INTRODUCTION
Acute hepatitis C (AHC) still raises many questions regarding its definition, its diagnosis and its management. Spontaneous clearance of hepatitis C virus (HCV) infection is generally observed within the first 3–4 months of infection [Reference Hofer1]. The precise moment of infection is, however, difficult to assess for most patients since AHC is mainly asymptomatic [Reference Alter2] and since serological markers specific to AHC are not available. Thus, most patients may have chronic disease at diagnosis. Consequently, data on risk factors mainly come from descriptive or case-control studies based on prevalent chronic cases. Early diagnosis of AHC is important to provide counselling, medical evaluation and therapy, when appropriate, to prevent chronic infection that occurs in 54–84% of cases [Reference Hoofnagle3]. Antiviral therapies are indeed less effective during chronic infection than during the acute phase [Reference Nomura4]. Nevertheless, management of AHC remains controversial regarding which patients should be treated, the appropriate time-point to start therapy and the most effective regimen [Reference Wedemeyer5, Reference Craxi and Licata6].
This study aimed to describe current epidemiological and clinical characteristics of AHC patients and their medical follow-up and outcome in real practice through a national surveillance system of newly referred HCV-infected patients.
METHODS
This retrospective study was conducted in 2005 on AHC patients identified through a national surveillance system of HCV infections between April 2000 and July 2004. This system, implemented in April 2000, is based on 26 hepatology reference centres scattered throughout France. These centres are university hepatology wards specialized in the management of hepatitis C and linked to a regional network of hepatogastroenterology departments in non-university hospitals. Patients included are newly referred (first contact) patients with positive anti-HCV antibodies attending any of the participating reference centres. This first contact can be either as an outpatient or as an in-patient. Patients can be referred by their general practitioner, by a specialist or by self-referral. For all included patients, the reference centres collect data on demographical, epidemiological, biochemical, clinical, morphological and histological items among which: dates of the last negative and first positive HCV antibodies test, circumstances of diagnosis (during check-up, blood donation, screening before or after blood transfusion, screening because of known risk exposure, monitoring following an occupational exposure, liver tests abnormalities, jaundice or other symptom), risk exposures for HCV transmission [blood transfusion or blood products, intravenous drug use (IDU) or nasal drug use, occupational exposure, medical procedure, other or unknown], results of biochemical and virological tests at diagnosis [alanine aminotransferase (ALT) level at diagnosis, HCV RNA serum status, HCV genotype]. Routine HCV genotyping was performed with either a line probe reverse hybridization assay (Inno-Lipa HCV; Innogenetics, Gent, Belgium) or by sequence analysis.
From these items, AHC cases were identified among patients referred to the reference centres before July 2004. A case of AHC was defined as a patient with positive HCV RNA by polymerase chain reaction (PCR) assay and elevated serum ALT levels with documented HCV antibodies seroconversion within a 6-month period or at least two of the four following criteria: (1) negative anti-HCV antibodies but positive HCV RNA; (2) documented HCV antibodies seroconversion within a 12-month period; (3) ALT level >10 times the upper limit of the normal (N) range (ALT>10N); (4) high-risk documented exposure to HCV within 4 months prior to diagnosis, which includes IDU, haemodialysis, needle-stick injury in a health-care setting and surgery in a country with high HCV endemicity. Any other viral or toxic aetiology or pre-existing liver disease was ruled out.
For each eligible case, a standardized questionnaire was used to check routine surveillance data and to collect from medical charts additional data on: risk exposures for HCV transmission during the presumed contamination period (defined as the 6 months prior to the first positive serology), results of biochemical and virological tests (ALT peak, ALT level during the year prior to the infection, HCV genotype, anti-HIV antibodies, hepatitis B surface antigen), medical follow-up (with or without antiviral therapy) and outcome with or without antiviral therapy (HCV RNA).
