Introduction
Psoriasis is a chronic, systemic, immune-mediated, inflammatory skin disease(1) which can have a substantial impact on quality of life (QoL) through both physical and psychological effects(Reference Griffiths, Armstrong and Gudjonsson2). It typically presents as raised, scaly plaques on the skin(Reference Griffiths, Armstrong and Gudjonsson2) which can cause painful and debilitating symptoms(1) and is associated with significant arthritic, cardiovascular, metabolic and psychological comorbidities(1,Reference Takeshita, Grewal and Langan3,Reference Parisi, Rutter and Lunt4) . Globally, there are an estimated 60 million people living with psoriasis (PLwP)(5).
Psoriasis affects males and females equally and is more common in adults compared with children(Reference Parisi, Rutter and Lunt4,Reference Michalek, Loring and John6) . The reported prevalence of psoriasis among adults varies globally, from 0·09%(Reference Gibbs7) to 11·43%(Reference Danielsen, Olsen and Wilsgaard8), and is more common in high-income countries and in regions with older populations(1,Reference Parisi, Rutter and Lunt4) . The highest prevalence of psoriasis is seen in Australasia (1·99%), western Europe (1·92%), central Europe (1·83%) and North America (1·50%)(Reference Parisi, Rutter and Lunt4). However, only 19% of countries have epidemiological data on psoriasis(Reference Parisi, Rutter and Lunt4,5) . In the United States psoriasis is one of the most common immune-mediated diseases, affecting 3% of adults(Reference Armstrong, Mehta and Schupp9), and in the United Kingdom psoriasis affects an estimated 2% of the population, approximately 1·1 million people(5).
There is no cure for psoriasis, and treatment is focused on symptom control. Studies show that PLwP who experience improvements in disease severity commonly experience improvements in QoL(Reference Mattei, Corey and Kimball10,Reference Puig, Thom and Mollon11) . However, satisfaction and adherence to some treatments are suboptimal due to side effects and dissatisfaction with the time taken and degree of improvement(1,Reference Stern, Nijsten and Feldman12,Reference Florek, Wang and Armstrong13) . Long-term efficacy of psoriasis treatments has also been highlighted as a concern(Reference Zhou, Chen and Bi14). Psoriasis imposes a significant economic burden, which increases with the number and onset of psoriasis-related comorbidities(Reference Feldman15,Reference Vanderpuye-Orgle, Zhao and Lu16) .
Comorbidities
Psoriatic arthritis (PsA) is the most prevalent comorbidity of psoriasis, affecting approximately 30% of people living with the disease(Reference Mease, Gladman and Papp17) and is more prevalent in those with severe psoriasis and those who have had the disease for a longer duration(Reference Ogdie, Ad Langan and Love18). Compared with the general population, PLwP have an increased risk of cardiovascular disease (CVD)(Reference Takeshita, Grewal and Langan3) and people with more severe psoriasis have increased odds of developing CVD, compared with those with mild-to-moderate psoriasis(Reference Armstrong, Schupp and Bebo19). It has been suggested that psoriasis may be an independent risk factor for CVD(Reference Takeshita, Grewal and Langan3). Multiple cardiovascular risk factors are also associated with psoriasis including, type 2 diabetes(Reference Armstrong, Harskamp and Armstrong20), obesity(Reference Armstrong, Harskamp and Armstrong21), metabolic syndrome(Reference Armstrong, Harskamp and Armstrong22), dyslipidaemia(Reference Ma, Schupp and Armstrong23) and hypertension(Reference Armstrong, Harskamp and Armstrong22). Furthermore, meta-analyses have also associated psoriasis with non-alcoholic fatty liver disease(Reference Candia, Ruiz and Torres-Robles24), certain cancers(Reference Trafford, Parisi and Kontopantelis25) and inflammatory bowel disease(Reference Alinaghi, Tekin and Burisch26).
Psoriasis also has a substantial psychological impact. PLwP are 1·5 times more likely to have symptoms of clinical depression compared with healthy controls(Reference Dowlatshahi, Wakkee and Arends27). Living with a chronic condition, social stigmatisation and low self-esteem play a significant role in the development of depression in PLwP(1), and emerging evidence suggests that systemic inflammation could also be playing a role in this relationship(Reference Liang, Cohen and Ho28).
Aetiology and pathophysiology
The onset of psoriasis is multifactorial and is theorised to occur due to a combination of genetic and environmental factors which trigger a dysregulated immune response, which activates and sustains a cycle of inflammation(Reference Zeng, Luo and Huang29,Reference Campanati, Marani and Martina30) . Multiple components of the adaptive and innate immune systems are involved in this process(Reference Griffiths, Armstrong and Gudjonsson2,Reference Campanati, Marani and Martina30) . The inflammatory cascade in psoriasis starts when plasmacytoid dendritic cells are activated, which promotes myeloid dendritic cell maturation through production of interferon (IFN) -α, IFN-y, tumour necrosis factor (TNF)-α and interleukin (IL)- 1β(Reference Armstrong and Read31). This leads to the activation and production of multiple cytokines, chemokines and antimicrobial peptides that promote an ongoing proinflammatory response. These include TNF-α, IL-6, IL-12 and IL-23, which activate T helper (Th)1, Th17 and Th22 cells(Reference Zhou, Chen and Cui32), which help to sustain the self-driving cycle of inflammation by producing TNF-α, IFN-y, IL-17 and IL-22(Reference Zeng, Luo and Huang29–Reference Armstrong and Read31,Reference Nestle, Kaplan and Barker33,Reference Mahil, Capon and Barker34) . This response leads to epidermal keratinocyte hyperproliferation and maintains a continual cycle of inflammation(Reference Zeng, Luo and Huang29,Reference Campanati, Marani and Martina30,Reference Nestle, Kaplan and Barker33,Reference Korman35) . The key role that the IL-17/IL-23 axis plays in psoriasis, as well as specific cytokines such as TNF-α, is demonstrated by the efficacy of biological medications which target these specific cytokines and pathways(Reference Griffiths, Armstrong and Gudjonsson2) (Fig. 1).

Fig. 1. Diagrammatic overview of the immune response, keratinocyte hyperproliferation and self-sustaining cycle of inflammation in psoriasis. pDC, plasmacytoid dendritic cells; mDC, myeloid dendritic cells; IL, interleukin; TNF, tumour necrosis factor; IFN, interferon; Th, T-helper cells; Tregs, regulatory T cells.
Compared with healthy controls, PLwP have increased serum levels of proinflammatory cytokines(Reference Dowlatshahi, Van Der Voort and Arends36,Reference Bai, Zheng and Dong37) , continual elevated levels of which lead to chronic subclinical systemic inflammation(Reference Korman35), hence why psoriasis is now seen as a systemic disease rather than solely dermatological(Reference Korman35). The systemic inflammation seen in psoriasis is theorised to contribute to the pathogenesis of many of the associated comorbidities(Reference Griffiths, Armstrong and Gudjonsson2,Reference Korman35,Reference Rendon and Schäkel38,Reference Hwang, Nijsten and Elder39) .
Lifestyle management for psoriasis
People living with psoriasis often look to lifestyle changes to manage their symptoms. The James Lind Alliance Priority Setting Partnership on psoriasis identified the top research priority for the disease as ‘Do lifestyle factors such as diet, dietary supplements, alcohol, smoking, weight loss and exercise play a part in treating psoriasis?’ in 2018(40). Lifestyle factors such as smoking, alcohol intake and stress have been shown to affect disease severity(1), but there is limited knowledge on the role of diet in managing psoriasis. Evidence suggests that diet can modulate immunological and inflammatory responses(Reference Katsimbri, Korakas and Kountouri41) and certain nutrients or dietary patterns could potentially worsen or alleviate psoriasis symptoms(Reference Kanda, Hoashi and Saeki42). However, there are no specific dietary guidelines for psoriasis.
There is a growing body of scientific literature regarding the role of diet in the management of psoriasis, alongside an increasing amount of ‘popular’ dietary advice(Reference Millsop, Bhatia and Debbaneh43–Reference Afifi, Danesh and Lee45). Studies have shown that in PLwP dietary modification is common, and that many are self-initiating dietary changes(Reference Millsop, Bhatia and Debbaneh43–Reference Afifi, Danesh and Lee45). It is therefore important for HCPs to familiarise themselves with the current literature on diet and psoriasis(Reference Millsop, Bhatia and Debbaneh43). By doing so, they will be able to provide informed support, combat misinformation and discuss the role of diet in managing psoriasis with PLwP(Reference Millsop, Bhatia and Debbaneh43,Reference Debbaneh, Millsop and Bhatia46) . This is particularly important considering the associated comorbidities(1).
Objectives of this review
The aim of this scoping review is to provide a comprehensive overview of the available evidence on the role of diet in the management of psoriasis. It will summarise the literature on dietary intake, the perceived role of diet in psoriasis management and evidence from dietary intervention studies on the impact of psoriasis symptoms. Additionally, this review will consider relevant grey literature on the role of diet in the management of psoriasis. A scoping review was determined as the most appropriate method given the broad study objective that will explore a range of sources, study designs and outcome measures.
Methodology
This scoping review was conducted according to the updated methodological guidance for the conduct of scoping reviews of the Joanna Briggs Institute(Reference Peters, Marnie and Tricco47). The search was conducted by:
-
(1) Searching PubMed and SCOPUS using relevant key words and phrases. The key words used were: Psoriasis AND diet* OR nutrition* OR eat OR ‘dietary patterns’ OR ‘dietary intake’ OR ‘dietary behaviours’ OR ‘dietary habits’.
-
(2) Searching appropriate grey literature. Grey literature is defined as ‘information produced on all levels of government, academia, business and industry in electronic and print formats not controlled by commercial publishing i.e., where publishing is not the primary activity of the producing body’(48). For this scoping review we included grey literature produced by psoriasis organisations, nutritional societies and health authorities, and reports and guidelines on psoriasis management. The grey literature search strategy used was developed using methods from Godin et al.(Reference Godin, Stapleton and Kirkpatrick49) for applying systematic search strategies to identify grey literature. Targeted searching of the identified resources, using appropriate search terms, was then undertaken.
-
(3) Screening reference lists of relevant papers, reports and guidelines.
-
(4) Searching for specific dietary modifications found to have been followed by people living with psoriasis (PLwP) individually as they emerged from the studies included in the review. The terms searched for on PubMed and SCOPUS were Psoriasis AND the following: dairy-free, vegan, vegetarian, paleolithic, Pagano, ketogenic diet, low carbohydrate–high protein, red meat and nightshades.
Findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist for scoping reviews (PRISMA-ScR)(Reference Tricco, Lillie and Zarin50) (see Table 1 for checklist). PRIMSA diagram details the search and selection process applied during this scoping review. Studies were identified via database searches of PubMed and SCOPUS, and other methods. The grey literature was identified solely via other methods as detailed in the diagram (Fig. 2).
Table 1. The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist(Reference Tricco, Lillie and Zarin50)


Fig. 2. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and other sources(Reference Page, Moher and Bossuyt167).
Inclusion and exclusion criteria
Papers assessed for inclusion in this review were selected on the basis of relevance by title and abstract initially, and then full paper review. The review considered all methodologies of relevant studies; however, only those written in English, focused solely on psoriasis (all types of psoriasis were included), involving dietary approaches alone, and conducted in or addressing humans over 18 years old were included. The database search included papers published during the last 20 years, from 2002 until October 2022. The grey literature search was conducted between April and November 2022.
Presentation of findings
The literature varied widely in methodology and type. As a result, this scoping review provides an overview of the current evidence according to three main themes: (1) dietary intakes of people living with psoriasis (PLwP), (2) the perceived role of diet in the management of psoriasis and (3) dietary approaches to manage psoriasis symptoms.
Theme 1: dietary intakes of people living with psoriasis (PLwP)
This theme reviews studies that explored the dietary intakes and habitual supplement use of people living with psoriasis (PLwP) (Table 2). The search identified nine studies that explored the dietary intakes of PLwP(Reference Afifi, Danesh and Lee45,Reference Polo, Corrente and Miot51–Reference Wasiluk, Stefanska and Ostrowska58) . Among these, seven performed studies comparing the dietary intakes of PLwP with healthy controls(Reference Afifi, Danesh and Lee45,Reference Yazdanpanah, Vahabi-Amlashi and Nematy52–Reference Barrea, Macchia and Tarantino57) , one compared the dietary intake of PLwP with adults with other chronic inflammatory conditions and recommended national dietary guidelines(Reference Wasiluk, Stefanska and Ostrowska58) and six included studies compared the dietary intakes of PLwP depending on levels of psoriasis severity(Reference Polo, Corrente and Miot51–Reference Kashani, Moludi and Fateh54,Reference Yamashita, Morita and Ito56,Reference Barrea, Macchia and Tarantino57) . The search also identified two studies that investigated the habitual supplement use of PLwP compared with controls(Reference Yousefzadeh, Mahmoudi and Banihashemi59,Reference Wilson60) . All studies were cross-sectional, seven used food frequency questionnaires (FFQs) to assess dietary intake(Reference Afifi, Danesh and Lee45,Reference Polo, Corrente and Miot51–Reference Yamashita, Morita and Ito56) , one used 3 × 24-h dietary recall(Reference Wasiluk, Stefanska and Ostrowska58) and one study used a 7-d food recall(Reference Barrea, Macchia and Tarantino57).
Table 2. Summary of included studies under Theme 1: dietary intakes of people living with psoriasis

FFQ, food frequency questionnaire; PASI, psoriasis area and severity index; PLwP, people living with psoriasis; BMI, body mass index; E-DII, energy-adjusted dietary inflammatory index; NHANES, US National Health and Nutrition Examination Survey; BDHQ, Japanese diet history questionnaire based on diets in Japan.
Several common significant differences in dietary intakes of food groups were observed between controls and PLwP. Three studies found that fat intake was significantly higher in PLwP compared with controls(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) . A further study compared dietary intakes of PLwP with the recommended dietary guidelines in Poland and found that the mean dietary intakes of fat in PLwP were 148% of the recommended dietary intakes(Reference Wasiluk, Stefanska and Ostrowska58). However, when compared with adults with other chronic inflammatory diseases, no significant difference in fat intake was observed. The absence of a healthy control group in this study meant that the findings are impossible to compare(Reference Wasiluk, Stefanska and Ostrowska58).
Carbohydrate intake was also found to be significantly higher in PLwP compared with controls in two studies(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Barrea, Macchia and Tarantino57) . However, intake differences depended on the type of carbohydrate. Yazdanpanah et al.(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52) found that total carbohydrate intake was significantly higher in PLwP compared with controls. Barrea et al. found that that total and simple carbohydrate intakes were significantly higher in PLwP compared with controls, whereas complex carbohydrate intake was significantly lower in PLwP when compared with controls(Reference Barrea, Macchia and Tarantino57). This was the only study to assess carbohydrate intake dependent on type and was conducted in all white males (n = 82), using a 7-d food recall, which makes the results difficult to compare(Reference Barrea, Macchia and Tarantino57).
Fibre intake was found to be significantly lower in PLwP compared with controls in three studies(Reference Afifi, Danesh and Lee45,Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) . A further study found that fibre intake of PLwP was only 53·3% in females (n = 17) and 65% in males (n = 22) of Polish recommended dietary guidelines (30 g/d). However, no significant difference was observed when compared with the dietary intakes of adults with other chronic inflammatory conditions, and no healthy controls were included in this study(Reference Wasiluk, Stefanska and Ostrowska58).
Findings on the dairy and sugar intakes of PLwP compared with controls were contrasting. Two studies found that sugar intake was significantly lower in PLwP in the United States(Reference Afifi, Danesh and Lee45,Reference Johnson, Ma and Kanada55) , whereas one study conducted in Japan found that PLwP consumed significantly more sugar than controls(Reference Yamashita, Morita and Ito56). Regarding dairy intake, a large study in the United States found that PLwP consumed significantly less dairy compared with controls from the 2009 to 2010 National Health and Nutrition Examination Survey (NHANES)(Reference Afifi, Danesh and Lee45). However, dairy intake was found to be significantly higher in PLwP compared with controls in a study conducted in Thailand(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53). Only one study found that PLwP consumed significantly less protein than controls(Reference Barrea, Macchia and Tarantino57). However, this study was conducted in all white males (n = 82), using 7-d food recall, which makes the results difficult to compare.
Regarding the intake of specific foods, several differences were observed between PLwP and controls. One study reported that PLwP had significantly higher intake of pulses compared with controls(Reference Yamashita, Morita and Ito56), and significantly higher intakes of legumes were also reported in PLwP compared with controls(Reference Afifi, Danesh and Lee45). Single studies reported that fruit and vegetables intakes were significantly higher in PLwP(Reference Afifi, Danesh and Lee45), as well as coconut milk and soft drinks(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53) compared with controls, whereas olive oil, eggs, berry fruits, brown rice/Riceberry, pickled foods and tree nuts(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53), and meat(Reference Yamashita, Morita and Ito56) intake were reported to be significantly lower in PLwP compared with controls. Differences in fish and seafood intakes between PLwP and controls were found in two studies; however, results were contrasting(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53,Reference Yamashita, Morita and Ito56) .
Differences in dietary intakes of specific nutrients between PLwP and controls were also reported in several studies. Polyunsaturated fatty acid (PUFA) intake was reported to be significantly higher in PLwP compared with controls in two studies(Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) . Barrea et al. also found that n-6:n-3 PUFA ratio intake was significantly higher whereas n-3 PUFA intake was significantly lower in PLwP compared with controls(Reference Barrea, Macchia and Tarantino57). However, Kashani et al. reported that both linoleic acid and linolenic acid intakes were higher in PLwP compared with controls(Reference Kashani, Moludi and Fateh54). Regarding monounsaturated fatty acids (MUFA) intake, the two studies that found significant differences in dietary intakes between PLwP and controls reported contrasting results(Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) . A further study found that females with psoriasis consumed significantly more MUFA compared with females with other chronic inflammatory diseases(Reference Wasiluk, Stefanska and Ostrowska58). However, no significant difference was seen in MUFA consumption in the male group, and there was no healthy control group to compare intakes with. Contrasting findings on vitamin A(Reference Kashani, Moludi and Fateh54,Reference Johnson, Ma and Kanada55) and calcium intake(Reference Afifi, Danesh and Lee45,Reference Kashani, Moludi and Fateh54) of PLwP and controls were also reported. Single studies reported that PLwP consumed significantly higher amounts of cholesterol(Reference Barrea, Macchia and Tarantino57), vitamin B12, vitamin D(Reference Yamashita, Morita and Ito56) and iron(Reference Kashani, Moludi and Fateh54), and significantly lower amounts of vitamin E and folate(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52) compared with controls.
One study explored the inflammatory potential of diets consumed by PLwP (n = 75) compared to age-, sex- and BMI-matched controls (n = 74) using FFQs and an energy-adjusted dietary inflammatory index (E-DII) as a predictive tool for inflammation potential of diets. They found that PLwP had a significantly higher energy-adjusted dietary inflammatory index with a median score of 0·10 (−1·59 to 0·83), a more pro-inflammatory diet, compared with controls where the median score was −2·14 (−2·96 to 1·00)(Reference Kashani, Moludi and Fateh54).
Differences in dietary intake between those with lower psoriasis severity and those with more severe psoriasis were also reported in several studies. Those with lower psoriasis severity had significantly higher intakes of fibre(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Barrea, Macchia and Tarantino57) , complex carbohydrates(Reference Barrea, Macchia and Tarantino57), vegetables(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53), MUFA, n−3 PUFA(Reference Barrea, Macchia and Tarantino57) and vitamin E(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52) compared with those with higher psoriasis severity. Furthermore, those with higher psoriasis severity had significantly higher intakes of total energy, saturated fatty acids, total PUFA, n-6 PUFA, n-6:n-3 PUFA ratio, simple carbohydrates(Reference Barrea, Macchia and Tarantino57), confectionery(Reference Yamashita, Morita and Ito56) and red meat(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53) compared with those with lower psoriasis severity. A single study reported that a high energy-adjusted dietary inflammatory index (E-DII) score was associated with increased severity of psoriasis(Reference Kashani, Moludi and Fateh54). Polo et al. found an inverse association with adherence to a ‘fresh diet’, characterised by predominantly fresh foods and a high consumption of fruits and vegetables, and cutaneous activity(Reference Polo, Corrente and Miot51). However, no definition of cutaneous activity was given, and PASI was recorded separately in this study.
Of note was that the definitions and methods for determining psoriasis severity varied between studies, and several studies did not include any definition of what constituted as lower or higher severity. Therefore, it is difficult to compare or understand the effects of these dietary intakes on psoriasis symptom severity.
Habitual supplement use
Two studies explored supplement use of PLwP compared with controls, over a 30-d period. These had mixed results. In the United States no significant difference in supplement use was found between PLwP (n = 184) and matched controls (n = 6027)(Reference Wilson60), whereas in Iran, a significantly higher proportion of PLwP (n = 138) used supplements over the previous 30-d period, compared with controls (n = 138). However, no difference was reported between supplement use and psoriasis severity(Reference Yousefzadeh, Mahmoudi and Banihashemi59).
