Introduction
Scientific research has unravelled several biologically active components from the age-old Ayurvedic medicine and kitchen spice turmeric (Curcuma longa L.). Curcumin, the yellow polyphenolic pigment in turmeric, has received much scientific scrutiny over the years and has been proven to be the major active component responsible for the pharmacological effects of turmeric. Turmeric is considered ‘Generally Recognized As Safe’ (GRAS) by the US Food and Drug Administration, and curcumin is safe and well tolerated even when administered at high doses of 10–12 g in healthy volunteers(Reference Lao1).
Today there are thousands of publications reporting on the functional and therapeutic benefits of curcumin mediated through its pleiotropic mechanisms of action in the context of many chronic diseases. Its distinct chemical structure as α,β-unsaturated diketone moiety flanked by two phenolic groups, [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], makes curcumin a unique pharmacophore with high reactivity and great binding affinity towards several molecular targets such as transcription factors, enzymes and receptors involved in oxidative stress, apoptosis and inflammatory pathways which further translate to the observed neuroprotective, cardioprotective, gastroprotective, hepatoprotective and anticancer effects when a proper dosage of the bioactive form is delivered(Reference Salehi2–Reference Sharifi-Rad13).
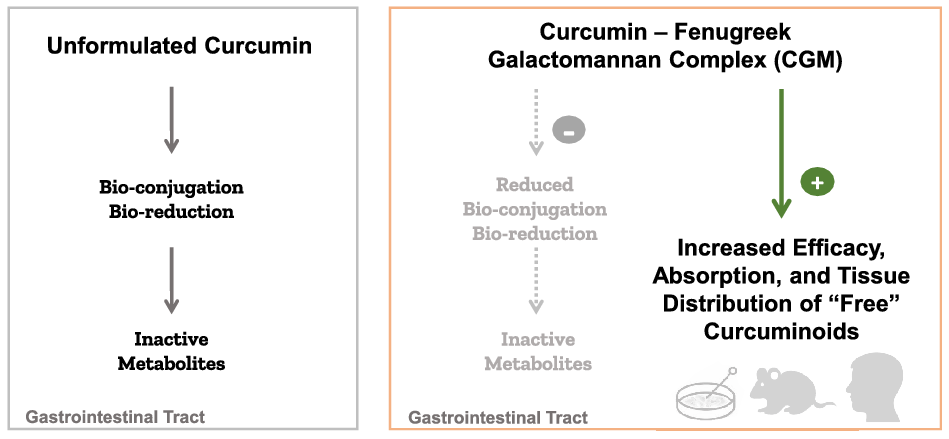
Despite the interesting pharmacodynamics, curcumin has been notorious for its poor pharmacokinetics. Curcumin is a class IV Biopharmaceutics Classification System molecule with low solubility (<10 ng/ml), low permeability and low oral bioavailability(Reference Wahlang, Pawar and Bansal14). Curcumin’s H-bond donating and accepting capacity, Michael acceptor type ligand character and metal-chelating properties also contribute to its in vivo instability(Reference Heger15). Moreover, curcumin undergoes rapid intestinal biotransformation into inactive metabolites, mainly the glucuronide and sulphate conjugates, which contribute to the major limitations of curcumin in translation to therapeutic benefits (Fig. 1)(Reference Nelson16–Reference Kurita and Makino19). A key role in mediating the efficacy of any substance that undergoes rapid biotransformation is the absorption of the bioactive and permeable form; thus, drug delivery systems capable of providing the unconjugated or ‘free’ curcuminoids have major therapeutic implications.

Fig. 1. Oral administration of curcumin results mainly in conjugated curcumin detected in plasma, and intravenous or intraperitoneal administration results mainly in reduced curcumin metabolites, while there are minor levels of free curcumin detected in plasma following any administration method.
To our knowledge, this review is the first critical overview of the current pre-clinical and clinical research conducted with curcumagalactomannoside (CGM), a novel curcumin formulation designed to overcome the pharmacokinetic challenges of curcumin and improve the bioavailability and tissue distribution of the unconjugated ‘free’ curcuminoids. Running a search across for the terms ‘CGM’, ‘curcumin’, ‘CurQfen’ and ‘curcumagalactomannoside’ in PubMed, MEDLINE and EMBASE databases along with Google Scholar yielded thirty-two results (5 May 2022). We have excluded duplicates and manuscripts that have not explicitly evaluated CGM formulation, which resulted in twenty-six study-specific manuscripts (two in vitro, twelve in vivo and twelve clinical studies) included in this review.
The first section describes CGM formulation technology, highlighting the therapeutic relevance of improving bioavailability of the bioactive ‘free’ curcuminoids as well as some of the technical challenges in quantifying these actives. The following sections summarise current published literature with CGM evaluating pharmacokinetics, safety and efficacy of CGM in various models and health/disease conditions.
CGM curcumin formulation
CGM is a patented formulation of curcumin with galactomannan biopolymer, a soluble dietary fibre component of another GRAS-listed traditional Indian medicinal spice, fenugreek (Trigonella foenum graecum). CGM contains 40% turmeric root extract standardised to 35% total curcuminoids (sum of curcumin, demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC)) and 60% fenugreek galactomannan dietary fibre, without any other phytochemicals found in fenugreek (M/s Akay Natural Ingredients Private Limited, Cochin, India, trademarked as CurQfen®).
FENUMAT® patented technology
In the manufacturing process, curcuminoid molecules are allowed to interact and uniformly impregnate the homogeneous gel phase of fenugreek galactomannans. Curcuminoids are effectively encapsulated in the hydrophobic pockets created by the conformationally restricted molecular chains of galactose and mannose (1:1 ratio) that behave like a soft hydrogel scaffold. The result is a self-emulsifying non-covalent molecular complex of curcumin and fenugreek galactomannans that converts the insoluble crystalline curcumin to amorphous, water-dispersible and stable ‘curcumagalactomannoside’. The microparticle with a size of less than 150 µm renders it a reversible hydrogel with high swelling index, optimum hydrophobic–hydrophilic balance and amphiphilicity that confers both protection from intestinal conjugation and an increase in the absorption of ‘free’ curcuminoids into bloodstream(Reference Krishnakumar, Dinesh Kumar, Kuttan and Maliakel20,Reference Song21) . As a water-only-based process, FENUMAT® formulation technology is considered the first ‘green’ approach in phytonutrient delivery. This technology also enabled the co-delivery and enhanced bioavailability of low-dose curcumin with other known lipophilic phytonutrients such as boswellic acids(Reference Abhilash22). Subsequently, FENUMAT® technology was successfully employed for gastric irritation prevention and sustained intestinal delivery of the pungent capsaicinoids from red chili pepper (Capsicum annum L.)(Reference Joseph23). FENUMAT® could also deliver gummy- and essential-oil-rich components of Ferula asafoetida oleo gum resin, an Ayurvedic remedy for functional dyspepsia(Reference Vijayasteltar24), and fixed oils such as vitamin-E-rich sunflower oil in free-flowing water-soluble granular powders(Reference Vismaya25).
Therapeutic relevance of biologically active ‘free’ curcuminoids
Several studies have highlighted the significance of the native unconjugated or ‘free’ forms of curcuminoids, owing to their ability to cross cellular membranes, blood–brain barrier permeability and potential in vivo antioxidant, anti-inflammatory, anti-proliferative, neuroprotective, anti-amyloid and neurogenesis effects(Reference Begum26–Reference Ireson29). Although some nano formulations provide ‘free’ curcuminoids upon intravenous administrations, most of the oral curcumin formulations yield only the conjugated metabolites in plasma(Reference Kumar30). Oral administration of native unformulated curcumin with 95% purity at high doses of 10–12 g could detect only less than 10 ng/g ‘free’ curcuminoids in some subjects(Reference Lao1). Garcea et al. mentioned that doses of (unformulated) curcumin required to provide sufficient tissue levels to exert pharmacological activity are probably not feasible in humans(Reference Garcea31). So, formulations that can deliver high levels of native unconjugated ‘free’ curcuminoids into systemic circulation and further into tissues at convenient doses have great relevance for functional benefits.