Three types of dates were considered: the date of diagnosis (defined as the first positive serology), the date of first referral and the date of initiation of antiviral therapy. Spontaneous viral clearance was defined as at least one negative HCV RNA during the follow-up period. Patients who had undetectable HCV RNA at the end of therapy were considered as having an end-of-treatment virological response (EoT). Patients who had undetectable HCV RNA 6 months after the end of treatment were classified as having a sustained virological response (SVR).
Data were analysed using Stata version 8.2 (StataCorp, College Station, TX, USA). Fisher's exact test, χ2 test, Student's t test and Wilcoxon's test were used. A P value of <0·05 was considered to indicate statistical significance.
RESULTS
Patients' characteristics
Among the 16 244 HCV cases recorded between April 2000 and July 2004, 61 (0·38%) fulfilled the definition criteria of AHC. Forty-four patients (72%) had documented HCV antibodies seroconversion within a 6-month period. Among the 17 other included patients, 14 had a high-risk documented exposure to HCV within 4 months prior to diagnosis; 11 had an ALT level >10N; six patients had documented HCV antibodies seroconversion within 12 months (within 9 months for four); four had negative anti-HCV antibodies but a positive HCV RNA (Table 1). Half of the AHC patients were referred within 1 month following diagnosis. Thirty-eight were males (62%) and mean age was 39 years (median 38 years, range 19–78 years) (Table 2).
Table 1. Inclusion criteria of the 61 acute hepatitis C patients at first referral in hepatology reference centres, France, 2000–2004
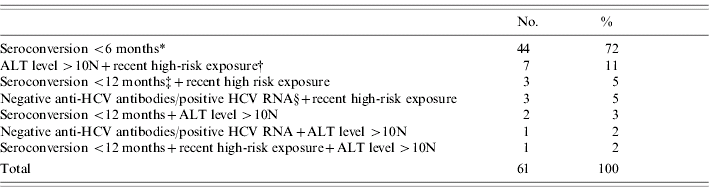
ALT, Alanine aminotransferase; N, normal; HCV, hepatitis C virus.
* Documented HCV antibodies seroconversion within a 6-month period.
† High-risk documented exposure to HCV in the 4 months preceding the diagnosis among: intravenous drug use (six patients), needle-stick injury in a health-care setting (three patients), haemodialysis (four patients), surgery in a country with high HCV endemicity (one patient).
‡ Documented HCV antibodies seroconversion within a 12-month period.
§ Negative anti-HCV antibodies but positive HCV RNA.
Table 2. Baseline characteristics of the 61 acute hepatitis C patients at first referral in hepatology reference centres, France, 2000–2004
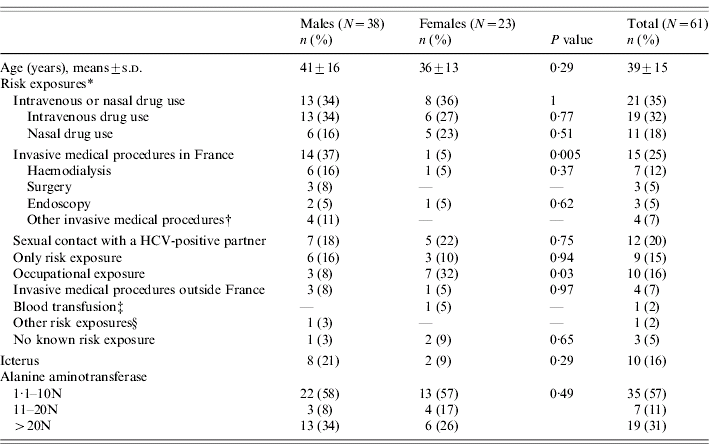
HCV, hepatitis C virus; N, normal.
* Risk exposures for HCV transmission during the presumed contamination period. Total >100% because 13 patients reported more than one risk exposure.
† Arteriography, biopsy.
‡ Information on the ascending transfusional investigation not available.
§ Tattooing, travel in an endemic country.