Theme 2: the perceived role of diet in the management of psoriasis
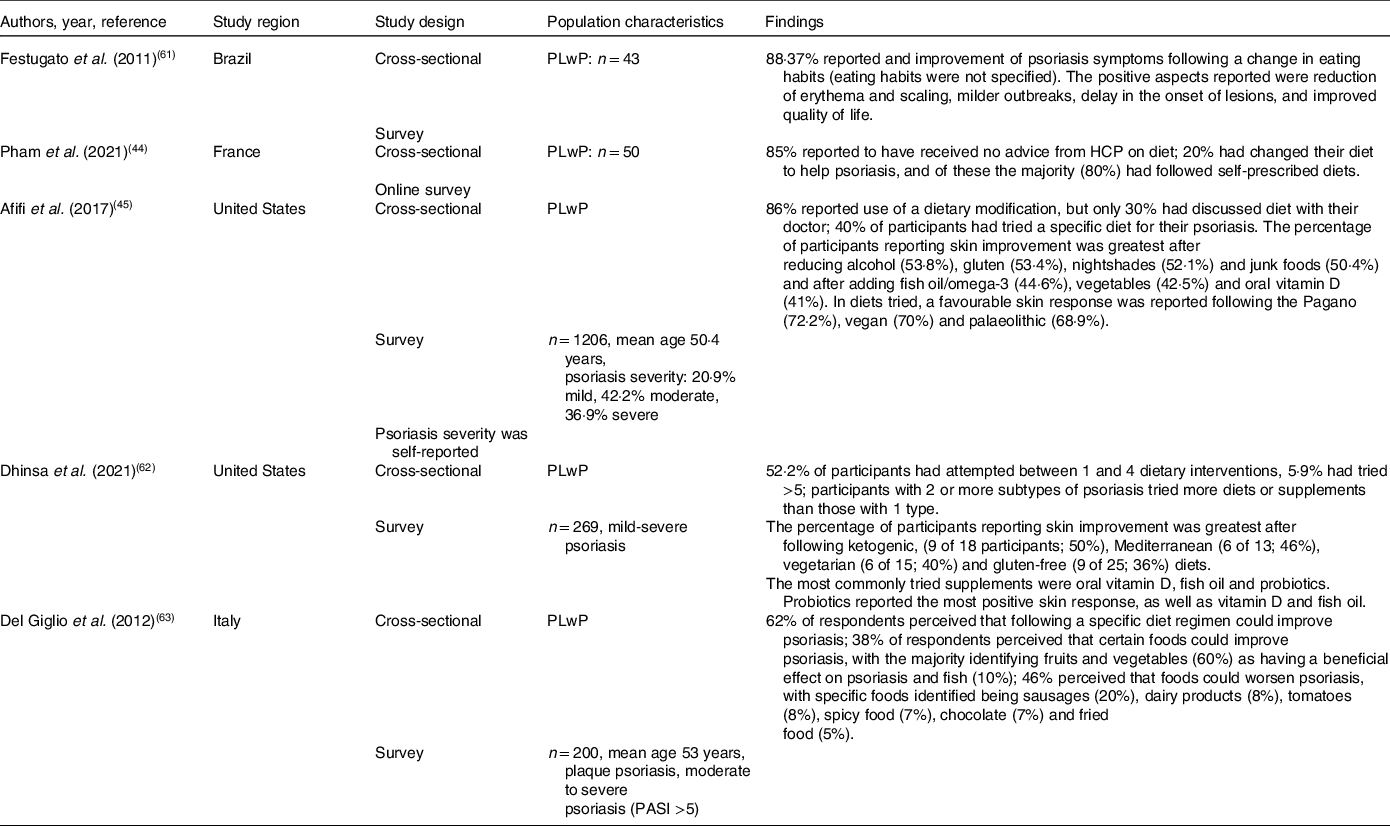
This theme comprises studies which explored the perceived role and use of diet in the management of psoriasis. Five studies were identified under this theme(Reference Pham, Sokol and Halioua44,Reference Afifi, Danesh and Lee45,Reference Festugato61–Reference Del Giglio, Gisondi and Tessari63) , all of which were cross-sectional surveys and focused solely on the perceptions and experiences of people living with psoriasis (PLwP). No studies exploring the perceptions of healthcare professionals (HCPs) were identified in this review (Table 3).
Table 3. Summary of included studies under Theme 2: the perceived role of diet in the management of psoriasis
PLwP, people living with psoriasis; HCP, healthcare professional; PASI, psoriasis area and severity index.
Diet was perceived by the majority of PLwP to have an impact on their psoriasis symptoms in several studies. A survey on perceptions of dietary approaches to manage psoriasis of PLwP (n = 200) found that 62% of respondents perceived that following a specific diet could improve psoriasis, and 38% perceived that consuming specific foods could improve psoriasis(Reference Del Giglio, Gisondi and Tessari63). A further study exploring dietary modifications and perceived effects on psoriasis symptoms over the past 2 years in PLwP (n = 43) found that 88·37% of respondents reported an improvement of psoriasis symptoms following a change in eating habits(Reference Festugato61). Although Afifi et al. found that in PLwP (n = 1206) 43·2% of respondents were not sure how diet affected their skin, 17·4% felt diet was slightly helping their skin, 16·7% felt diet was significantly helping their skin, and 2·2% reported that their skin condition was completely controlled by diet(Reference Afifi, Danesh and Lee45).
People living with psoriasis commonly reported that they had made changes to their diet, the majority of which were self-prescribed. Afifi et al. found that in PLwP (n = 1206) most respondents (86%) reported using a dietary modification of some kind; of these, 40% reported following a specific diet to help their psoriasis, but only 30·7% of those that had changed their diet had discussed diet with a dermatologist(Reference Afifi, Danesh and Lee45). A further study of 269 PLwP found that over half (52·2%) of participants had attempted between one and four dietary interventions, with 5·9% having tried more than five different dietary interventions(Reference Dhinsa, Wu and Gibbons62). This study also found that participants with two or more subtypes of psoriasis had tried following more diets or taking more supplements than those with only one. Additionally, an online survey exploring the dietary perceptions of PLwP (n = 50) found that most respondents (85%) reported that they had not received any advice from HCPs on diet. Overall, 20% had changed their diet to help psoriasis; of these, the majority (80%) had followed self-prescribed diets(Reference Pham, Sokol and Halioua44).
The dietary changes made by PLwP were often restrictive, either following elimination diets or removing specific foods from diets. Afifi et al. found that a higher number of PLwP reported removing foods from their diets than those that reported trialling dietary additions(Reference Afifi, Danesh and Lee45). Only three studies reported on specific dietary modifications trialled by PLwP and perceived symptom response. The most common dietary modification tried by PLwP reported across studies was reducing gluten or following a gluten-free diet(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . Further diets trialled by PLwP were vegetarian, palaeolithic, ketogenic(Reference Dhinsa, Wu and Gibbons62), Mediterranean, low-carbohydrate–high-protein and the Pagano diet alongside reducing or removing dairy(Reference Afifi, Danesh and Lee45). Common dietary components excluded were nightshades, alcohol and junk food(Reference Afifi, Danesh and Lee45). Dietary additions reported to have been trialled by PLwP were increased consumption of fruit, vegetables and fish as well as vitamin D, omega-3/fish oil and probiotic supplements(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) .
The dietary modifications perceived to have a beneficial effect on symptoms were dairy free, vegan(Reference Afifi, Danesh and Lee45), vegetarian, palaeolithic, the Pagano diet(Reference Afifi, Danesh and Lee45), the ketogenic diet, the Mediterranean diet (MD) and a gluten-free diet (GFD)(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . Reducing red meat, gluten, nightshades, alcohol and junk foods were also perceived to improve psoriasis skin symptoms by PLwP.(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . Respondents also reported improvement in skin symptoms after adding or increasing certain foods to their diet; fish, fruit and vegetables and supplements, specifically, omega-3, vitamin D and probiotics(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . A further study in PLwP (n = 43) found that the majority (88·37%) of respondents reported an improvement of psoriasis symptoms following a dietary change(Reference Festugato61). However, the study did not specify which dietary changes were perceived to make a difference. The positive aspects reported after changing diet were reduction of erythema and scaling, milder outbreaks, delay in the onset of lesions, and improved quality of life(Reference Festugato61).
Dietary components were also perceived to be able to negatively affect psoriasis symptoms. In a study on those with moderate-to-severe psoriasis with a psoriasis area and severity index (PASI) >5 (n = 200), 46% perceived that foods could worsen psoriasis. Specific foods identified by participants were sausages, dairy products, tomatoes, spicy food, chocolate and fried food(Reference Del Giglio, Gisondi and Tessari63). However, Afifi et al. reported that 37% of respondents reported that they did not recognise any dietary triggers which may worsen their psoriasis(Reference Afifi, Danesh and Lee45).
Popular literature
To be comprehensive, this review searched all diets and dietary modifications reported to have been tried by PLwP that were identified in the literature under theme 2 that had not been identified in the initial searches. PubMed and SCOPUS were searched using Psoriasis AND each of the diets or dietary modifications tried, using the same inclusion and exclusion criteria as described in the methods. No additional relevant results were found on PubMed or SCOPUS. This indicates that most of the diets that PLwP try, as reported in theme 2, have not been substantiated with any scientific evidence in relation to psoriasis management.
Theme 3: dietary approaches for managing psoriasis symptoms
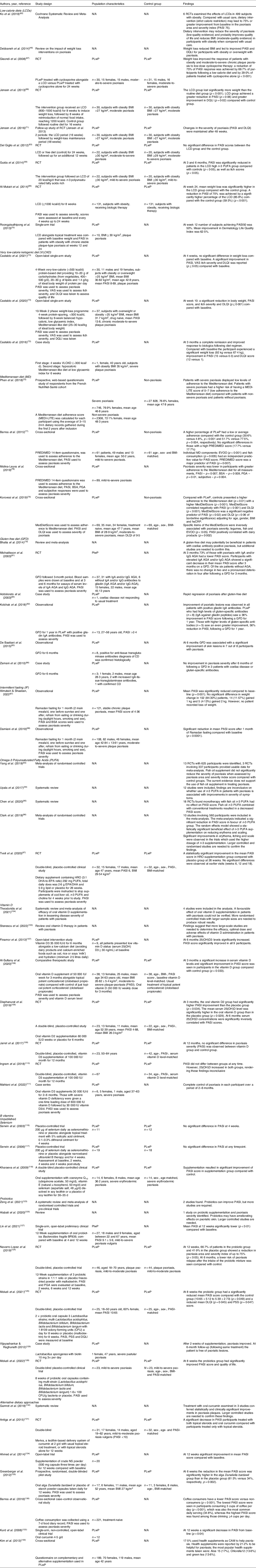
This theme included studies that explored specific dietary approaches and their impact on psoriasis symptoms. Dietary approaches were defined as specific dietary modifications followed to try to alleviate psoriasis symptoms through peer-reviewed investigations (Table 4).