The main active constituents in turmeric roots are curcuminoids, consisting of curcumin, DMC and BDMC. When isolated from dried turmeric rhizomes by typical solvent extraction process, these molecules are generally obtained as a complex (commonly referred to as ‘curcumin’) of no less than 95% purity, with 70–80% (w/w) curcumin as most abundant, 12–15% (w/w) DMC and 2–5% (w/w) BDMC(Reference Pancholi32). Following oral administration of unformulated curcumin, most of the ingested curcumin remains unabsorbed due to its hydrophobicity and insolubility as evidenced by 1% bioavailability compared with intraperitoneal administration, with the remaining fraction undergoing rapid intestinal/hepatic metabolism to conjugated metabolites such as glucuronides and sulphates (Fig. 1 (Reference Kurita and Makino19,Reference Fança-Berthon33,Reference Prasad34) ). Intravenous or intraperitoneal curcumin administration leads mainly to enzymatically reduced curcumin metabolites such as di-, tetra-, hexa- and octa-hydrocurcumin(Reference Xu11,Reference Prasad, Tyagi and Aggarwal35) . Curcumin metabolites have a limited contribution to the observed biological effects of curcumin, especially the conjugated metabolites. Glucuronides and sulphates are bulky water-soluble molecules which undergo rapid renal elimination and possess low cellular permeability. While some of the enzymatic metabolites and rapid degradation products of curcumin (tetrahydrocurcumin, ferulic acid or vanillin) may partially account for the observed activity of curcumin(Reference Pandey36–Reference Shoji38), the majority of its conjugated metabolites have shown significantly weaker relative absorption, tissue permeability, blood–brain barrier permeability and anti-inflammatory, antioxidant and anti-proliferative activities(Reference Sandur27,Reference Pal28,Reference Prasad34,Reference Shoji38) . For example, curcumin monoglucuronide, a major conjugated metabolite of curcumin, has shown ten-fold lower antioxidant activity(Reference Choudhury39), anti-inflammatory(Reference Sandur27) properties and no anti-proliferative effects in comparison with ‘free’ curcumin(Reference Pal28,Reference Pal40) .
First-generation techniques to overcome curcumin metabolism and glucuronidation issues to enhance its oral bioavailability included the use of inhibitors such as piperine (black pepper extract) and permeability enhancers such as turmeric oils or turmerones(Reference Pancholi32). Both piperine and turmeric oil have been shown to boost curcumin bioavailability primarily by inhibiting UDP-glucuronyltransferase, glucuronidase/sulfatase, cytochrome P450, hepatic aryl hydrocarbon hydroxylase and mixed-function oxygenases(Reference Volak41,Reference Srinivasan42) . These are essential endogenous drug metabolism and detoxification enzymes, and blocking their activity may compromise the body’s defence and immunity. Yet another mechanism of these adjuvants was an expected increase in the intestinal permeability of drugs, which also renders toxins into systemic circulation. Furthermore, piperine has been shown to have reproductive toxicity and embryotoxic effects when administered as a high bolus dose in animals(Reference Ziegenhagen43). Subsequent approaches to enhance the curcuminoids solubility included the use of synthetic emulsifiers in nano forms. These second-generation formulations were reported to enhance the plasma curcuminoids levels, but mainly as their conjugated metabolites(Reference Pancholi32). Thus, there were safety concerns with the adjuvant-based methods to enhance bioavailability, and the existing methods to enhance solubility were only increasing the levels of conjugated metabolites without increasing the bioactive ‘free’ forms and would not guarantee an improvement in clinical efficacy. Regarded as a third-generation bioavailable curcumin, CGM formulation reportedly addresses both the bioavailability and cellular uptake issues of ‘free’ curcuminoids.
Technical challenges in the assessment of ‘free’ curcumin bioavailability
Aside from differences in study populations, analytical instruments and dosage regimens, some of the key issues contributing to the high heterogeneity of curcumin bioavailability reported in various studies stem from the different methodologies used to assess pharmacokinetics. A common practice in most pharmacokinetic studies is that the blood samples are subjected to hydrolysis with β-glucuronidase and sulfatase enzymes that convert the predominantly circulating forms of conjugated curcumin glucuronides and sulphates into ‘free’ curcuminoids prior to quantification of plasma curcuminoids. Thus, the true amount of ‘free’ bioactive curcumin cannot be quantified and the observed bioavailability is mainly that of the artificially un-conjugated curcumin metabolites, which may lead to misrepresentation of the formulation biological effects since there are significant differences in the activity of ‘free’ curcuminoids versus conjugated metabolites(Reference Stohs44,Reference Liju45) .
Another key issue is reporting enhanced bioavailability of a curcumin formulation by calculating the cumulative area under the curve (AUC) of all native curcuminoids: curcumin, DMC and BDMC in the unformulated and in the formulation following enzymatic hydrolysis. The standard unformulated curcumin has significantly lower amounts of DMC and BDMC, possibly even undetectable in plasma, while in formulations with enhanced absorption these additional curcuminoids can be detected as their glucuronide and sulphate conjugated forms. Thus, enzymatic hydrolysis of plasma samples results in an increase in total curcuminoids concentration or number of folds of bioavailability but does not typically represent the true bioavailability of the bioactive ‘free’ curcuminoids(Reference Szymusiak46,Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47) .
Curcumin bioavailability is also estimated by calculating maximum plasma concentration per mg (C max/mg) and total area under plasma concentration curve per mg (AUC/mg) of administered formulation after enzymatic hydrolysis(Reference Stohs44). A higher ratio is regarded as better bioavailability but is not a good estimation of the ‘true’ bioavailability, since formulations containing low curcumin content usually yield high ratios(Reference Douglass and Clouatre48). Furthermore, because these calculations use total plasma concentration of the sum of both free and conjugated curcumin metabolites, they cannot determine the bioavailability of ‘free’ curcuminoids.
Another potential source of errors is the use of the more limited and less sensitive HPLC techniques with ultraviolet/visible or fluorescence detectors instead of more sensitive techniques such as ultraperformance liquid chromatography electrospray ionisation triple quadrupole tandem mass spectrometry (UPLC–QQQ–ESI–MS/MS). To address these issues, in their pharmacokinetic study evaluating the bioavailability of CGM, Kumar et al. employed the gold standard UPLC–QQQ–ESI–MS/MS technique and measured the ‘free curcuminoids ratio’ (FCR)(Reference Kumar30). FCR is a direct measure of the ‘free’ curcuminoids in circulation versus curcumin conjugates and gives the fraction of the absorbed curcumin in the bioactive ‘free’ form and the fraction absorbed as conjugated metabolites after a single dose. Higher FCR ratio is correlated with better bioavailability of the ‘free’ curcuminoids.
It is not possible to compare the ‘free’ curcuminoids bioavalability of CGM with other bioavailable curcumin formulations because to our knowledge, a pharmacokinetic study with such parallel testing has not been reported. However, several studies that have investigated oral administration of unformulated curcumin and other bioavailable curcumin formulations, yielded insignificant plasma levels and tissue distribution of the physiologically relevant ‘free’ curcuminoids, or employed the enzymatic treatment of samples so the observed bioavailability enhancement would be mainly that of the conjugated metabolites, such as glucuronides and sulphates (Reference Lao1,Reference Garcea31,Reference Jager49–Reference Schiborr51) . Overall, CGM is distinct from other bioavailable curcumin formulations due to its all-natural food-grade status and water-based manufacturing process without synthetic emulsifiers, excipients or processing aids.