Thirteen patients (21%) reported more than one risk exposure for HCV transmission during the presumed contamination period and three patients (5%) no known exposure. Main reported risk exposure was IDU or nasal drug use (35%). Drug users (DUs) had been mostly tested for HCV because of their known risk factor (14/21). Invasive medical procedures in France were reported by 25% and sexual contact with a HCV-infected partner by 20% of patients (Table 2). For nine patients (six men and three women), sex with a HCV-infected partner was the only reported risk exposure identified during the presumed period of contamination. Five patients (two women and three men), reported sexual contacts with a HCV-infected partner and four (one woman and three men; among whom two had documented co-infection with HIV) reported sex with a HCV and HIV co-infected partner. Genotype of the partner, sexual preferences, practices or the concomitance of a sexually transmitted infection could not be collected. An occupational exposure was suspected for ten health-care-worker patients (16%), among whom nine reported a needle-stick injury. Transmission related to blood transfusion was suspected for one patient but could not be confirmed.
At diagnosis, 10 patients (16%) had jaundice, 57 (93%) documented positive anti-HCV antibodies and all patients were positive for HCV RNA (case definition). Viral genotype was determined for 54 patients (89%). Genotype distribution differed by risk exposures; the most frequent genotypes were 3, 4, and 1 (undetermined subtype) for patients reporting drug use, 2 and 1b for patients with a history of invasive medical procedures in France and 3 and 4 for patients who reported having sexual contact with a HCV-infected partner in the 6 months prior to diagnosis (Table 3). Among the 53 patients for whom this information was available, seven (13%) had a documented co-infection with another virus (Table 4).
Table 3. Distribution of genotypes of acute hepatitis C patients by reported risk exposures to HCV during the 6 months prior to diagnosis, France, 2000–2004
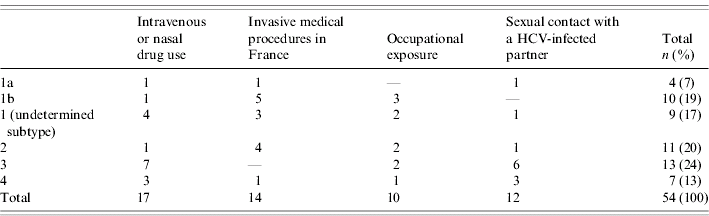
HCV, hepatitis C virus.
Table 4. Characteristics of seven acute hepatitis C patients with documented co-infection, France, 2000–2004
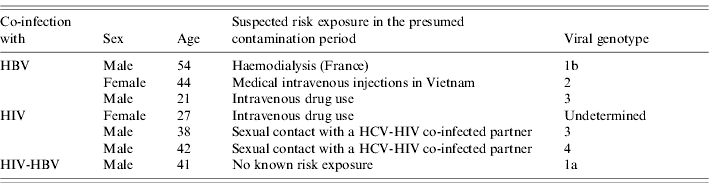
HBV, hepatitis B virus; HCV, hepatitis C virus.
Medical follow-up and outcome with or without antiviral therapy
At first referral, a follow-up without therapy was decided upon for 21 patients (35%) whereas antiviral therapy was initiated in 39 patients (65%) (Fig.). One patient was lost to follow-up. No clear-cut difference in terms of sex, age, risk exposures, genotype, jaundice or delay between diagnosis and referral appeared between patients with surveillance alone and patients for whom therapy was initiated (data not shown). Among 21 patients managed without initial antiviral therapy, six were later treated because they were still viraemic at 3 months (one patient), 6 months (three patients) and >6 months (two patients) after diagnosis, five were lost to follow-up, three remained under surveillance and seven spontaneously recovered. Among these latter, spontaneous clearance of HCV RNA occurred within 3 months after diagnosis for six patients and 1 year after diagnosis for one patient. Patients with jaundice during the acute phase were more likely to have a spontaneous viral clearance than patients without jaundice although this difference is not statistically significant (4/5 vs. 3/11 respectively, P=0·1). Icteric patients who spontaneously recovered had undetectable HCV RNA within a delay of 47–85 days after diagnosis. The rate of spontaneous viral clearance was not associated with genotype, age, or gender (data not shown).