Table 4. Summary of included studies under Theme 3: dietary approaches in the management of psoriasis symptoms
PLwP, people living with psoriasis; RCT, randomised control trial; BMI, body mass index, PASI, psoriasis area and severity index; DLQI, dermatology life quality index; LCD, low calorie diet; n-3 PUFA, omega-3 polyunsaturated fatty acid; VLCKD, very low-calorie ketogenic diet; MD, Mediterranean diet; EVOO, extra-virgin olive oil; BSA, body surface area; PGA, physician global assessment; GFD, gluten-free diet; IgA, immunoglobulin A; IgG AGA, immunoglobulin G antigliadin antibodies; IF, intermittent fasting; HRO, herring roe oil; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IU, international units; PSS, psoriasis severity scale; CAM, complementary and alternate methods; NS, Nigella sativa.
The findings are presented under each relevant subtheme.
-
1. Specific diets
-
2. Dietary supplementation
-
3. Alternative dietary approaches
1. Specific diets in the management of psoriasis
This review found that a handful of specific diets had been studied regarding the management of psoriasis: low-calorie diets (LCDs), very low-calorie ketogenic diets (VLCKD), intermittent fasting (IF), the Mediterranean diet (MD) and gluten-free diet (GFD).
Low-calorie diets (LCDs)
Low-calorie diets (LCDs) are dietary interventions that restrict energy intake with the goal of weight loss. All LCD studies identified were conducted in people living with psoriasis (PLwP) who were living with obesity or overweight, defined as a BMI ≥25 kg/m2. Diets prescribed ranged from 500 kcal/d to 1600 kcal/d. This review did not include studies on the impact of medication, exercise or surgery for weight loss on psoriasis severity.
The beneficial effect of LCD on psoriasis severity in subjects with obesity is supported in recent systematic reviews(Reference Debbaneh, Millsop and Bhatia46,Reference Ford, Siegel and Bagel64) . A Cochrane review of lifestyle changes in the treatment of psoriasis identified six randomised control trials (RCTs) that evaluated the effects of a low-calorie diet in 499 participants with obesity(Reference Ko, Chi and Yeh65). The review found that low-calorie diets may lead to an improvement ≥75% from baseline psoriasis area and severity index in PLwP with obesity, compared with usual care. However, more RCTs with larger sample sizes are needed. The Cochrane review meta-analysis also found that known risk factors of the associated comorbidities of psoriasis were significantly reduced in the LCD group compared with the control groups at week 16(Reference Al-Mutairi and Nour66–Reference Guida, Napoleone and Trio69).
Several RCTs have found that LCDs significantly improve psoriasis severity in subjects who are living with overweight or obesity compared with controls(Reference Al-Mutairi and Nour66,Reference Gisondi, Del Giglio and Di Francesco67) . Improvement in severity was also seen in an observational study at 12 weeks(Reference Roongpisuthipong, Pongpudpunth and Roongpisuthipong70). Only one study explored the long-term impact of an LCD on psoriasis severity and found that, after 48 weeks, weight loss in patients with psoriasis continued to have positive effects on symptom severity(Reference Jensen, Christensen and Zachariae71).
However, an LCD followed by participants with obesity (BMI ≥30 kg/m2) for 24 weeks found no significant difference in PASI scores between the LCD group and the control(Reference Del Giglio, Gisondi and Tessari63). However, baseline BMI was higher than in other studies, and the intervention LCD group may not have lost enough weight to produce the beneficial effect. In another study, although the LCD and control groups did not show a statistically significant difference in severity, the trend was towards reduced severity(Reference Jensen, Zachariae and Christensen68).
Very low-calorie ketogenic diet (VLCKD)
The main requirement to be defined as a ketogenic diet is carbohydrate restriction. In the studies identified in this review, the ketogenic diets also had a very low energy content (300–500 kcal/d) and were conducted only in PLwP with obesity or overweight. No systematic reviews on VLCKD and psoriasis symptoms were identified in this review. Three studies were identified that had explored the effects of a VLCKD on psoriasis severity(Reference Castaldo, Galdo and Rotondi Aufiero72–Reference Castaldo, Pagano and Grimaldi74).
A single-arm open-label trial (n = 37) found that weight loss following a VLCKD (<500 kcal/d; 1·2 g of protein/kg of ideal body weight/d) for 4 weeks followed by a balanced LCD (25–30 kcal/kg of ideal body weight per day) for 6 weeks significantly improvement in psoriasis area and severity index (PASI) and itch severity(Reference Castaldo, Rastrelli and Galdo73) in drug-naive adults with an overweight BMI and stable plaque psoriasis. Castaldo et al. (2021) explored the effect of a 4-week VLCKD of <500 kcal/d, providing 10–20 g of carbohydrates (from vegetables, 400–500 g/d), 20–30 g of lipids, and 1·4 g per kg of ideal body weight of protein per day, on the psoriasis severity of participants (n = 30) with overweight or obesity(Reference Castaldo, Pagano and Grimaldi74). After 4 weeks there was a significant improvement in PASI, itch severity and dermatology life quality index (DLQI) (p ≤ 0·05). However, no significant difference in weight loss compared with baseline was reported at 4 weeks(Reference Castaldo, Pagano and Grimaldi74). One case study of a female with severe psoriasis and obesity following a VLCKD(Reference Castaldo, Galdo and Rotondi Aufiero72) was also identified. Following a psoriasis relapse after treatment, the patient was put on a VLCKD of a protein-based enteral nutrition liquid of approximately 300 kcal/d, containing a protein content of 1·2 g/kg of ideal body weight, for 4 weeks. Compared with baseline, the patient lost 11 kg, and a significant reduction in psoriasis severity was observed (>80% PASI) after 4 weeks.
Mediterranean diet (MD)
The Mediterranean diet (MD) is typically high in fruits and vegetables, legumes, whole grains, fish, nuts and monounsaturated fatty acids (MUFA) such as extra-virgin olive oil (EVOO), with a moderate intake of meat, dairy and alcohol(Reference Phan, Touvier and Kesse-Guyot75). Four studies explored MD in the management of psoriasis(Reference Phan, Touvier and Kesse-Guyot75–Reference Molina-Leyva, Cuenca-Barrales and Vega-Castillo78). These were all cross-sectional studies that assessed the association between a score reflecting adherence to the MD and psoriasis severity. The higher the score, the higher the adherence to a MD. No randomised control trials (RCTs) were found to have been conducted on MD and psoriasis severity. Three of the studies also compared MD adherence of PLwP compared with controls(Reference Barrea, Macchia and Tarantino57,Reference Phan, Touvier and Kesse-Guyot75,Reference Korovesi, Dalamaga and Kotopouli76) .
Controls presented a significantly higher adherence to a Mediterranean diet compared with PLwP in all three case–control studies identified(Reference Phan, Touvier and Kesse-Guyot75–Reference Barrea, Balato and Di Somma77). Barrea et al. (Reference Barrea, Balato and Di Somma77) found that psoriasis participants exhibited statistically significant differences compared with controls, in the consumption of certain individual MD dietary components. Controls consumed significantly more EVOO, fruit, fish and nuts and significantly less red meat than those with psoriasis(Reference Barrea, Balato and Di Somma77).
Regarding psoriasis severity and MD adherence, those with less severe psoriasis had a higher adherence to a Mediterranean diet in all four studies. Barrea et al.(Reference Barrea, Balato and Di Somma77) used PREDIMED score to assess MD adherence in people with mild to severe psoriasis (n = 62). The study concluded that that PREDIMED score was a major predictor of psoriasis severity determined by PASI (p = 0·007). Individual MD components were also shown to have an independent predictive value for PASI score, higher consumptions of EVOO (p < 0·001) and fish (p = 0·005) were significantly associated with lower psoriasis severity scores(Reference Barrea, Balato and Di Somma77). A summary of individual foods associated with higher or lower psoriasis severity is presented in Table 5.
Table 5. Summary of individual foods included in the studies identified that were associated with lower or higher psoriasis severity.

PLwP, people living with psoriasis; BMI, body mass index, PASI, psoriasis area and severity index; DLQI, dermatology life quality index; VAS, visual analogue scale; MD, Mediterranean diet; EVOO, extra-virgin olive oil; BSA, body surface area; FFQ, food frequency questionnaire.
A large national-cross sectional study in PLwP in France (n = 3557) found that a higher percentage of participants with severe psoriasis had a MEDI-LITE score of 0–7 (low adherence to the Mediterranean diet) compared with those without severe psoriasis. Mediterranean diet score was also found to be negatively correlated with PASI (p = 0·001)(Reference Phan, Touvier and Kesse-Guyot75). In a smaller study (n = 69) using MedDietScore to assess MD adherence in PLwP, MedDietScore was a significant negative predictor of PASI (p = 0·02) adjusting for age, gender and BMI. Higher consumption of legumes, fish and EVOO (p < 0·05) were found to be associated with lower PASI scores, whereas higher dairy product consumption was positively correlated with psoriasis severity (p = 0·002)(Reference Korovesi, Dalamaga and Kotopouli76). The severity of psoriasis was lower in participants with greater adherence to the Mediterranean diet assessed using PASI (p = 0·007), body surface area (BSA) (p = 0·009) and practitioner global assessment (PGA) (p = 0·01) in a further cross-sectional study on PLwP using PREDIMED questionnaire to assess adherence to the Mediterranean diet(Reference Molina-Leyva, Cuenca-Barrales and Vega-Castillo78).
Gluten-free diet (GFD)
A gluten-free diet (GFD) eliminates gluten, a protein found in wheat, barley and rye. Psoriasis is associated with an increased risk of coeliac disease, compared with the general population(Reference Bhatia, Millsop and Debbaneh79,Reference Ungprasert, Wijarnpreecha and Kittanamongkolchai80) . Coeliac disease is a chronic condition affecting the small intestine, which is activated by the consumption of gluten. Studies suggest that psoriasis and coeliac disease share common genetic and inflammatory pathways(Reference Bhatia, Millsop and Debbaneh79). Gluten-specific serum antibody levels followed by a biopsy is used to diagnose coeliac disease. In those without a coeliac disease diagnosis, gluten-specific antibodies are higher in PLwP compared with controls. However, whether there is an association between higher antibody levels and greater psoriasis severity is unclear(Reference Bhatia, Millsop and Debbaneh79).