CGM studies
This section discusses the fourteen pre-clinical and twelve human clinical trials that have evaluated the bioavailability, safety and the effects of CGM formulation on cognitive, cardiovascular and hepatic function, as well as in various inflammation-driven conditions (Fig. 2). Tables 1 and 2 summarise the pre-clinical and clinical studies with CGM that are discussed in detail below.

Fig. 2. CGM results in higher bioavailability and tissue distribution of free curcuminoids with improved effects on inflammatory, oxidative stress and endogenous antioxidant pathways in multiple organ systems. AChE, acetylcholine esterase; CAT, catalase; CRP, C-reactive protein; Dopa, dopamine; Glu, glutamate; GPx, glutathione peroxidase; GSH, Reduced Glutathione; HDL, High density lipoprotein; IL-1β, interleukin-1β; IL-6, interleukin-6; i-NOS, inducible nitric oxide synthase; MMP-2 & 9, matrix metalloproteinase-2 & 9; NF-κB, nuclear factor kappa B; Ser, serotonin; sVCAM, solublevascular cell adhesion molecule; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α.
Table 1. Pre-clinical studies

* Abbreviations: AA, adjuvant induced arthritic control; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cat, catalase; CGM, curcumaglactomannosides; GGT, gamma-glutamyl transferase; CHN, chondroitin sulphate; GLN, glucosamine hydrochloride; GGT, gamma-glutamyl transferase; GSH, reduced glutathione; LPS, lipopolysaccharide; MS, multiple sclerosis; RC, radiation control; PP, pulse pressure; SOD, superoxide dismutase; SS, sulfasalazine; UC, ulcerative colitis; USC, unformulated standard curcumin.
Table 2. Clinical studies

* Abbreviations: AC, active-controlled; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BBB, blood–brain barrier; BID, two times per day; BMI, body mass index; BP, blood pressure; BPP, brachial pulse pressure determined by subtracting diastolic BP from systolic; BUN, blood urea nitrogen; Cat, catalase; CGM, curcumaglactomannosides; GSH, reduced glutathione; CHN, chondroitin sulphate; CO, crossover; DB, double blinded; EEEG, electroencephalography; FEN, fenugreek galactomannan fibre; GGT, gamma-glutamyl transferase; GLN, glucosamine hydrochloride; KPS, Karnofsky Performance Scale; LDL, low-density lipoproteins; ND, not detected; OA, osteoarthritis; OL, open label; PC, placebo controlled; PP, pulse pressure; RC, radiation control; RCT, randomised clinical trial; SA, single arm; SOD, superoxide dismutase; USC, unformulated standard curcumin; QoL, quality of life; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index; TC, total cholesterol; UC, ulcerative colitis; SS, sulfasalazine.
Pharmacokinetics
Absorption and tissue distribution of significant amounts of bioactive forms at the target site are crucial for functional benefits. Begum et al. indicated an optimal tissue concentration of 1–2 µM of ‘free’ curcumin to elicit any favourable biochemical transformations in vivo (Reference Begum26). Krishnakumar et al. showed a 20-fold increase in curcumin bioavailability in rats administered with 250 mg/kg body weight (b.w.) CGM by oral gavage and a 12·9-fold and 15·8-fold increase in bioavailability at the 250 mg and 1500 mg CGM dose in humans compared with unformulated curcumin dosage of 1000 mg(Reference Krishnakumar, Dinesh Kumar, Kuttan and Maliakel20). HPLC method of estimation of plasma curcumin content, the small sample size of only eight male subjects, and the different equivalent curcuminoids content in the administered doses were the major limitations of this pilot study.
In a subsequent study using triple quadruple tandem mass spectrometry, Krishmakumar et al. showed that rats administered CGM at 200 mg/kg body weight (b.w.) curcumin content by oral gavage had twenty-five-fold increased plasma bioavailability of ‘free’ curcuminoids with a C max of 341·57 ± 30·88 ng/ml and an elimination half-life of 3·7 h compared with the equivalent amount of unformulated standard curcumin(Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47). CGM led to significant uptake of ‘free’ curcuminoids into various tissues, with 12- to 347-fold higher levels of the native unconjugated curcuminoids (sum of curcumin, DMC and BDMC) in the brain, liver, kidney, heart and spleen tissue for a longer duration of time (5 h versus 30 min) compared with standard curcumin, which showed curcumin at a concentration of only 1·4 ± 0·8 ng/g of brain tissues(Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47). However, this study did not attempt to investigate the regional distribution of ‘free’ curcuminoids in brain and further the improved brain tissue uptake was not correlated with any functional assessment. Another caveat was that only male rats were used in this single dose intake study.
Subsequently Kannan and colleagues addressed some of the caveats from these previous studies by evaluating the blood–brain barrier permeability, brain regional distribution of ‘free’ curcuminoids using UPLC–ESI–QQQ–MS/MS method concomitant with cognitive testing with single and repeated doses of CGM in rats. The authors showed that both single and 28 d repeated CGM administration at 100 mg/kg b.w. curcuminoid content yielded significant amounts of ‘free’ curcuminoids in brain, especially in the hippocampus. Correlated with the increased levels of ‘free’ curcuminoids measured in the brain, the animals in the CGM group also showed improvements in behavioural tests, locomotor activity and spatial memory errors that were significantly better compared with the unformulated curcumin group administered equivalent amount of curcuminoids for 28 d, p < 0·05(Reference Kannan52). A limitation of this study was that the authors did not measure regional brain tissue distribution of ‘free’ curcumioids with unformulated curcumin intake but only used equivalent curcuminoids intake in the functional behavioural testing part of the study.
Several studies report better pharmacological effects and enhanced permeability of ‘free’ curcuminoids as compared with their metabolites(Reference Begum26–Reference Ireson29,Reference Szymusiak46,Reference Jager49,Reference Pan, Huang and Lin53) . Significantly increased bioavailability of ‘free’ curcuminoids with CGM administration has also been demonstrated in clinical studies. Pharmacokinetics of ‘free’ curcuminoids following the oral administration of high (1000 mg) and low (250 mg) doses of CGM and an equivalent dose of unformulated curcuminoids was assessed in a large, randomised, double-blinded human trial with fifty healthy volunteers using UPLC–ESI–QQQ–MS/MS. In this study, plasma levels of ‘free’ curcuminoids were measured without enzymatic pre-treatment with glucuronidase/sulfatase that converts conjugated curcumin metabolites to ‘free’ curcumin(Reference Kumar30). The high dose of CGM provided over 45·5-fold increase in ‘free’ curcuminoid bioavailability (24·8-fold for the low-dose), with significant improvement in all the pharmacokinetic parameters compared with unformulated standard curcumin which did not yield detectable levels of plasma curcuminoids at the low dose. Further comparison of plasma with and without enzymatic treatment revealed significantly higher levels of ‘free’ curcuminoids in plasma (74 ± 8%) as compared with conjugated forms of curcuminoids (26 ± 12%). The improved pharmacokinetic parameters, such as 440 ng/ml C max versus 13 ng/ml with unformulated curcumin and more than 3 h of elimination half-life, can lead to increased absorption and in vivo stability for cellular uptake, as well as favourable pharmacological effects via interaction with intracellular components(Reference Kumar30). A weakness of this study was that the relative distribution of ‘free’ and conjugated curcuminoids in plasma following the oral administration of CGM was not compared in parallel with administration of equivalent amounts of unformulated curcumin.