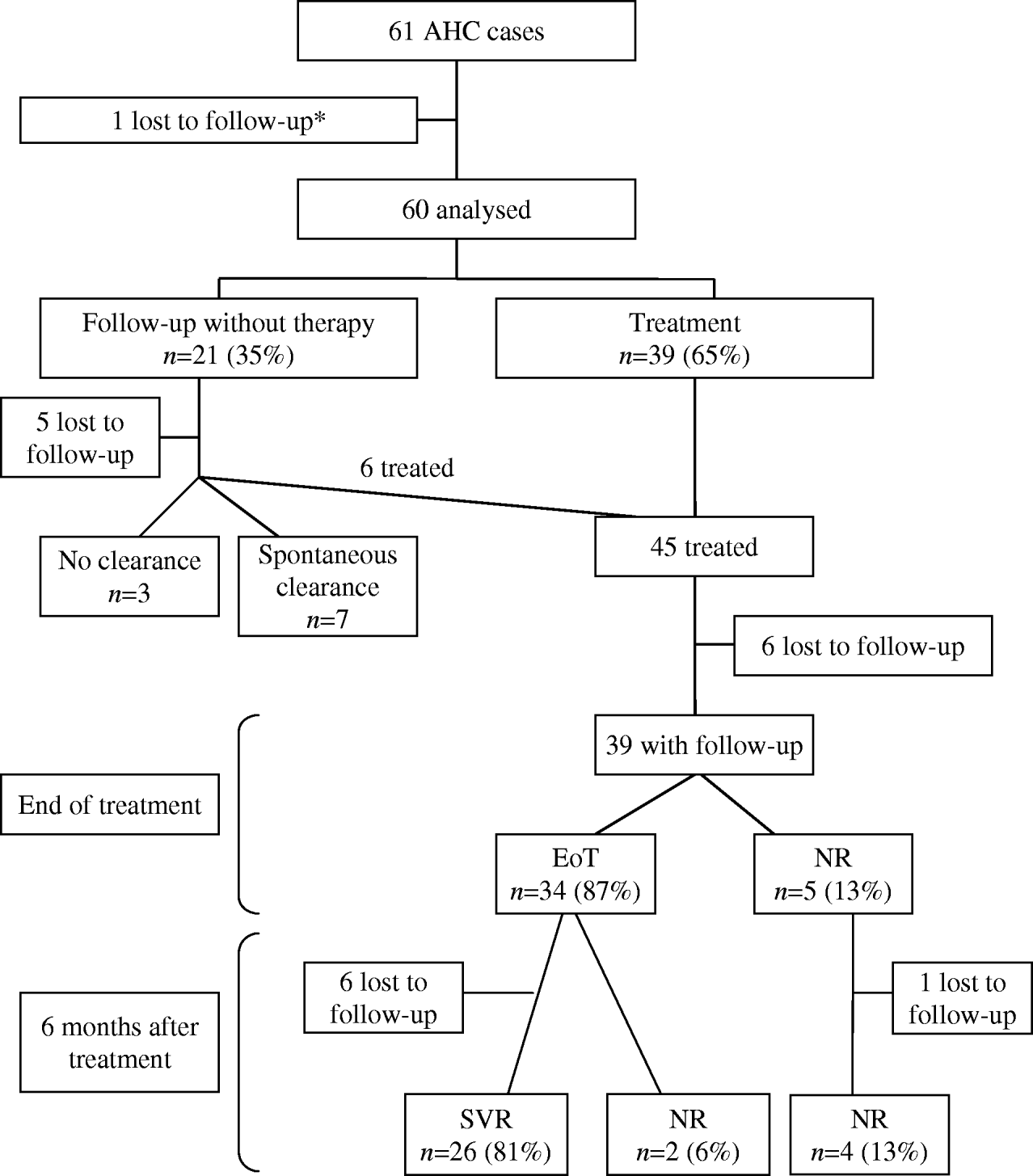
Fig. Schematic overview of the 61 acute hepatitis C patients newly referred to the reference centres, France, 2000–2004. EoT, End-of-treatment virological response (undetectable HCV RNA at end of therapy); NR, no response to antiviral therapy (positive HCV RNA at end of therapy); SVR, sustained virological response (undetectable HCV RNA 6 months after end of therapy). * Included in the epidemiological and clinical characteristics at referral.