Two systematic reviews on diet and psoriasis found that a GFD may be beneficial in reducing psoriasis severity in those with coeliac disease or gluten-specific antibodies(Reference Ford, Siegel and Bagel64,Reference Bhatia, Millsop and Debbaneh79) . From the findings of their review, Bhatia et al. recommended that healthcare professionals (HCPs) screen patients with psoriasis for symptoms of gluten sensitivity, followed by gluten-specific antibody tests(Reference Bhatia, Millsop and Debbaneh79). Those with positive antibody tests should then be advised to trial a GFD for symptom management(Reference Bhatia, Millsop and Debbaneh79). However, there was no suggestion on the length of GFD trial.
Several studies have shown the beneficial impact of following a GFD on psoriasis severity in participants with coeliac disease or gluten-specific antibodies. A study on psoriasis patients (n = 39) with elevated gluten-specific antibodies showed a significant decrease in mean PASI score after 3 months on a GFD compared with a control group(Reference Michaëlsson, Gerdén and Hagforsen81). Those with moderate-to-severe psoriasis showed an even greater PASI reduction than those with mild psoriasis. The control group consisted of PLwP but without coeliac disease or gluten-specific antibodies, who also followed a GFD. In this group there was no change in disease severity, and in two participants there was a substantial worsening of psoriasis severity.
A further study by Ref. (Reference Kolchak, Tetarnikova and Theodoropoulou82), found that PLwP who had high levels of gluten-specific antibodies (n = 8) (IgA against gliadin peptides) saw a 36% improvement in PASI score following a GFD for 1 year. Those with higher levels of gluten-specific antibodies (n = 5) saw an even greater improvement, 56% reduction in PASI, following a GFD for 1 year. A GFD also significantly improved psoriasis symptoms in nine patients with coeliac disease compared with baseline at 3 months and was maintained at 6 months(Reference De Bastiani, Gabrielli and Lora83). Complete clearance of psoriatic skin symptoms following a GFD for 1 month has also been reported in individual case studies(Reference Addolorato, Parente and De Lorenzi84,Reference Tahiri, Azzouzi and Squalli85) . However, these data are based on small, uncontrolled studies.
One study found no improvement in psoriasis severity after 6 months of following a GFD in three patients with coeliac disease or gluten-specific antibodies(Reference Zamani, Alizadeh and Amiri86). However, this was another small uncontrolled study.
Intermittent fasting (IF)
More recently intermittent fasting has been studied in the management of psoriasis. Two studies have explored this dietary approach, using fasting during Ramadan to explore the effects on psoriasis(Reference Almutairi and Shaaban87,Reference Damiani, Watad and Bridgewood88) .
Almutairi and Shaaban (2022) assessed the effects of Ramadan fasting on psoriasis severity of 121 people with stable chronic plaque psoriasis in Kuwait(Reference Almutairi and Shaaban87). Participants followed traditional Ramadan fasting for 1 month, which consists of refraining from eating, drinking or smoking during daylight hours. Participants consumed two main meals a day, one before sunrise and one after. At 1 month, no participant recorded any weight loss, but mean PASI was significantly reduced compared with baseline(Reference Almutairi and Shaaban87). A further study(Reference Damiani, Watad and Bridgewood88) also investigated the impact of Ramadan fasting on psoriasis severity in participants with moderate-to-severe psoriasis (n = 108). Following the month of fasting, a significant reduction in mean PASI score was observed compared with baseline(Reference Damiani, Watad and Bridgewood88).
One pilot study exploring the effects of modified intermittent fasting, the 5:2 diet (consuming normally for 5 d and restricting calorie intake on 2 non-consecutive days) on psoriasis severity was also found(Reference Grine, Hilhorst and Michels89). Preliminary study findings presented at the European Academy of Dermatology and Venerology Spring Symposium, show a significant reduction in scaling and thickness in patients with mild psoriasis after following a 5:2 diet(Reference Grine, Hilhorst and Michels89).
Other diets
Vegetarian, vegan and plant-based diets have been discussed in the literature as diets with potential to help alleviate psoriasis symptoms(Reference Marta90,Reference Rastmanesh91) . However, this is based on the assumptions that following these diets would result in increased consumption of fruits, vegetables and antioxidants and the reduced consumption of saturated fats(Reference Marta90). Whilst cross-sectional studies have shown that following a MD, which is characterised by high fruit and vegetable consumption and low saturated fat intake, could help lessen psoriasis severity(Reference Phan, Touvier and Kesse-Guyot75,Reference Barrea, Balato and Di Somma77) , this is not the same as following a vegan or vegetarian diet. Following a vegan, vegetarian and plant-based diet does not always result in increased fruit and vegetable consumption. So far, no studies have been undertaken explicitly exploring vegetarian, vegan or plant-based diets in the management of psoriasis.
2. Supplementation in the management of psoriasis
Several supplements have been studied in the management of psoriasis: omega-3 polyunsaturated fatty acids (PUFA), vitamin D, selenium, B vitamins and probiotics.
Omega-3 polyunsaturated fatty acids (PUFA)
Several recent systematic reviews and meta-analysis have been conducted to evaluate the effects of omega-3 PUFA supplementation on psoriasis severity, with conflicting results. Most studies gave omega-3 PUFA as fish oil supplements.
A systematic review based on thirteen randomised control trials (RCTs) and a meta-analysis of three RCTs found that fish oil supplementation did not significantly reduce the severity of psoriasis assessed by psoriasis area and severity index (PASI) compared with controls, concluding that the current evidence does not support the use of fish oil supplement in treating psoriasis(Reference Yang and Chi92). This was in line with a previous systematic review(Reference Upala, Yong and Theparee93).
Another systematic review found that supplementation with fish oil omega-3 PUFA alone had no effect on PASI score. However, when combined with traditional psoriasis treatments, a significant reduction in PASI score was observed compared with controls(Reference Chen, Hong and Sun94).
In contrast, a recent 2019 meta-analysis found that supplementation of omega-3 PUFA did significantly reduce PASI score. Significant improvements in specific psoriasis skin symptoms, erythema, itching and scale, were observed in trials which used higher doses of omega-3 PUFA supplementation (>1800 mg/d)(Reference Clark, Taghizadeh and Nahavandi95). The positive effects of high doses of omega-3 on psoriasis symptoms were in line with a recent study on the effect of herring roe oil (HRO) on psoriasis severity(Reference Tveit, Brokstad and Berge96). A significant improvement in mean PASI score with HRO supplementation of 2600 mg eicosapentaenoic (EPA)/docosahexaenoic (DHA) per day, was observed compared with placebo treatment, at week 26. The authors of this study theorised that the beneficial effects of HRO were due to its EPA and DHA acids ratio of 3:1, compared with omega-3 PUFA from fish oils, which is typically 1:1.
Omega-3 PUFA supplementation has been shown to have beneficial effects on the co-morbidities associated with psoriasis(Reference Khan, Lone and Khan97).
Vitamin D
Topical vitamin D is a widely used treatment for plaque psoriasis(Reference Griffiths, Armstrong and Gudjonsson2). Lower levels of serum vitamin D have been reported in psoriatic patients compared with controls(Reference Gisondi, Rossini and Di Cesare98,Reference Hambly and Kirby99) . A small, but significant, inverse correlation between serum 25(OH)D and the severity of psoriasis has also been reported(Reference Chandrashekar, Krishna Kumari and Rajappa100,Reference Filoni, Vestita and Congedo101) , hence the interest in oral vitamin D and psoriasis management.
So far, studies have shown mixed results on the effectiveness of oral vitamin D supplementation in the management of psoriasis. Systematic reviews have found no clear evidence to support vitamin D supplementation in the management of psoriasis symptoms(Reference Millsop, Bhatia and Debbaneh43,Reference Ford, Siegel and Bagel64,Reference Stanescu, Simionescu and Diaconu102) . A recent meta-analysis found that a favourable effect of oral vitamin D supplementation in patients with psoriasis could not be verified(Reference Theodoridis, Grammatikopoulou and Stamouli103). However, more RCTs are required to confirm these conclusions. There is evidence indicating that vitamin D supplements for the treatment of psoriasis should not be prescribed in participants with normal serum levels of vitamin D(Reference Megna, Ferrillo and Barrea104). It is unclear from the literature whether those with deficient or insufficient vitamin D levels have an improved skin response compared with those with optimal levels.
An RCT assessing the effect of oral vitamin D2 on psoriasis severity found that D2 supplementation significantly increased the serum vitamin D level and significantly improved PASI scores in patients with psoriasis compared with the placebo group at 3 months. There was no significant difference in baseline serum 25(OH)D vitamin D between groups, and some vitamin D insufficiency was seen in both groups(Reference Disphanurat, Viarasilpa and Chakkavittumrong105). In a study that gave high doses of oral vitamin D3 (35 000 IU/d) to PLwP (n = 9) and low vitamin D status (≤30 ng/mL), significant improvements in psoriasis severity were observed at 6 months compared with baseline(Reference Finamor, Sinigaglia-Coimbra and Neves106). However, this was a small, uncontrolled study and participants were also required to follow a low-calcium diet (excluding dairy) over the course of the study. A further study also found a significant improvement in PASI score in participants given oral vitamin D supplement of 50 000 IU per week for 3 months alongside usual treatment compared with the control group who just received usual treatment(Reference Al-Sultany107).
However, several RCTs have shown no beneficial effect of oral vitamin D supplementation on psoriasis severity. No significant difference was found in people with mild psoriasis over 12 months of vitamin D3 supplementation, or in those with plaque or moderate-to-severe psoriasis over 3 months compared with controls(Reference Jarrett, Camargo and Coomarasamy108,Reference Ingram, Jones and Stonehouse109) .
A recent series of case studies showed complete control of psoriasis with a high daily dose of 30 000 IU of vitamin D3 over a period of 2–6 months. Only two participants presented with severe vitamin D deficiency and were given a one-off loading dose of 600 000 IU vitamin D, all others had optimal levels(Reference Mahtani and Nair110). Other uncontrolled studies have also indicated that oral vitamin D supplementation for ≥6 months can significantly improve PASI score(Reference Theodoridis, Grammatikopoulou and Stamouli103).
Epidemiological studies have demonstrated a strong association between vitamin D insufficiency and risk of several psoriasis-associated comorbidities, including cardiovascular disease (CVD) and metabolic syndrome(Reference Judd and Tangpricha111).
B vitamins
Vitamin B12 deficiency has been associated with psoriasis(Reference Brazzelli, Grasso and Fornara112). However, studies so far have focused on intramuscular doses of vitamin B12 and have been shown to be ineffective. Two systematic reviews on dietary approaches to psoriasis did not recommend vitamin B12 supplementation in the management of psoriasis due to the lack of studies(Reference Millsop, Bhatia and Debbaneh43,Reference Ford, Siegel and Bagel64) . Vitamin B12 is an important cofactor in the metabolism of homocysteine, elevated levels of which have been associated with increased risk of CVD(Reference Škovierová, Vidomanová and Mahmood113). This review also found one ongoing RCT on the effect of high doses of vitamin B2 (riboflavin) on psoriasis severity that is yet to be published(Reference Gudjonsson114).