Pandaran et al. investigated the pharmacokinetics of ‘free’ curcuminoids following CGM administration of 500 mg as a single dose and 500 mg twice per day as a repeated dose for 30 d in healthy volunteers(Reference Pandaran Sudheeran54). Significantly higher relative absorptions and improved pharmacokinetics of total ‘free’ curcuminoids were observed with administration of CGM both as single dose (30·7-fold, p < 0·001) and repeated dose (39·1-fold, p < 0·001) compared with the unformulated curcumin. There was a significantly higher absorption (C max) and bioavailability with the repeated-dose regimen but no accumulation of plasma curcuminoids. The plasma concentration timeline followed the absorption, distribution and elimination phases, indicating its safe pharmacokinetics(Reference Pandaran Sudheeran54).
Liju et al. also reported the improved bioavailability of CGM from various food matrices (honey, soups, chocolate, yogurt) in healthy volunteers(Reference Liju45). In their study CGM was seven to ten times more bioavailable when incorporated into various foods or beverages at 100 mg CGM per serving size, as compared with unformulated curcumin. There was a good compatibility of CGM and no sensory issues in the various foods tested(Reference Liju45).
Safety
Recently, a few cases of hepatotoxicity have been reported among some long-term consumers of curcumin. Pancholi et al. reviewed the various related adverse events reported so far and speculated that added adjuvants known to inhibit essential detoxification pathways, adulteration with synthetic curcumin, or contaminations with heavy metals, chromate, illegal dyes, mycotoxins, non-steroidal anti-inflammatory agents, polyaromatic hydrocarbons and pyrrole alkaloids could be the main potential causes of the observed hepatotoxicity(Reference Pancholi32). Interestingly, among the reported cases of hepatotoxicity, twelve were women and only three were men. Moreover, the subjects reported with toxic manifestations were older (mean 55+ years) individuals under polypharmacy. Currently there are only a few safe, all-natural, food-grade curcumin formulations that have been shown to provide significant levels of bioactive ‘free’ curcuminoids in plasma and tissues(Reference Jamwal55,Reference Dei Cas and Ghidoni56) .
Pancholi and colleagues evaluated the safety and tolerance of long-term oral administration of CGM in healthy volunteers. Daily supplementation of 1000 mg CGM for 90 d did not cause any adverse effects or any clinically meaningful variations in the vital signs, haematological parameters, lipid profile and renal function markers of the volunteers. Liver function enzymes and bilirubin also stayed within the normal ranges after 90-d CGM supplementation(Reference Pancholi32). Some caveats with this study were the relatively younger study population (mean 31·32 years) that were healthy and not undergoing any pharmacological treatments. Considering the reported hepatotoxicity studies, it would be important to investigate the safety of long-term CGM intake in older subjects.
In animals, acute and chronic administration of CGM showed a no-observed-adverse-effect level (NOAEL) of 2000 mg/kg b.w. Furthermore, Ames test indicated no mutagenic character for CGM in concentrations of up to 5 mg per plate(Reference Liju45). Toxicology studies together with the pharmacokinetic and several clinical trials support CGM formulation safety for regular human consumption in doses of up to 1000 mg/d.
Neuroprotection
Neuroinflammation and abnormal protein aggregates are characteristic features of many neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Multiple studies report that curcumin administration can protect from neuroinflammation, β-amyloid and tau aggregates(Reference Maiti and Dunbar57–Reference Reddy61).
On the basis of the observed improved blood–brain barrier permeability and brain distribution of bioactive ‘free’ curcuminoids, CGM has been tested for neuroprotective and cognitive benefits in animal models(Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47). Sunny et al. investigated the neuroprotective efficacy of CGM versus unformulated standard curcumin (USC) in a rat model of lipopolysaccharide (LPS)-induced neuroinflammation and neurotoxicity(Reference Sunny, Ramalingam, Das, Maliakel, Krishnakumar and Ittiyavirah62). LPS administration causes a detrimental increase in neuro-inflammation, acetylcholinesterase (AChE) activity, stimulation of serotonergic and glutamatergic neurotransmission, and reduced levels of dopamine in the brain leading to cognitive impairments as seen in neurodegenerative disorders. Oral administration of 200 mg/kg b.w. CGM for 28 d showed significant cognitive improvement and reduced inflammation in the brain compared with unformulated curcumin: 80% increase in number of entries in EPM test and 62·71% increase in the time spent (from 4·5% to 12·07%) in CGM compared with LPS group (p < 0·001) and 93·3% improved Y-maze behaviour in CGM versus LPS group. Unformulated standard curcumin (USC) group also showed improvements in the cognitive behavioural studies, but the percentage difference in reference, working memory errors and Y-maze test were 33·39%, 39·63% and 23·3% less respectively, as compared with CGM. CGM and USC significantly reduced AChE activity by 25% and 14%, respectively CGM reduced brain levels of glutamate from 3·17 ± 0·11 to and 2·35 ± 0·087, p < 0·001. CGM showed a 50% increase tissue levels of serotonin and dopamine that were depleted by LPS treatment, while the USC group showed a 10% relative increase. Furthermore, LPS-induced up-regulation of NF-κB, a key transcription factor regulating immunity and inflammation, was significantly down-regulated with CGM treatment. Histopathological analysis of the brain tissue showed significant reduction in inflammation, normal astrocyte morphology and minimal oedema and necrosis in CGM group, while USC also showed reduced oedema but enlarged astrocytes still present(Reference Sunny, Ramalingam, Das, Maliakel, Krishnakumar and Ittiyavirah62). It was unclear from the study design whether animals were administered multiple doses of LPS (after 7 and 14 d supplementation with CGM or USC) before the behavioural testing and whether the animals continued treatment for 28 d. With repeated injection of LPS there is also an increase in β-amyloid toxicity which is a hallmark of Alzheimer’s disease(Reference Lee63). Future studies are needed to determine CGM’s effect on Aβ levels or α/β-secretase activity. Another caveat of the study may be the lack of analysis of brain tissue lysates from different cortical and hippocampal regions.
In a mouse model of cuprizone-induced multiple sclerosis, cuprizone treatment leads to decrease in body weight, oligodendrocyte death and subsequent reversible demyelination(Reference Ittiyavirah64,Reference Torkildsen65) . Oral administration of 200 and 400 mg/kg b.w. CGM or unformulated curcumin significantly prevented the body weight loss induced by cuprizone treatment, in such a way that the low-dose CGM was similarly effective as the high-dose USC: average body weight 19·76 ± 0·2 g in 200 mg/kg in CGM group compared with 18·11 ± 0·2 g in 400 mg/kg USC group, and 11·35 ± 0·17 g in cuprizone-treated animals, (p < 0·01)(Reference Ittiyavirah64). Histopathology analysis showed less demyelinated lesions in the high-dose CGM group compared with unformulated curcumin- and cuprizone-treated groups. A major limitation of this study was the lack of quantification of demyelination and specific immunohistochemical myelin protein staining in the histology analysis.