Forty-five patients received antiviral therapy after a median of 81 days after diagnosis. Six patients were lost to follow-up after initiation of therapy (Fig.). Thirty-four out of the remaining 39 patients had undetectable HCV RNA at EoT (87%) whereas five were non-responders. In this non-responder group, treatment was interrupted in two patients (because of side-effects for one patient and inefficacy of treatment for the other). At 6 months after treatment, 26 out of 32 patients with a complete follow-up had undetectable HCV RNA, giving an overall SVR rate of 81%. No association was found between SVR and genotype.
DISCUSSION
In our study, 61 patients, <1% of newly referred HCV patients between April 2000 and July 2004, fulfilled the criteria of acute infection according to our case definition, confirming that hepatitis C is rarely diagnosed (or managed) in its acute phase. In an Australian prospective survey conducted between 1997 and 2000, this proportion reached 2·8%, possibly because of a less specific case definition [Reference Robotin7].
As in most studies [Reference Hofer1, Reference Jaeckel8–Reference Morin11], AHC patients were rather young (mean age 39 years). Male gender predominated. Risk exposure distribution illustrates the changes in routes of transmission that occurred in the last 15 years. In developed countries, since screening of blood donations was introduced in the early 1990s, the major mode of transmission is IDU. In France, despite 10 years of harm-reduction policy, HCV transmission remains very high among DUs. In 2004, the seroprevalence of HCV infection among DUs was estimated at 59·8% (95% CI 50·7–68·3) [Reference Jauffret-Roustide12]. The proportion of 35% of reported DUs in our study is consistent with previous case series of newly acquired HCV patients identified through hospitals in which drug use concerned 10–38% of patients [Reference Hofer1, Reference Jaeckel8–Reference Morin11, Reference Martinez-Bauer13]. In studies with larger recruitment (laboratories, medical practitioners, hospitals), this proportion, however, reached 70–82% [Reference Robotin7, Reference Wu14]. Among the 21 patients who reported drug use, 14 (67%) were screened for HCV for this reason, thus emphasizing the importance of regular testing of DUs.
History of invasive medical procedure in France was reported by 25% of AHC cases. This proportion is consistent with the results of another French retrospective study conducted between 1990 and 1997 in general hospitals [Reference Morin and Pariente9] and with the preliminary results of a French registry of AHC implemented in 1999 [Reference Morin11]. In developed countries, thanks to recommendations on enhanced hygiene measures, the relative contribution of health-care-related transmission of HCV infection has dropped since the early 1990s. However, several outbreaks of HCV infection related to lapses in aseptic techniques during invasive medical procedures were recently reported [Reference Delarocque-Astagneau15–Reference Desenclos18]. In this study, suspected health-care procedures were mainly surgery, haemodialysis and endoscopy a finding consistent with previous studies [Reference Delarocque-Astagneau15, Reference Bronowicki17, Reference Delarocque-Astagneau19, Reference Mele20]. For endoscopy, however, one recent survey showed a very low or null risk of HCV transmission by endoscopy if internationally approved cleaning and disinfection procedures are used [Reference Ciancio21].
Occupational transmission was suspected for ten patients (16%). In other case series, this proportion ranged between 0·4% and 32% [Reference Robotin7–Reference Morin11]. Our study emphasizes that this route of transmission is not negligible.