Selenium
Reviews have not found any significant improvement in psoriasis severity with selenium supplementation(Reference Millsop, Bhatia and Debbaneh43,Reference Ford, Siegel and Bagel64,Reference Serwin, Wasowicz and Gromadzinska115,Reference Serwin, Wasowicz and Chodynicka116) . A small number of studies have evaluated the effect of selenium supplementation on psoriasis severity(Reference Serwin, Wasowicz and Gromadzinska115–Reference Kharaeva, Gostova and De Luca117). One study found a significant beneficial effect of selenium on PASI score compared with controls. However, the supplement was combined with coenzyme Q10 and vitamin E(Reference Kharaeva, Gostova and De Luca117).
Probiotics
Recent studies have drawn attention to the role that the gut microbiome plays in the pathogenesis of dermatological conditions, including psoriasis(Reference Benhadou, Mintoff and Schnebert118). Psoriasis is associated with inflammatory bowel disease (IBD), and studies have shown that the gut microbiome is altered in psoriasis compared with controls(Reference Schade, Mesa and Faria119,Reference Hidalgo-Cantabrana, Gómez and Delgado120) . It has also been reported that patients with moderate-to-severe psoriasis have a lower gut microbial diversity than patients with mild disease(Reference Dei-Cas, Giliberto and Luce121). As a result, probiotic supplementation has become a recent research focus in the management of psoriasis.
A systematic review on the effectiveness of probiotic supplements in psoriasis found that probiotics significantly reduced PASI scores in psoriasis compared with controls after 12 weeks of supplementation and may be an effective treatment for alleviating psoriasis symptoms. However, these findings were based on only two RCT studies that explored probiotic supplementation and PASI, and larger-scale RCTs are needed to confirm this(Reference Zeng, Yu and Wu122).
Several studies have shown probiotic supplementation to have a beneficial effect on psoriasis severity(Reference Navarro-López, Martínez-Andrés and Ramírez-Boscá123–Reference Moludi, Fathollahi and Khedmatgozar127). A recent RCT found that consuming a probiotic drink containing Lactobacillus strains for 8 weeks significantly reduced PASI and psoriasis symptom scale (PSS) scores compared with the placebo group(Reference Moludi, Khedmatgozar and Saiedi124). A further double-blind placebo-controlled trial (n = 46) found that after 8 weeks of multi-strain probiotic oral supplementation PASI and quality of life scores had significantly improved compared with the placebo group(Reference Moludi, Fathollahi and Khedmatgozar127). Additionally, single-arm trial (n = 27) reported significant reduction in PASI compared with baseline at 12 weeks of probiotic supplementation(Reference Lin, Zeng and Deng126). One case study also reported that supplementation of a probiotic containing Lactobacillus strains had a strong alleviating effect on skin symptoms in a patient with pustular psoriasis after 15 d. The patient continued with probiotic supplementation, and after 6 months psoriasis severity had reduced further(Reference Vijayashankar and Raghunath128).
3. Alternative dietary approaches in the management of psoriasis
This review identified several studies that explored alternative dietary approaches in the management of psoriasis. These were defined as non-traditional dietary approaches, the majority of which were small studies. One cross-sectional questionnaire was identified that explored the use of complementary alternative methods used by people living with psoriasis (PLwP). This study found that health supplements were reported by 21·2% to be helpful for psoriasis; the most popular health supplements taken were aloe (17%), chlorella (13·6%) and green tea (13·6%)(Reference Kim, Park and Chin129).
Several small studies have been conducted on oral curcumin, a phytochemical found in the spice turmeric, and psoriasis severity, with mixed results(Reference Antiga, Bonciolini and Volpi130–Reference Kurd, Smith and VanVoorhees132). A review of the RCTs suggested that more studies are needed on the effects of oral curcumin on psoriasis severity before any conclusions can be made(Reference Gamret, Price and Fertig131).
The impact of oral Nigella sativa (NS) on psoriasis severity in sixty participants with mild-to-moderate psoriasis was investigated in an RCT. Participants were given an oral dose of NS (500 mg three times daily) for 12 weeks; at 12 weeks psoriasis area and severity index (PASI) score had decreased from baseline(Reference Ahmed Jawad, Ibraheem Azhar and Al-Hamdi133). However, whether this was significant or not was not clear. One RCT investigated oral capsules containing alga Dunaliella bardawil, a natural source of the retinoid precursor 9-cis β-carotene, in participants with mild plaque psoriasis (n = 34)(Reference Greenberger, Harats and Salameh134). Participants received capsules of the alga or a placebo, and at 6 weeks, the reduction in the mean PASI score was significantly higher in the alga group compared with the placebo group (p = 0·002).
The association between coffee consumption and severity of psoriasis was evaluated in a cross-sectional study of treatment naive PLwP (n = 221). Coffee consumers were found to have a significantly lower PASI score compared with non-consumers (p < 0·001), with the lowest PASI score seen in those consuming three cups of coffee per day, and the highest PASI score was found among those drinking four or more cups per day(Reference Barrea, Muscogiuri and Di Somma135).
Grey literature
The relevant grey literature sources identified and included in this review included reports, guidelines and other materials produced by a range of stakeholders in psoriasis management. Most of the grey literature identified, regarding psoriasis management, provided no dietary guidance for people living with psoriasis (PLwP), and most did not mention the word diet or nutrition at all. In those that did mention diet, the vast majority focused on weight loss and dietary approaches for comorbidities associated with psoriasis. For example, the National Institute for Heath and Clinical excellence (NICE) guidelines for psoriasis assessment and management mention reducing alcohol intake and losing weight, but only as modifiable risk factors for associated comorbidities(136). Several sources did provide further information regarding diet specific to psoriasis symptom management. The National Psoriasis Foundation (NPF) in the United States conducted a systematic review on the dietary recommendations for adults with psoriasis in 2018(Reference Ford, Siegel and Bagel64). Based on this, they recommend weight loss in PLwP with obesity or overweight as the only evidence-based dietary approach for psoriasis management on their website and suggested that a gluten-free diet (GFD) could provide relief in those with coeliac or gluten sensitivity. Several grey literature resources provided warnings about diets that claim to ‘cure’ psoriasis and misinformation that can be found online and included evidence-based dietary advice. However, overall, there was a lack of advice on who to go to for dietary support, the health impacts and the risks associated with following restrictive diets that claim to help psoriasis, and guidance for following a restrictive diet.
Discussion
In this study, we reviewed the current evidence on the role of diet in the management of psoriasis. We included all types of study designs that met the inclusion criteria, as well as relevant grey literature. This has enabled us to provide a comprehensive overview of the current evidence and a unique insight into the role of diet in the management of psoriasis regarding dietary intake of people living with psoriasis (PLwP), the use and perceived effectiveness of diet of PLwP, and dietary approaches for psoriasis management. We reviewed seventy-two peer-reviewed studies as well as seventy-seven relevant grey literature resources. The principal findings suggest that diet could play a role in psoriasis management; however, most evidence comes from small heterogeneous studies. Therefore, specific psoriasis dietary guidelines and recommendations cannot be made. The breadth of this scoping review also enabled us to map the research gaps and highlight areas for future research, to be able to better understand the role that diet plays in psoriasis management and improve dietary support for PLwP. The results of this scoping review were organised into three themes, alongside the grey literature. The discussions for each theme are presented below.
Theme 1: dietary intakes of people living with psoriasis
The studies included in this review suggest that the dietary intakes of PLwP differ from those of controls. The studies frequently found PLwP to have higher dietary intakes of fat(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) and lower intakes of fibre(Reference Afifi, Danesh and Lee45,Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Barrea, Macchia and Tarantino57) compared with controls. Studies also reported differences in intakes of sugar, dairy, pulses and legumes, vegetables and polyunsaturated fatty acids (PUFA) compared with controls. Furthermore, the evidence also suggests that the dietary intakes of people with less severe psoriasis differ from those with higher psoriasis severity.
High-fat diets (HFDs) have been shown to elicit low-grade systemic inflammation through elevated production of pro-inflammatory cytokines also seen in psoriasis, including interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α. HFDs also play a key role in the development and progression of multiple diseases, including cardiovascular disease (CVD), type II diabetes, atherosclerosis and some cancers(Reference Duan, Zeng and Zheng137). Murine studies have found that HFDs exacerbate the imiquimod induced psoriasiform dermatitis in mice(Reference Honda and Kabashima138,Reference Higashi, Yamakuchi and Fukushige139) , and in both mice with obesity and lean mice, those fed with HFDs developed a more severe early psoriasiform skin inflammation(Reference Herbert, Franz and Popkova140). This suggests that increased fat consumption could play a role in psoriasis symptom severity. Information on the specific fats consumed was lacking in the studies included in this review and would provide more insight into the potential mechanisms behind these dietary intakes.
Several studies also reported that PLwP had lower intakes of fibre compared with controls, and in psoriasis populations lower intakes of fibre were seen in those with more severe psoriasis compared with those with lower psoriasis severity(Reference Yazdanpanah, Vahabi-Amlashi and Nematy52,Reference Barrea, Macchia and Tarantino57) . Fibre has been shown to decrease levels of plasma inflammatory markers including C-reactive protein (CRP), IL-6 and TNF-α(Reference Kuo141), which play a key role in the pathophysiology of psoriasis. Dietary fibre also has a beneficial effect on the gut microbiome, and through short-chain fatty acid production produces immune and inflammatory regulation responses(Reference Kuo141). However, higher intakes of pulses and legumes, which are high in fibre, were reported in PLwP than in controls(Reference Afifi, Danesh and Lee45,Reference Yamashita, Morita and Ito56) . Additionally, a gluten-free diet (GFD) is associated with reduced fibre intake. Following a gluten-free diet in people with coeliac disease has been shown to improve psoriasis symptoms, and coeliac is seen more commonly in people with psoriasis compared with the general population(Reference Bhatia, Millsop and Debbaneh79). Following a GFD is also a common dietary modification trialled by PLwP(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) , which could explain the difference reported in fibre intakes between PLwP and controls. Following a GFD was also frequently perceived to improve psoriasis symptoms by PLwP. However, these studies did not include information on the coeliac status of participants, and lack of information on types and sources of fibre make it difficult to compare results and understand the potential mechanisms of action. Furthermore, fibre intake is associated with relevant health impacts and has appetite regulating and anti-obesogenic effects, and higher intakes have been associated with lower systemic inflammation(Reference Krishnamurthy, Wei and Baird142). Consuming adequate amounts of dietary fibre is also associated with multiple health benefits, including reduced CVD risk(Reference Lattimer and Haub143). Therefore, understanding the fibre intake in PLwP is also important due to the associated co-morbidities.