Another recent publication compared the effect of CGM and unformulated curcumin on carbofuran (CF)-induced neurotoxicity in rats. CF is a carbamate pesticide toxic to neurons involved in locomotor function(Reference Sindhu, Binitha, Nair, Maliakel, Ramadasan and Krishnakumar66). Rotarod experiments show significant decline in muscle coordination and strength in CF-treated animals including 19 s reduction in retention time on the rotarod (p < 0·01), 22 s reduction in grip strength (p < 0·05) and forty-fold increase in time to elicit pain response (p < 0·001). Both unformulated curcumin and CGM showed improvement in muscle strength; however, the CGM effect was significantly higher and almost reversed the motor deficit to the normal control level: CGM reverted grip strength similar to normal levels and decreased the pain threshold by 26 s (p < 0·001) compared with 5 s improved grip strength and maximum 10 s pain threshold decrease with the same dose of unformulated curcumin. Similarly, there was a significant improvement in CF-induced increase in the time to elicit pain response in the CGM-treated group compared with unformulated curcumin(Reference Sindhu, Binitha, Nair, Maliakel, Ramadasan and Krishnakumar66). Interestingly, CGM treatment also improved other aspects of CF-induced toxicity, such as reduced oxidative stress parameters and enhanced mitochondrial function with low dose of CGM comparable to high-dose USC and high dose of 250 mg CGM closer to normal controls (p > 0·05). Some limitations with this study include the use of only male rats and lack of assessment of the number and morphology of mitochondria in the brain(Reference Sindhu, Binitha, Nair, Maliakel, Ramadasan and Krishnakumar66). In summary, three different chemically induced neurotoxic animal models CGM showed superior functional benefits, as well as higher improvement in neurotransmitter levels, inflammatory markers, mitochondrial function and tissue histopathology as compared with unformulated curcumin. The observed benefits can be attributed to the better brain bioavailability and pharmacokinetics of ‘free’ curcuminoids with CGM(Reference Sandur27,Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47) .
In a randomised placebo-controlled clinical trial, Pandaran et al. investigated the effect of CGM or unformulated curcumin supplementation on endogenous oxidative stress biomarkers and quality of life in healthy subjects experiencing occupational stress-related anxiety and fatigue(Reference Pandaran Sudheeran54). Occupational stress and anxiety have been shown to increase formation of highly reactive free radicals and deplete levels of endogenous antioxidants with a negative impact on health(Reference Fedoce67,Reference Lobo68) . Repeated supplementation with 500 mg CGM twice per day for 30 d significantly increased plasma levels of antioxidant enzymes (superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), reduced glutathione (GSH)) and reduced lipid peroxidation. Changes in serum biomarkers correlated with significant improvements among 78% of subjects in perceived stress, anxiety and physical/mental fatigue scores and in quality of life. The enhanced efficacy with CGM supplementation versus unformulated curcumin was attributed to better antioxidant status and hence the modulation of oxidative stress with higher amounts of ‘free’ curcuminoids such as 30·7-fold higher bioavailability of ‘free’ curcuminoids with single dose of 500 mg CGM and 39·1-fold increase with 30-d repeated-dose CGM(Reference Pandaran Sudheeran54). The study did not show any adverse events, side effects or deviations in clinical laboratory parameters indicating its safety.
In another randomised, double-blinded, placebo-controlled clinical trial, Khanna and colleagues investigated the effects of CGM administration on brain waves in healthy volunteers(Reference Khanna69). Changes in brain waves measured by electroencephalogram have been used as an index of blood–brain barrier permeability and bioavailability of drugs or nutrients(Reference Bewernitz and Derendorf70). In this study, 500 mg of CGM twice daily for 30 d showed significant increases in α- and β-waves as compared with unformulated curcumin and placebo groups. Previous clinical studies have shown that increases in α-waves correlate with better cognitive performance(Reference Klimesch, Schimke and Pfurtscheller71), while increases in β-waves were associated with higher alertness/arousal and arithmetic calculation ability(Reference Fernandez72,Reference Lal and Craig73) . Studies show that α- and β-wave generation reflects cholinergic modulation in various areas of the brain. CGM’s effect on these waves together with modulation of the cholinergic system via AChE indicates CGM’s capacity to influence attention, learning and memory(Reference Sarlak, Oryan and Moghaddasi74,Reference Roopun75) . CGM group showed a 36% improvement in choice-based visual-reaction time compared with 15·36% in unformulated curcumin and 5·2% in placebo groups. The CGM-supplemented group also showed significant (29·8%) reduction versus placebo in α/β ratio, a validated indicator of fatigue/tiredness(Reference Tiago-Costa, Quelhas-Costa and Santos-Baptista76). A major limitation of the study was the relatively small number of just eighteen subjects, though the results were consistent with a previous larger clinical study in sixty subjects with occupational stress, indicating CGM’s ability to influence brain waves is consistent with brain penetration and several potential cognitive benefits including audio-visual and working memory improvements, as well as stress and fatigue reduction(Reference Pandaran Sudheeran54).
Promising results from an unpublished, randomised, placebo-controlled clinical trial in forty-eight patients with Alzheimer’s disease suffering from moderate dementia, indicate that 400 mg CGM supplementation twice daily for 180 d significantly improved cognitive and locomotor function (assessed by Mini-Mental State Examination and Geriatric Locomotive Function Scale score) compared with unformulated curcumin and placebo groups. Functional benefits were correlated with improvements in plasma levels of β-amyloid 42, Tau proteins, antioxidant markers SOD and GSH, and inflammatory markers IL-6, IL-1β, TNF-α and BDNF (unpublished results, R&D Internal Report 06/2021, Akay Natural Ingredients, Cochin, India). As in previous studies, the authors attribute the benefits observed with CGM to the improved blood–brain barrier permeability, brain bioavailability and brain tissue distribution of ‘free’ curcuminoids in this formulation. However, further larger clinical studies are warranted to confirm these results and to investigate the effects of long-term supplementation in various populations, including paediatrics and geriatrics.
Cardiovascular effects
Oxidative stress-induced intracellular redox imbalance plays a pivotal role in the pathogenesis of various cardiometabolic risk factors (hyperlipidaemia, hypertriglyceridemia, hyperglycemia, hypertension, obesity) leading to severe cardiovascular diseases. Enhancing the endogenous antioxidant defence mechanisms (enzymatic and non-enzymatic) with antioxidant supplementation has been shown to significantly reduce cardiometabolic risk factors(Reference Yin77). Due to its specific chemical structure, when provided at an effective dose and not compromised by its poor bioavailability, curcumin has substantial free radical scavenging and anti-atherosclerosis activities, improving several cardiovascular biomarkers such as homocysteine, C-reactive proteins, inflammatory cytokines, cholesterol, triglycerides, blood pressure and glycemia(Reference Li78–Reference Jin81). CGM also demonstrated a strong anti-inflammatory effect against oxidised LDL (ox-LDL)-induced inflammatory responses in vitro in human peripheral blood mononuclear cells with 85% inhibition of COX activity (12·5 μg/ml CGM), down-regulated reactive oxygen species, TNF-α, IL-6, INOS and VCAM-1 mRNA, and inhibited nuclear translocation of NF-kB and lipid peroxidation (p < 0·05)(Reference Saji82).
High levels of homocysteine and altered lipid metabolism are correlated with an increased risk of cardiovascular disease, heart attacks and strokes(Reference Baszczuk83). Lipid peroxidation is another important step in the pathogenesis of atherosclerosis(Reference Esterbauer, Wag and Puhl84). In two clinical trials, CGM supplementation has significantly reduced lipid peroxidation and enhanced levels of glutathione and superoxide dismutase(Reference Pandaran Sudheeran54,Reference Krishnareddy85) . The first study in young obese men receiving daily supplementation with 500 mg CGM for 12 weeks, without any dietary restrictions and exercise, showed a significant increase in HDL-cholesterol by 34% and reduction in homocysteine levels by 29% from 12·22 ± 2·29 µg/ml before to 8·62 ± 1·02 µg/ml compared with the placebo group, who saw an increase from 9·45 ± 0·84 mg/ml to 11·84 ± 1·63 mg/ml at the end of study, p = 0·04(Reference Campbell86).
