegetables(Reference Ahluwalia, Andreeva and Kesse-Guyot26), has been found to play the opposite role. The possible mechanism through which pro-inflammatory diets may weaken the hosts’ immune defences include increased levels of CRP and IL-6(Reference Tabung, Steck and Zhang13), leucocytes as well as neutrophils(Reference Wirth, Sevoyan and Hofseth20), endoplasmic reticulum-stress reactivities provoked by SFA(Reference Hotamisligil and Erbay27) and skewing of the redox balance(Reference Graffouillere, Deschasaux and Mariotti28).
In particular, several studies have shown that a higher DII score derived from a single 24 h is correlated with increased CRP in the US NHANES(Reference Shivappa, Wirth and Hurley29,Reference Shivappa, Wirth and Murphy30) . Nevertheless, there is relatively little knowledge about the effect of the inflammatory diet and immune system in kidney stone development. Shoag et al.(Reference Shoag and Eisner31) demonstrated a significantly positive correlation between CRP and kidney stone disease in younger patients. Then, they explained that the immune system and inflammatory pathways might play a significant role in the pathogenesis of nephrolithiasis in this age group, while this may not be applied to older people with renal stones.
In subgroup analysis, we revealed a relationship that the association between DII and recurrent nephrolithiasis in individuals with less than moderate and moderate physical activity was stronger than those with vigorous physical activity. It has been found that a regular exercise schedule could generate an overall reduction in inflammation at rest due to the anti-inflammatory environment produced by each exercise session combined with a reduction in visceral fat, which releases pro-inflammatory adipokines like TNF and IL-6(Reference Gleeson, Bishop and Stensel32). Moreover, Sorensen et al.(Reference Sorensen, Chi and Shara33) conducted a cohort study including 84 225 post-menopausal women in 2014; they found that participants with a level of physical activity over 10 metabolic equivalent/week decreased a significant 30 % risk of developing nephrolithiasis after multivariate adjustment. Our research showed that there is no association between DII and kidney stones in participants with vigorous physical activity, but a significant positive correlation has been found in the other two groups, which indicates that increased exercise may decline the positive relationship between DII and kidney stones. In other words, people with a high pro-inflammatory diet intake could reduce the risk of recurrent nephrolithiasis through more exercise.
To the best of our knowledge, our study is the first to use the food-based DII score to link the relationship between diet-related inflammation and risk of kidney stones. We used a large well-defined cohort with appropriate weighting of survey participants, thereby allowing wide spread application of the findings to the US population. However, a single 24-h dietary recall may not take into account within-person variations in dietary intakes and is imprecise for characterising an individual’s long-term intake habit, which means the kidney stone occurrence could have occurred years before the diet assessment(Reference Hebert, Hurley and Steck34). Moreover, since the data in our study derived from a cross-sectional survey, the temporality of DII and kidney stones was unclear. Albeit the associations are of biological plausibility, the findings should be interpreted with caution and confirmatory longitudinal studies or clinical trials are warranted. Then, we cannot completely exclude the residual confounding by unmeasured or unknown variables, although we have adjusted for several potential confounders. Additionally, differentiating individuals based on stone composition or metabolic phenotypes might further illuminate the relationship with DII and present different aetiologies and pathogeneses.
Conclusion
Our findings revealed that a pro-inflammatory diet with a higher DII score is correlated with increased odds of kidney stones incidence and recurrence. These results might be meaningful to advise the public health community about this possible dietary approach to prevention kidney stone formation and recurrence, but a further study should be designed.
Acknowledgements
Acknowledgements: The authors thank Dr Chi Chen, Changzhong Chen and Xing-Lin Chen for providing statistical methodology consultation. Financial support: This work was supported by the National Key Research and Development Program of China (grant no. 2017YFC0908003), National Natural Science Foundation of China (grant no.81902578 and 81974098), China Postdoctoral Science Foundation (2017M612971), Post-doctoral Science Research Foundation of Sichuan University (2020SCU12041), Post-Doctor Research Project, West China Hospital, Sichuan University (2018HXBH085), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2018C01). Authorship: C.C.Z., S.Q. and H.Y.B. contributed equally as first authors of this manuscript. Y.L. and Q.W. are responsible for the conception and design of the study. S.Q., H.Y.B. and X.N.Z. interpreted the analysis. C.C.Z., B.W.T., H.Y.W., J.K., X.T. and B.Y.C. were responsible for the acquisition of data. C.C.Z., S.Q. and H.Y.B. wrote the first draft of the manuscript and interpreted the data and wrote the final version. All authors critically revised the article for important intellectual content and approved the final version. Q.W. obtained public funding. Conflict of interest: All authors in the study declare no conflict of interests. Ethics of human subject participation: This study was conducted according to the guideline laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Institutional Review Board of the National Center for Health Statistics (NCHS). All participants provided written informed consent.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021000793