A significantly higher consumption of vegetables was reported in those with lower psoriasis severity compared with people with higher severity(Reference Ingkapairoj, Chularojanamontri and Chaiyabutr53). Vegetables are key sources of vitamins and polyphenolic compounds which have antioxidant and anti-inflammatory properties(Reference Katsimbri, Korakas and Kountouri41,Reference Block, Jensen and Dalvi144,Reference Salinthone, Kerns and Tsang145) . Flavonoids and carotenoids, polyphenolic compounds commonly found in vegetables, have been shown to enhance immune pathways and inhibit certain pro-inflammatory pathways(Reference Katsimbri, Korakas and Kountouri41). Specifically relevant to psoriasis, they have been shown to reduce pro-inflammatory cytokines IL-6 and TNF-α(Reference Katsimbri, Korakas and Kountouri41), which are involved in the pathophysiology of psoriasis(Reference Armstrong and Read31). Vegetables are also important sources of dietary fibre(Reference Dhingra, Michael and Rajput146). However, no studies identified specific vegetables consumed, so it is difficult to suggest potential pathways. Interestingly, Afifi et al. found that PLwP had a higher intake of fruits and vegetables compared with controls(Reference Afifi, Danesh and Lee45). This could be attributable to people with psoriasis following popular psoriasis dietary recommendations, which typically suggest that fruits and vegetables can improve psoriasis symptoms. This review has shown that dietary modifications among PLwP to try to manage psoriasis are common(Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . Dietary changes after diagnosis or to manage symptoms may also explain the contradictory findings regarding sugar, dairy and fish intakes of PLwP compared with controls. Removing or reducing dairy and sugar as well as following a vegetarian diet are recommended as dietary approaches to manage psoriasis in popular literature.
Two studies found that total polyunsaturated fatty acid (PUFA) intake was significantly higher in PLwP compared with controls(Reference Kashani, Moludi and Fateh54,Reference Barrea, Macchia and Tarantino57) . Although, when assessed on PUFA type, Barrea et al. found that n-3 PUFA intake was significantly lower in PLwP compared with controls, and lower intakes were associated with higher severity(Reference Barrea, Macchia and Tarantino57). n-3 PUFA are potentially potent anti-inflammatory agents(Reference Calder147). However, this was a small study conducted in treatment-naive males; therefore, generalisability and comparability are limited.
Although the studies identified suggest that dietary intake is different in PLwP and controls and between those with different severities, the evidence is limited. This review identified nine studies that have been conducted in only seven countries worldwide. All the studies were cross-sectional, and most had sample sizes under 200 people. Additionally, the methodologies varied substantially between studies, which impairs the ability to compare results between studies. Most of the studies used food frequency questionnaires (FFQs) to assess dietary intakes of participants that, although useful in these types of studies, rely on self-reported information, participant memory and perceptions of portion sizes, and foods may be missed if not presented on FFQ lists. It is also difficult to focus on the effect of one dietary component, as diet is a complex combination of different nutrients(Reference Hu148) and multiple other lifestyle factors can impact the development and severity of psoriasis(1). Longitudinal population-based studies are needed to further investigate a causal role between dietary intake and psoriasis, and effects on severity in PLwP. However, the studies identified in this review give an insight into the dietary intakes of PLwP and highlight important research gaps. Furthermore, the differences in dietary intakes could also impact general health and prompt further research in PLwP, due to the associated comorbidities that could also be exacerbated by the dietary intakes highlighted here, in particular, high fat and low fibre intakes(Reference McKeown, Fahey and Slavin149).
Theme 2: the perceived role of diet in the management of psoriasis
The belief that diet impacts psoriasis symptoms is common in PLwP, and many adjust their diets accordingly. However, most PLwP do not discuss diet with their healthcare professional prior to making dietary changes. This is concerning considering that most of the dietary changes tried were restrictive. This study also searched the scientific literature for evidence on the dietary approaches trialled by PLwP reported in the studies found under Theme 2. Except for the Mediterranean diet (MD) and GFD, no studies were identified that had explored the use of any of the dietary approaches reportedly followed in the management of psoriasis. This suggests that most dietary approaches tried by PLwP are unsubstantiated, self-prescribed and taken from the popular literature. Following fad diets long-term or restrictive diets without the guidance of healthcare professionals (HCPs) can result in micronutrient deficiencies (MND)(Reference Nazarenkov, Seeger and Beeken150,Reference Malik, Tonstad and Paalani151) . Micronutrient deficiencies have been reported in people with irritable bowel syndrome (IBS) who self-prescribe elimination diets without consulting HCPs(Reference Nazarenkov, Seeger and Beeken150,Reference Simons, Taft and Doerfler152) . Elimination diets could also result in further health impacts. Following a GFD was a common dietary modification trialled by PLwP(Reference Afifi, Danesh and Lee45). A GFD has been shown to be lower in dietary fibre and some essential micronutrients which have protective properties, such as cholesterol lowering and improved glycaemic control(Reference McKeown, Fahey and Slavin149), relevant to PLwP considering the associated comorbidities. Additionally, gluten-free foods are often more expensive(Reference Aljada, Zohni and El-Matary153). Furthermore, restrictive diets have also been linked to reduced quality of life (QoL), disordered eating and orthorexia(Reference Simons, Taft and Doerfler152).
The most common dietary modifications reported to improve psoriasis were reducing dairy, gluten, nightshades, alcohol and sugar. Apart from alcohol, and gluten in those with coeliac and gluten sensitivity, the mechanisms of how reducing these specific dietary components could improve psoriasis are unclear and have not been researched. Theories suggest that the potential pro-inflammatory impact of sugar consumption could be the reason behind this effect; high amounts of dietary sugars have been shown to promote T-cell-mediated inflammation(Reference Ma, Nan and Liang154). Dairy is commonly demonised in popular literature as being pro-inflammatory, most likely due to the saturated fat and lactose content of certain dairy products(Reference Nieman, Anderson and Cifelli155). However, a recent systematic review found that dairy products and dairy proteins have neutral to beneficial effects on biomarkers of inflammation(Reference Nieman, Anderson and Cifelli155). Nightshades are plants from the Solanaceae family, which include potatoes, tomatoes, peppers and aubergines. They contain solanine and alkaloids which have been linked with inflammation(Reference Afifi, Danesh and Lee45). However, no association between nightshades and inflammation is supported by scientific studies in humans. Furthermore, they are high in nutrients beneficial to health.
Overall, the evidence is limited; only five studies were identified under this theme in this review(Reference Pham, Sokol and Halioua44,Reference Afifi, Danesh and Lee45,Reference Festugato61–Reference Del Giglio, Gisondi and Tessari63) , all of which were cross-sectional surveys which relied on self-reported information and memory. Participants may have been more likely to have an interest in diet or believe that diet helps manage their psoriasis, which may have impacted results. Additionally, sample sizes were small, with only two studies with a sample size over 200, most participants were white females, and no studies included information on other factors known to affect psoriasis severity, including stress and smoking. Despite the limitations of these studies, the findings highlight important factors to consider in psoriasis care, as well as highlighting important research gaps.
Studies exploring the perceived role of diet in the management of psoriasis have been conducted in only four countries worldwide. None has been conducted in the United Kingdom, and this represents an important research gap as over 1·1 million people in the United Kingdom are estimated to be living with psoriasis. Instagram and online forums are commonly used by people with acne to seek information on nutritional suggestions to help their skin condition(Reference Smollich and Tischner156). However, no studies identified explored the sources of dietary information of PLwP or content of recommended dietary changes. Only three of the studies provided information on specific dietary modifications made. Further understanding the dietary recommendations suggested in the popular literature and duration of diets trialled could help HCPs understand the potential impact on nutrient status and ways to support PLwP. Following restrictive diets long term can lead to micronutrient deficiencies(Reference Nazarenkov, Seeger and Beeken150,Reference Malik, Tonstad and Paalani151) . Furthermore, specific symptom responses to dietary modifications have not been investigated. It was commonly reported that most PLwP do not discuss diet with an HCP prior to making dietary changes(Reference Pham, Sokol and Halioua44,Reference Afifi, Danesh and Lee45,Reference Festugato61) . Understanding the reasons behind this will give insight into patient support needs and enable HCP to better understand how to assist PLwP. Another notable gap in the literature are studies exploring the perceptions of HCPs involved in psoriasis management on the role of diet.
Theme 3: dietary approaches to manage psoriasis
The strongest evidence for dietary methods in the management of psoriasis symptoms is for low-calorie diets (LCDs) in subjects with obesity or overweight. The link between obesity and psoriasis is well recognised(Reference Takeshita, Grewal and Langan3,Reference Kumar, Han and Li157) . Several studies have demonstrated a relationship between increased BMI and increased psoriasis severity(Reference Debbaneh, Millsop and Bhatia46,Reference Ford, Siegel and Bagel64,Reference Ko, Chi and Yeh65) . Excess body weight is also associated with increased incidence of psoriasis(Reference Kumar, Han and Li157,Reference Wolk, Mallbris and Larsson158) and reduced response to psoriasis treatments(Reference Bardazzi, Balestri and Baldi159). The relationship is theorised to be a result of increased pro-inflammatory cytokine release due to increased adipose tissue. Weight reduction in subjects with obesity reduces adipose tissue and, consequently, inflammation(Reference Al-Mutairi and Nour66). Limited research has been conducted on ketogenic diets and psoriasis. Two open-label single-arm studies(Reference Castaldo, Rastrelli and Galdo73,Reference Castaldo, Pagano and Grimaldi74) and one case study(Reference Castaldo, Galdo and Rotondi Aufiero72) were identified in this review, all of which used very low-calorie ketogenic diets, between 300 and 500 kcal/d, and were conducted only in subjects with obesity or overweight. Although significant improvements in psoriasis severity were observed in these studies(Reference Castaldo, Galdo and Rotondi Aufiero72–Reference Castaldo, Pagano and Grimaldi74) it is currently unclear whether this was due to the very low calorie content or the specific ketogenic properties (protein-based diet with low carbohydrate intake) of the diets followed. Further RCTs are needed to fully assess the additional benefits of VLCKD versus other non-ketogenic diets with the same calorie intake. This is in line with conclusions of a narrative review on nutritional management of VLCKD in psoriasis(Reference Barrea, Caprio and Camajani160). Furthermore, no studies have been conducted on ketogenic diets in PLwP without overweight or obesity.