In another clinical study in a similar population of healthy obese men, daily supplementation of 500 mg CGM with no exercise or dietary modifications led to significant improvement in arterial stiffness in the ‘responder’ subgroup with higher aortic stiffness at baseline. The CGM responder group (6/11 subjects) showed a higher effect on carotid–femoral pulse wave velocity compared with non-responders with lower levels at baseline (6·81 ± 0·83 m/s versus 5·84 ± 0·41 m/s, p = 0·045)(Reference Campbell87). Even small increases in aortic stiffness, as measured by 1·0 m/s increase in carotid–femoral pulse wave velocity, has been associated with a 14% increase in cardiovascular events, 15% increase in cardiovascular mortality and 15% increase in all-cause mortality(Reference Vlachopoulos, Aznaouridis and Stefanadis88). Thus, the 1 m/s relaxation observed in the CGM responder subgroup is a clinically meaningful improvement. The authors suggest that the change in brachial pulse pressure observed in the CGM group was likely due to functional rather than structural changes in the artery, as structural changes would have been expected to cause an overall change in the carotid–femoral pulse wave velocity. The CGM responders also had a significant increase in the plasma levels of the anti-inflammatory cytokine IL-13. Previous work suggested these cytokines may be protective against cardiovascular pathologies via vasoactive properties and modulation of collagen(Reference Tang89,Reference Cardilo-Reis90) . The observed beneficial effect of CGM on several cardiovascular markers indicates its potential in the maintenance or improvement of cardiovascular health in younger obese individuals; however, further larger and longer studies in older populations are warranted. Studies in combination with dietary restrictions and exercise may provide a better understanding of the possible role of CGM to maintain healthy arteries or ‘de-stiffen’ arteries.
An unpublished clinical study showed promising results of CGM supplementation in diabetic patients on metformin treatment. In this study, 400 mg CGM supplementation daily led to a significant decrease in the postprandial blood glucose spike, significant reduction in diabetes-related side effects such as thirst, dry mouth and fatigue, and improvement in quality of life (unpublished results, R&D Internal Report 08/2021, Akay Natural Ingredients, Cochin, India).
Hepatoprotection
Many natural treatments for liver dysfunction target the overactivated inflammatory and oxidative stress cascade(Reference Hong91). Curcumin has multiple therapeutically relevant molecular targets in the liver, serving as a powerful antioxidant against free-radical damage(Reference Bielak-Zmijewska92) as well as anti-inflammatory agent via inhibition of NF-κB(Reference Saji82,Reference Sandur93–Reference Deguchi95) and reduction of pro-inflammatory cytokines(Reference Tasneem96,Reference Chin97) . In a previously mentioned clinical trial, CGM treatment significantly increased endogenous antioxidant enzymes and hence the detoxification capacity in subjects with occupational stress(Reference Pandaran Sudheeran54).
In an animal model of alcohol-induced liver damage, 250 mg/kg b.w. CGM significantly reversed the detrimental effects of ethanol on liver enzymes, lipid peroxidation, inflammation and endogenous antioxidants(Reference Mohan98). Chronic alcohol consumption has been shown to induce oxidative stress-mediated inflammation along with up-regulation of NF-kB, TNF-α, IL-6, IL-1β and CRP and activation of matrix metalloproteases (MMP-2 and MMP-9) and toll-like receptors (TLR4), which are involved in the pathogenesis of alcoholic liver disease via degradation of extracellular matrix and activation of immune responses, respectively(Reference Banerjee99,Reference Gustot100) . Both CGM and standard curcumin treatment significantly down-regulated the ethanol-induced increased levels of TNF-α and IL-6, supporting the anti-inflammatory role of curcumin. Ethanol-induced up-regulation of TLR4 and MMP-2 and MMP-9 were also significantly counterbalanced in both curcumin treatment groups. However, CGM formulation was superior to standard curcumin in regulating oxidative stress, liver function and inflammatory markers, as well as TLRs and MMPs expression almost to control levels, highlighting the therapeutic importance of higher bioavailability of ‘free’ curcuminoids with CGM. The enhanced protective effect of CGM was also evident from the improved histopathology observed of liver tissues of CGM-treated animals(Reference Krishnakumar, Maliakel, Gopakumar, Kumar, Maliakel and Kuttan47,Reference Mohan98) .
In a double-blinded placebo-controlled clinical trial, Krishnareddy et al. showed that 250 mg CGM supplemented twice per day for 56 d improved hepatic function in subjects with chronic alcohol consumption of more than six alcohol units per week (one unit is equivalent to 150 ml of wine or 360 ml of beer or 45 ml of 40% (v/v) alcohol) and elevated levels of two biomarkers of liver disease, serum transaminases and gamma-glutamyl transferase (GGT). Benefits were apparent from 28 d of treatment and led to an average 36% and 29% decrease in ALT and GGT transaminases, respectively, from baseline by the end of the study. There was also up to 25% increase in levels of endogenous antioxidant enzymes (GSH, SOD, and GPx) and a significant reduction in markers of systemic inflammation (IL-6 and CRP) as compared with both baseline and placebo groups(Reference Krishnareddy85). Interestingly, the subjects were not allowed to consume more than three units of alcohol per week for the duration of the study, but this 50% reduction in alcohol intake did not elicit improvements in liver function markers in the placebo group. Taken together, both pre-clinical and clinical studies show that CGM can potentially attenuate the alcohol-induced alterations in several biomarkers of liver function and offer hepatoprotective effects in younger males. It would be important to confirm and further investigate the effect of CGM supplementation in larger studies including women, as well as subjects with other chronic liver diseases such as non-alcoholic liver diseases.
Anti-inflammatory effects
The anti-inflammatory activity of curcumin has been well documented. The molecular mechanisms underlying this effect include inhibition of enzymes and factors that mediate pro-inflammatory pathways such as cyclooxygenases, lipoxygenases, inducible nitric oxide synthase and NF-κB(Reference Jurenka101–Reference Menon and Sudheer103).
In an in vitro study, Saji et al. explored the anti-inflammatory effect of CGM against ox-LDL-induced inflammatory responses in human peripheral blood mononuclear cells (hPBMCs). Gene expression analysis showed that ox-LDL treatment increased expression of several pro-inflammatory biomarkers such as iNOS, TNF-α, IL-6, VCAM-1, LOX, PGE2, total COX and lipid peroxidation along with nuclear translocation of NF-κB. CGM treatment down-regulated the activation of these cell adhesion molecules and pro-inflammatory cytokines, indicating its potential anti-inflammatory effect via the NF-κB signalling pathway(Reference Saji82).
Sheethal et al. investigated the effect of CGM in an acetic-acid-induced animal model which mimics some of the features of ulcerative colitis disease(Reference Sheethal104). Ulcerative colitis is a chronic inflammatory bowel disease characterised by reduced antioxidant capacity and increased inflammation. In this experimental model CGM significantly increased endogenous antioxidant enzymes (SOD, GPx, CAT, normalised GSH and reduced myeloperoxidase (MPO) activity, p < 0·05) and decreased oxidative damage thiobarbituric acid reactive substances (TBARS) and the level of pro-inflammatory markers (total COX, iNOS, TLR4, TNF-a, IL-6, CRP) as compared with both sulfasalazine standard therapy and unformulated curcumin groups, restoring the heavily inflamed and damaged colon to almost normal levels with mild inflammation(Reference Sheethal104). A limitation of this study was that the experimental model does not provide the opportunity to evaluate both the acute and chronic mucosa repair mechanisms, so further studies with chronic exposure to different chemical agents such as dextran sulphate sodium or 2,4,6-trinitrobenzene sulfonic acid 34 may be more relevant to the complex human pathological phenotype of ulcerative colitis.