Twenty percent of AHC patients reported a sexual contact with a HCV-infected partner. For nine cases (15%), this was the only reported risk exposure. This proportion varies between 2% and 25% in other studies [Reference Hofer1, Reference Robotin7–Reference Morin and Pariente9, Reference Morin11, Reference Martinez-Bauer13, Reference Alter22]. Sexual transmission of HCV is still a controversial issue. Although, several case-control studies found an association between HCV infection and either the number of sexual partners [Reference Alter23, Reference Mele24] or HCV-positive/at-risk partner [Reference Balasekaran25, Reference Murphy26], a prospective cohort study of monogamous heterosexual couples with one infected with HCV indicated a null or very low risk of sexual transmission of HCV [Reference Vandelli27]. Sexual transmission of HCV may, however, be facilitated by concomitant sexually transmitted infections with genital erosive lesions or by traumatic sexual practices among HIV-infected men who have sex with men [Reference Gambotti28, Reference Ghosn, Leruez-Ville and Chaix29]. Some of the patients who reported a sexual exposure may also have shared drug use with their partner.
The distribution of genotypes confirms the results of previous studies that showed that intravenous DUs are mainly infected by genotypes 3 and 1a and patients with history of nosocomial exposure by genotypes 1b and 2 [Reference Payan30]. Moreover, the high proportion of genotype 4 among DUs (18%) is consistent with the increase of the relative proportion of genotype 4 previously described [Reference Payan30–Reference Castera32].
The high frequency of documented co-infections (13%) is consistent with the preliminary results of a French registry of AHC which showed that 10% of patients were co-infected with another virus [Reference Morin11]. The majority of these seven patients reported at-risk behaviours: IDU or sexual exposure with co-infected partners.
Our study contains some limitations. One major limitation is that the characteristics of these AHC patients can not be generalized to all newly HCV-infected persons in the same period of time, since our study population was restricted to AHC patients newly referred to hepatology wards in hospitals. This mode of recruitment may have an impact on the distribution of risk exposures by overestimating the number of patients exposed to invasive medical procedures and health-care workers with needle-stick injury, and by underestimating the number of DUs. Furthermore, the proportion of patients who reported sexual contact with a HCV-positive partner appears rather high. One reasonable hypothesis based on the predominance of genotype 3 among these patients is that some of them may have shared drug use with their partner. Since it did not include a control group, this study does not allow the interpretation of risk exposures in terms of causality and their relative frequencies. However, our results are consistent with the result of a recent incident case-control study that showed the role of IDU and endoscopy at the end of the 1990s [Reference Delarocque-Astagneau19].
In comparison with other studies [Reference Robotin7, Reference Morin and Pariente9, Reference Morin11, Reference Wu14], our case definition of AHC, similarly to that used in a German clinical trial [Reference Jaeckel8], might be more specific because the period for HCV seroconversion was clearly stated and of shorter duration. Thus, almost three quarters of our study cases seroconverted within a 6-month period. Moreover, this study was based on a structured surveillance system which had shown a good internal sensitivity (73–100% of patients seen in the 26 reference centres in 2001 meeting the case definition criteria for notification were included; data not shown). Risk exposures were explored in the presumed contamination period. Finally, the proportion of jaundice observed (16%) is consistent with the knowledge on AHC clinical spectrum [Reference Orland, Wright and Cooper33].
In the present study, seven out of 16 AHC patients spontaneously recovered which is very similar to recently reported figures [Reference Morin and Pariente9, Reference Gerlach34]. As expected, spontaneous viral clearance mainly occurred within 3 months after diagnosis. The occurrence of spontaneous recovery 1 year after diagnosis in one case is, however, consistent with previous studies that showed that infection can exceptionally resolve spontaneously at 2 years and even 45 months after contamination [Reference Villano35, Reference Larghi36]. Our results are in agreement with previous studies that showed that the presence of jaundice during the acute phase is associated with spontaneous viral clearance [Reference Santantonio37]. This suggests that in real clinical practice, therapy initiation could be delayed in patients waiting for probable spontaneous viral clearance. In 2002, the French Consensus Conference suggested a waiting period of about 12 weeks after the onset of jaundice before the initiation of therapy in symptomatic AHC cases [Reference Lerebours, Marcellin and Dhumeaux38]. More recently, some authors have suggested that a good time to start therapy could be between 70 and 100 days after exposure, corresponding to 20–50 days after onset of symptoms [Reference Wedemeyer5]. The possibility of comparison is limited by the fact that the authors often referred to different types of delay.