A gluten-free diet (GFD) in coeliac or gluten-sensitive populations of people with psoriasis also seems to have a beneficial effect on symptom severity. Overall, evidence suggests that patients with psoriasis with gluten-related antibodies may benefit from a GFD; however, larger trials are still lacking(Reference Kolchak, Tetarnikova and Theodoropoulou82). Additionally, it should be acknowledged that not all people living with psoriasis are also living with obesity or overweight, or have a sensitivity to gluten. This leaves a large proportion of patients without any evidence-based dietary advice. A recent Cochrane review highlighted the need for more studies on the effects of diets other than LCDs on psoriasis severity, dietary interventions in people without obesity, and in people with mild psoriasis(Reference Ko, Chi and Yeh65).
A greater adherence to a Mediterranean diet (MD) shows a positive trend in helping to manage psoriasis. This dietary pattern is anti-inflammatory and is associated with significant reductions in both IL-6 and IL-1β levels(Reference Koelman, Egea Rodrigues and Aleksandrova161), key pro-inflammatory cytokines in psoriasis(Reference Armstrong and Read31), which may explain the reason for these findings. Certain individual foods components of the MD (extra-virgin olive oil, fruit, vegetables and fish(Reference Barrea, Balato and Di Somma77)) have been associated with lower psoriasis severity (Table 5). The MD is a dietary pattern typically high in fruits and vegetables, legumes, whole grains, fish, nuts and monounsaturated fatty acids (MUFA) such as extra-virgin olive oil (EVOO), with a moderate intake of meat, dairy and alcohol(Reference Phan, Touvier and Kesse-Guyot75) shown to have anti-inflammatory effects(Reference Koelman, Egea Rodrigues and Aleksandrova161). Dietary patterns are complex combinations of foods and nutrients that act synergistically; they account for inter-relations of foods, represent the cumulative exposure to different diet components, and may have stronger effects on health than any single component(Reference Schulze and Hoffmann162,Reference Schulze, Martínez-González and Fung163) . Therefore, it is difficult to attribute effects to single dietary components(Reference Schulze and Hoffmann162–Reference Jacobs, Tapsell and Temple165). It is also important to note that these findings are based on cross-sectional studies, which cannot establish a cause-and-effect relationship between adherence to the Mediterranean diet or its individual components and psoriasis severity.
Regarding supplementation, high doses of omega-3 with an increased eicosapentaenoic (EPA):docosahexaenoic (DHA) ratio of 3:1 have shown potential for alleviating psoriasis symptoms(Reference Tveit, Brokstad and Berge96). A higher ratio of EPA:DHA is associated with further reductions in C-reactive protein (CRP) compared with lower ratios(Reference AbuMweis, Abu Omran and Al-Shami166). CRP is a pro-inflammatory biomarker shown to be elevated in psoriasis(Reference Dowlatshahi, Van Der Voort and Arends36), which may explain some of the beneficial effect seen in higher ratio supplementation. Probiotic supplementation also shows promising results for alleviating psoriasis symptoms; however, the studies identified for the review were small and heterogeneous. Additionally, limited evidence is available on long-term follow-ups, specific strain, amount, dosage or duration of consumption for probiotics. Overall, the evidence for dietary supplementation in managing psoriasis is inconclusive, and no evidence on optimal dose for any supplement is apparent. Larger controlled studies are needed to elucidate any dietary approach that is helpful, specifically in PLwP without obesity and coeliac- or gluten-sensitivity-free populations. This is in line with findings from several systematic reviews on diet and psoriasis(Reference Ford, Siegel and Bagel64,Reference Zeng, Yu and Wu122) .
Grey literature
Considering the limited evidence for specific dietary approaches for psoriasis management, it is understandable that there are no specific dietary guidelines for the management of psoriasis. This could also be why most of the grey literature provides very limited dietary information specific to psoriasis management and instead focuses on dietary advice for associated comorbidities. However, this review has highlighted that, despite the lack of guidelines, PLwP do modify their diet to try to manage their psoriasis symptoms, often without consulting a healthcare professional (HCP)(Reference Pham, Sokol and Halioua44,Reference Afifi, Danesh and Lee45,Reference Dhinsa, Wu and Gibbons62) . The diets trialled are often restrictive and could have detrimental effects on health and wellbeing. Therefore, it is important for HCPs and PLwP to understand the potential harms of following restrictive diets, especially considering the associated comorbidities. Stakeholders and those responsible for providing support for PLwP should provide more specific guidance on the potential harms of popular diets, in particular highlighting the risks of following restrictive diets, dietary aspects to consider if following elimination diets and the importance of consulting an HCP regarding dietary modifications. However, this review has highlighted that there is limited research on the use of popular diets and support that PLwP would like regarding diet. This is especially true for the United Kingdom where, even though there are an estimated 1·1 million PLwP, no studies were identified that have explored the perceived role of diet in the management of psoriasis, dietary information acquisition and dietary support wanted by PLwP in the United Kingdom, which highlights an important research gap. Further understanding dietary information acquisition and advice being suggested could enable stakeholders to provide more support to PLwP by increasing awareness of the potential health impacts of following popular diets.
Limitations of the review
Although the search strategy and inclusion criteria used in this scoping review followed PRSIMA-ScR systematic methods for scoping reviews, there are several limitations. Only literature written in English was included; this could have excluded relevant studies and guidelines conducted in other languages. The challenges of searching for grey literature could result in bias, and the reproducibility of grey literature searching is difficult. The strategy used to search grey literature in this review was based on the methodology of Godin et al.(Reference Godin, Stapleton and Kirkpatrick49), and pre-defined targeted search terms were used to try to reduce bias and improve reproducibility, alongside using incognito mode during google searches. However, website searching is dependent on the specific website and correct functioning. Additionally, this review did not contact experts in the field of psoriasis management to ask for additional reports or unpublished studies they were aware of. This may have resulted in some relevant studies and grey literature being omitted. Including studies that investigated the nutritional status of PLwP may have also made this review more comprehensive.
Despite these limitations, this review provides a comprehensive summary of the current available evidence and grey literature regarding the role of diet in the management of psoriasis. This review goes beyond previous review articles on diet and psoriasis, by including grey literature, studies on the dietary perceptions of people living with psoriasis and systematically searching for common dietary modifications used by PLwP on PubMed and SCOPUS as they emerged from the literature.
Conclusion
This scoping review provides a comprehensive overview of evidence of the role of diet in the management of psoriasis. It is the first study to review such a wide evidence base on the role of diet in managing psoriasis: exploring the dietary intakes of people living with psoriasis (PLwP), perceptions and use of diet in PLwP, the dietary approaches and the grey literature on psoriasis management.
Overall, there is limited evidence on all themes identified in this review, and the methodology and outcome measures of the studies identified vary widely. Dietary intakes of PLwP warrant homogeneous longitudinal studies to elucidate a causal relationship between diet and psoriasis status. In the absence of dietary guidelines, PLwP are self-prescribing dietary modifications suggested in the popular literature. These are often restrictive and could have detrimental effects on health and wellbeing. No studies have been conducted on sources of dietary information for people with psoriasis. There is also an absence of studies investigating the effects of popular dietary recommendations on psoriasis symptoms, patient experience and the perceptions of healthcare professionals (HCPs) on the role of diet in managing psoriasis. None has been conducted in the United Kingdom.
Some dietary methods have been shown to improve psoriasis severity, but only in specific populations: low-calorie diets in people with obesity or overweight, and gluten-free diets in those with coeliac disease or gluten sensitivity. The evidence suggests that diets with anti-inflammatory properties, particularly the Mediterranean diet (MD), may have beneficial effects on psoriasis through moderating specific inflammatory pathways in psoriasis(Reference Kanda, Hoashi and Saeki42,Reference Koelman, Egea Rodrigues and Aleksandrova161) . However, this is based on cross-sectional studies(Reference Phan, Touvier and Kesse-Guyot75–Reference Molina-Leyva, Cuenca-Barrales and Vega-Castillo78), and larger intervention studies are needed before any cause and effect can be ascertained regarding psoriasis or psoriasis severity. Other dietary approaches lack high-quality evidence to support their use. Larger controlled trials in PLwP without obesity or overweight, and coeliac or gluten-sensitive free populations, are necessary prior to any dietary recommendations being made for psoriasis management.
Several studies identified in this review highlighted individual foods that were associated with psoriasis severity. However, it is difficult to attribute effects to single dietary components, as dietary patterns are complex combinations of foods and nutrients which work synergistically(Reference Schulze and Hoffmann162,Reference Schulze, Martínez-González and Fung163) . Caution should be used when singling out specific foods as ‘good’ or ‘bad’ for certain conditions, without a robust evidence base, as this is an oversimplification which may lead to unhealthy eating behaviours(Reference Tapsell, Neale and Satija164,Reference Jacobs, Tapsell and Temple165) . Deleterious dietary recommendations should also consider the food that will be substituted as a result of cutting out specific foods(Reference Schulze, Martínez-González and Fung163–Reference Jacobs, Tapsell and Temple165). It is also important to note that these findings are based on cross-sectional studies, which cannot establish a cause-and-effect relationship between these individual foods and psoriasis severity.
In the absence of dietary guidelines or evidence-based dietary recommendations for psoriasis, nutritionists and healthcare professionals should provide dietary support to PLwP in other ways, beyond standard healthy eating guidance. This should include highlighting the potential negative impacts of popular restrictive diets and the importance of discussing dietary modifications with healthcare professionals with nutritional expertise.
Grey literature resources for HCPs and PLwP should provide more comprehensive advice on diet specific to psoriasis. This should include information on the risks of following restrictive or elimination diets and the importance of discussing dietary modifications with an HCP. However, to make this advice as beneficial as possible, further research is needed to understand dietary information acquisition of PLwP, commonly recommended diets in the popular literature and the perceptions of PLwP on the dietary support available. Understanding the role of diet in the management of psoriasis from HCPs’ point of view would also enable advice to be more comprehensive. With the significant comorbidities associated with psoriasis, understanding dietary behaviours, perceived skin response, information acquisition and patient experience will play a key role in holistic patient care for people with psoriasis.
Financial support
Poppy Hawkins received funding from the University of Hertfordshire, QR-funded PhD Studentship, titled ‘The role of diet in the management of psoriasis’ 2021–2024, supervised by Dr Rosalind Fallaize ([email protected]), Dr Kate Earl ([email protected]) and Dr Athanasios Tektonidis ([email protected]).
Competing interests
The authors declare no conflict of interest.
Authorship
Poppy Hawkins: developed search strategy, performed scoping review, screening and interpretation of included literature, and drafted the manuscript. Rosalind Fallaize, Kate Earl and Athanasios Tektonidis: contributed to study design and drafting of the manuscript. All authors included reviewed and approved the final manuscript.