Saji et al. demonstrated the acute and chronic anti-inflammatory effects of CGM using a carrageenan-induced paw oedema acute model and an adjuvant induced arthritic model of chronic inflammation(Reference Sangeeth Saji, Jose, Ratheesh, Sandya and Asha105). In the carragenaan model of acute inflammation, 100 mg/kg b.w. CGM showed paw oedema inhibition of 82% compared with 72% with the unformulated standard curcumin. In the adjuvant induced chronic arthritic model, 30 d supplementation with 100 mg/kg b.w. CGM significantly increased the levels of antioxidant enzymes SOD and GPx, CAT and GSH compared with indomethacin, down-regulated several inflammatory biomarkers such as plasma CRP and COX2, lowered TNF-α and IL-6, and reduced the massive infiltration of polymorphonuclear cells contributing to soft tissue oedema. Further radiological analysis showed that the CGM-treated arthritic rats had significant improvement in the degenerative joint changes such as sub-chondral erosion and joint space narrowing(Reference Sangeeth Saji, Jose, Ratheesh, Sandya and Asha105).
Two clinical trials evaluated the anti-inflammatory potential of CGM treatment in subjects with symptomatic knee osteoarthritis and swelling in the knee joint(Reference Khanna106,Reference Thomas107) . Osteoarthritis is a degenerative disease that involves deterioration of the cartilage which acts as a cushion between the bones in a joint. This tissue damage triggers the inflammatory signalling cascade and overproduction of pro-inflammatory cytokines that cause swelling, pain and stiffness, and functional impairment of joints. Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are part of the conventional therapy but are also typically associated with adverse gastrointestinal, renal and cardiovascular effects upon prolonged use or high doses. Natural components of cartilage, glucosamine and chondroitin are marketed as dietary supplements for joint support, but there are conflicting reports on their efficacy, including joint pain relief mainly due to their lack of anti-inflammatory efficacy, and typically require longer duration of treatment for the beneficial effect to be felt(Reference Zhu108–Reference Clegg110).
In one of the randomised, double-blinded, active-controlled studies, Khanna et al. compared the efficacy of standard 500 mg glucosamine hydrochloride (GLN) plus 415 mg chondroitin sulphate (CHN) with 400 mg CGM plus 500 mg GLN administered twice daily in improving pain and quality of life in patients with confirmed osteoarthritis. The study used multiple validated questionnaires Visual Analog Scale (VAS), Karnofsky Performance Scale (KPS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) to evaluate pain, stiffness and physical activities along with the measurement of anti-inflammatory markers. After 4 weeks of supplementation (day 28) the CGM–GLN-treated group showed an 81% increase in treadmill performance, 32% reduction in WOMAC pain score, 17% reduction in stiffness score and 30% reduction in physical activity difficulty score compared with the CHN–GLN group, indicating relatively faster action of CGM. After 12 weeks at the end of the study, the CGM–GLN-treated group showed almost double the efficacy compared with CHN–GLN, with significant four-fold improvement in treadmill walking score (p < 0·001) versus CHN–GLN group (mean difference 301·35 ± 74·46 m from baseline, p < 0·001 in CGM–GLN versus mean difference of 89·78 ± 19·25 m from baseline in CHN–GLN), significant improvements in walking performance (mean difference of 24·19 ± 6·20 in CGM–GLN compared with 16·56 ± 7·45 in CHN–GLN group), pain intensity (60·46% reduction VAS in CGM–GLN, p < 0·001 and 21·25% in CHN–GLN from baseline), stiffness and physical function. There was also significant 54·52%, 59·08% and 22·03% reduction in serum inflammatory markers IL-1β, IL-6 and soluble vascular cell adhesion molecule-1 (sVCAM) from baseline compared with 23·17%, 21·38% and 6·82%, respectively, in the active-control group. Interestingly, the subjects in the CGM–GLN-supplemented group also showed a decrease in body weight which may be beneficial for reduction in pain(Reference Khanna106). Lack of a placebo group and information on dropout and responder rates were some of the major limitations of the study. Moreover, co-administration of CGM with GLN did not allow for estimation of the effect due to CGM supplementation.
In the second study in patients with osteoarthritis, Thomas et al. compared the safety and efficacy of CGM supplementation alone versus the standard GLN–CHN combination. The group administered 400 mg CGM per day for 42 d showed superior improvements compared with the active-control group in VAS, KPS and walking performance (47·02%, 21·43% and 206% in CGM compared with 24·67%, 23·84% and 85·69% in CHN–GLN, respectively) as well as pain, stiffness, physical function and WOMAC scores (31·17%, 32·93%, 46·44% and 35% in CGM compared with 26·62%, 25·97%, 12·56% and 16·46% in CHN–GLN, respectively) and reduction in IL-1b, IL-6 and sVCAM (54·52%, 59·08% and 22·03% in CGM compared with 23·17%, 21·38% and 6·82% in CHN–GLN, respectively). Furthermore, the number of subjects that required ‘rescue’ NSAIDs/analgesics during the study period was 31·43% in CGM compared with 52·35% in the CHN–GLN group. Again, a significant beneficial effect was observed from day 28 onwards. CGM supplementation was considered safe and did not cause any adverse effects in the subjects who completed the study(Reference Thomas107). While the study included several validated questionnaires to compare the pain, stiffness, physical activity and quality of life along with anti-inflammatory markers, the reported 15% dropout rate that was not further described in detail in the manuscript limits the enthusiasm. Another caveat was the relatively short 6-week duration of the study and the lack of a placebo group and double-blinded study design due to the different dosage levels in the two groups (400 mg CGM once daily versus 900 mg CHN–GLN twice per day).
It is worth mentioning that both of the above studies reported a reduction in body mass index (BMI) in CGM-treated obese individuals that was attributed mainly to the fenugreek fibre provided in the CGM formulation(Reference Khanna106,Reference Thomas107) . In addition, most clinical studies for the treatment of osteoarthritis use high doses of at least 1000 mg of curcumin per day(Reference Daily, Yang and Park111). Taking into account the negative side effects associated with chronic use of acetaminophen and NSAIDs for the management of pain and inflammation, a low daily dose of 400 mg of CGM warrants further investigations in larger double-blinded, placebo-controlled clinical trials as a potentially safer alternative in osteoarthritis and body weight management.
Aerobic performance
In a randomised placebo-controlled study, Goh et al. examined the effects of CGM on aerobic performance in healthy untrained young volunteers with no regular exercise or dietary change. Participants completed a maximal graded exercise on a cycle ergometer before and after 28 d of daily supplementation with either 500 mg CGM, equivalent 300 mg fenugreek soluble fibre (FEN), or placebo. This test is commonly used to determine submaximal endurance performance and maximal endurance performance as measured by the ventilatory threshold (VT) and peak oxygen consumption (VO2 peak), respectively. VT increased 6·2% (increase 0·094 litres/min) and 6·7% (increase 0·099 litres/min) from baseline for CGM and FEN, respectively, and decreased in the placebo group (−2·2%, decrease 0·034 litres/min). Only four of the eighteen participants in the FEN group and two of the fourteen participants in the CGM group showed a real change in the mean VT response. These results suggest that fenugreek galactomannan fibre is biologically active and can provide significant benefit even at a dose of 300 mg/d. The authors attributed these results to galactomannan mucilage fibre ability to improve insulin sensitivity and increase NEFA availability(Reference Goh112). Interestingly, increases of 6·2–6·7% in the VT in this study are consistent with 4·1–5·4% increases previously reported for the gas exchange threshold after 28 d of supplementation with 3 g/d arginine(Reference Camic113), which were also not accompanied by changes in maximal endurance. A limitation of the study was that authors did not measure any physiological markers aside from resting blood pressure, heart rate and patient self-reported medical history to confirm the metabolic status of the participants. Further, longer studies in trained athletes or including exercise and a higher-dose treatment arm are recommended to better understand the influence of CGM and fenugreek galactomannan fibre on peak oxygen consumption.