Our observational results from an unselected AHC population are, however, based on rather small numbers and warrant further studies.
In conclusion, this study documented the main characteristics of AHC patients identified through a national surveillance system, especially risk exposures in the presumed contamination period and allowed us a pragmatic assessment of the medical follow-up of AHC cases and the outcome in the real practice. It confirms the major role of drug use in HCV transmission and hence the necessity to strengthen efforts for the prevention of HCV transmission among DUs. It also highlights the role of invasive medical procedures and occupational exposure, stressing the need for strict adherence to hygiene measures and spreading needle-stick prevention. Our results support the recommendation that initiation of therapy could be delayed in symptomatic AHC cases since the majority of these spontaneously recover.
APPENDIX
Hepatitis C Surveillance System Steering Committee (in alphabetical order)
J. P. Bronowicki, P. Couzigou, O. Goria, D. Guyader, P. Hillon, P. Marcellin, J. P. Miguet, F. Roudot-Thoraval, J. P. Zarski.
Participating hepatology reference centres
CHU de Fort de France (Dr A. Edouard); CHU de Bordeaux hôpitaux de Haut Leveque (Prof. Couzigou, Dr J. Foucher); CHU de Clermont-Ferrand (Prof. G. Bommelaer, Dr A. Abergel, Dr S. Ughetto); CHU de Dijon (Prof. P. Hillon, Dr A. Minello); CHRU Pontchaillou, Rennes (Dr H. Daniélou, Y. Desille, Prof. D. Guyader); Hôpital Trousseau, Tours (Prof. E. H. Metman, Dr L. d'Alteroche); CHU de Reims hôpital Robert Debré (Prof. G. Thiefin, Dr S. Lévy, Dr B. Bernard-Chabert); CHU de Besançon (Prof. J. P. Miguet, Dr P. Mercet); CHU de Caen (Prof. M. T. Dao, Dr C. Guillemard); CHU Rouen, hôpital Charles Nicolle (Prof. Lerebours, Dr O. Goria); Région Ile de France (réseau Paris Nord) CHU Bichat Beaujon, Clichy (Prof. P. Marcellin, Dr M. P. Ripault); CHU Créteil (réseau sud est) (Prof. D. Dhumeaux, Dr C. Hezode); réseau ouest, CHU Necker, Paris (Prof. S. Pol, Dr B. Nalpas); CHU de Montpellier (Prof. D. Larrey, Dr P. Fabbro-Peray); CHU de Limoges (Prof. B. Pillegand, Dr V. Loustaud-Ratti); CHR de Metz (Dr J. J. Raabe); CHU de Nancy (Prof. J. P. Bronowicki, Dr Tricon); CHU Purpan, Toulouse (Prof. J. P. Pascal, Dr K. Barange, Dr L. Alric); CHRU de Lille (Prof. J. C. Paris, Dr V. Canva-Delcambre); CHU de Nantes (Prof. Galmiche, Dr J. Gournay); CHU d'Angers (Prof. P. Cales, Dr I. Hubert-Fouchard); CHU D'Amiens (Prof. D. Capron); Hôpital Jean Bernard Poitiers (Prof. C. Silvain); CHU de Nice (Prof. P. Rampal, Prof. A. Tran); CH Hotel Dieu, Lyon (Prof. Trepo, P. Guilloreau Merle); CHU Grenoble (Prof. J. P. Zarski, Dr V. Leroy).
ACKNOWLEDGEMENTS
The authors thank Jean-Claude Desenclos for helpful comments and review of the manuscript, and Corinne Pioche for support in the management of the database.
DECLARATION OF INTEREST
None.