Other cellular effects
Antioxidant, anti-inflammatory and epigenetic regulation mechanisms modulated by curcumin are well documented(Reference Hassan114,Reference Sharifi-Rad115) . The potential effect of CGM as an epigenetic modulator has been recently evaluated in vivo. Using whole transcriptome analysis (RNA-seq), Banik et al. identified 559 differentially expressed genes in the liver tissue of mice with LPS-induced inflammation treated with CGM for 14 d versus untreated. Subsequent gene expression and pathway analysis showed that thirty-three genes which were highly up-regulated in disease conditions were significantly down-regulated by CGM, and thirty-two genes highly down-regulated in various disease conditions were significantly up-regulated following CGM treatment(Reference Banik116). These results suggest that CGM could restore expression patterns of genes up-regulated or down-regulated in many chronic disease conditions. As uncontrolled inflammation plays a major role in the development of various chronic diseases and most chronic diseases are caused by perturbations in multiple molecular pathways, the authors suggest that targeting a single gene/protein/pathway might not be sufficient in tackling these diseases. Interestingly, this study highlights the pleiotropic action of CGM and potential to modulate multiple cellular targets such as nucleotides and transcription factors that modulate gene expression and pathways known to be involved in the development of several chronic diseases(Reference Banik116). Some limitations of this study were the use of only female mice, single intraperitoneal injection of LPS that can cause an acute inflammatory response, and the examination of solely liver tissue. Further studies with repeated exposure to LPS to produce chronic systemic inflammation, evaluation of multiple organs and including male animals would provide better information on the systemic gene modulatory effects of CGM in chronic diseases.
Radiation therapy is an important treatment against various types of cancer that has unfortunately deleterious effects on both tumour as well as surrounding healthy cells. There are opposing views regarding the use of supplementation with antioxidants during radiation therapy, with some radiation oncologists advising against it for fear that cancer cells may also benefit and render radiation less effective, but antioxidants can reduce the side effects of radiation therapy in normal cells(Reference Prasad117). A recent study evaluated the radioprotective effects of CGM in an animal model of radiation-induced damage(Reference Liju118). Ionising gamma radiation significantly reduces leucocytes and bone marrow cells and increases formation of cytotoxic reactive oxygen and nitrogen species as well as genetic instability. CGM showed better protection against radiation-induced bone marrow damage compared with standard curcumin treatment by restoring monocyte alpha esterase activity and haemoglobin levels (50% reduction in percentage of radiation-induced micronuclei increase in bone marrow in CGM compared with only 34% in USC group and a 60% reduction in polychromatic to normochromic (P/N) erythrocyte ratio upon radiation recovered by 2·1 times in CGM group, p > 0·01 compared with 1·2 times in USC, p > 0·05). The CGM group also showed higher levels of antioxidant enzymes (21% reduced hepatic GPx activity compared with 25% in unformulated curcumin and 42% in radiation control group) and prevented the radiation-induced increase in lipid peroxidation and infiltration of inflammatory exudates in intestinal mucosa. Furthermore, CGM treatment reduced the degree of chromosomal defects and DNA damage and yielded a better survival rate compared with unformulated curcumin (4-fold reduction in DNA damage in CGM, p < 0·05 compared with 2·5-fold in USC group, p < 0·01 and 83% reduction in radiation-induced chromosomal aberration in CGM vs 66% in USC group, p < 0·05)(Reference Liju118). Depending on the type, size, location of cancer and/or goal of the radiation therapy, multiple consecutive treatments may be required (‘Targeted Therapy to Treat Cancer’, originally published by the National Cancer Institute, 30 March 2022); thus, the single exposure to radiation used in this study may be considered a limitation of its translatability to clinical trials.
A recent study shows the anti-tumour potential of CGM via effects on cell cycle and apoptosis in vitro. Exposure to 25 μg/ml CGM showed higher cytotoxic activity and inhibition of cancer cell survival in HeLa cells compared with the drug doxorubicin. CGM induced apoptosis and cell cycle arrest as shown by increased levels of Bax and cleaved caspase-8 protein in cancer cells(Reference Ratheesh119). Previous studies have shown that curcumin can selectively induce apoptosis and inhibit cell growth and invasion in cancer cells but not in normal cells, highlighting the potential of exploring CGM’s anti-cancer potential in human interventions(Reference Jiang120–Reference Borek123).
Concluding remarks
CGM curcumin formulation with a proprietary fenugreek galactomannan dietary fibre called FENUMAT has consistently shown significantly higher bioavailability of ‘free’ curcuminoids in multiple animal models and human clinical studies when compared with equivalent doses of unformulated standard curcumin. In agreement with the pharmacokinetic studies, all the pre-clinical and clinical studies with CGM (brain, liver, heart and joint health) have also shown superior efficacy when compared with the unformulated standard curcumin, highlighting the therapeutic relevance of the improved bioavailability and tissue uptake of the ‘free’ curcuminoids. Furthermore, the whole genome transcriptome study provided the mechanistic evidence for CGM’s ability to carry out the pleiotropic effects by revealing the interaction of ‘free’ curcuminoids with various cellular targets leading to the regulation of several genes, including ones involved in the pathogenesis of cancers.
It has also been suggested that curcumin’s overall health benefits derive from its direct influence on the gastrointestinal microbiome(Reference Lopresti124,Reference Jabczyk125) . Interestingly, the fenugreek galactomannan used in the formulation of CGM is a soluble dietary fibre with significant prebiotic potential. It is also a functional dietary fibre with hypolipidaemic, hypoglycaemic and gastroprotective properties(Reference Vijayasteltar24). So, CGM would be expected to have higher impact on the microbiome and may bring additional benefits from galactomannan fibre compared with standard unformulated curcumin. Further clinical studies evaluating specific changes in gut microbiome and metabolites with long-term supplementation are required to confirm the effects of CGM on the microbiome.
Overall, scientific evidence on CGM curcumin addresses the issues of poor bioavailability and tissue distribution of the bioactive ‘free’ curcuminoids and demonstrates superior efficacy compared with standard unformulated curcumin. Clinical studies with the all-natural CGM curcumin formulation demonstrate efficacy at daily doses as low as 400 mg and safety at doses of up to 1000 mg/d, highlighting its potential in prevention and long-term management of different chronic diseases.
Acknowledgements
The authors would like to acknowledge Dr Balu Maliakel and Dr Shayna Sandhaus for manuscript review and valuable suggestions.
This research received no external funding.
C.M. and A.G.S. are employed by Life Extension company that sells CGM ingredient in dietary formulations, and I.M.K. works for Akay, which is a CGM ingredient manufacturer and supplier.
Author contributions
C.M. drafted the manuscript and created figures and the graphical abstract; C.M., I.M.K. and A.G.S. performed critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Author information
Cristina Matthewman is a Research Scientist at Life Extension Inc., Fort Lauderdale, Florida. She earned an MS in Biophysics and Medical Physics from University of Physics, Bucharest, Romania and a PhD in Neuroscience from University of Miami Miller School of Medicine, FL. Her current research interests are natural ingredients with health benefits. I. M. Krishnakumar is Chief Research Officer at Akay Natural Ingredients Ltd, Kerala, India. He earned a PhD in Chemistry from Mahathma Gandhi University, Kerala, India and received postdoctoral research fellowships from Houston Medical School, University of Texas, Houston and Burnham Institute of Medical Research, San Diego, CA. He is a bio-organic chemist interested in natural bioactive molecules, delivery forms and pharmacological effects. Andrew G. Swick is Chief Scientific Officer at Life Extension Inc., Fort Lauderdale, Florida. He earned an MS from the University of Nebraska, and a PhD from the University of Wisconsin and received postdoctoral fellowship from the University of North Carolina Lineberger Cancer Research Center and Johns Hopkins University School of Medicine. His current research interests focus on the use of nutraceuticals for disease prevention and maximisation of health span.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Not applicable.