Epidemiological studies have suggested that a diet rich in cruciferous vegetables such as broccoli, cabbage and watercress is associated with reduced risk of multiple cancer types(Reference Higdon, Delage and Williams1). The potential anti-cancer effect of high cruciferous vegetable intake has been linked to the presence of glucosinolates within these foods. Following the release of the plant enzyme myrosinase by chewing or cutting, hydrolysis of glucosinolates gives rise to a number of products including isothiocyanates (ITC), indoles, thiocyanates and nitriles(Reference Zhang, Yao and Li2, Reference Zhang3). Over 100 glucosinolates which give rise to chemically distinct hydrolysis products have been identified. Following absorption, ITC are rapidly conjugated to glutathione (GSH) via the action of glutathione-S-transferase enzymes and metabolised predominantly via the mercapturic acid pathway(Reference Zhang, Yao and Li2, Reference Zhang3). Several studies have indicated that the potential cancer-protective effects of a high-cruciferous vegetable diet are modulated by sequence variations within glutathione-S-transferase enzymes, most notably GSTM1 and GSTT1(Reference Brennan, Hsu and Moullan4–Reference Spitz, Duphorne and Detry7). These variants may be linked to the enhanced protective effects of a high-cruciferous vegetable diet via potential effects on ITC metabolism(Reference Steck and Hebert8). The mechanisms of action of the potential anti-cancer activity of ITC are complex, and at present, are incompletely understood. However, alterations of carcinogen metabolism via inhibition of phase I enzymes and induction of phase II enzymes, as well as direct modulation of pathways controlling key cancer hallmarks, such as proliferation, resistance to apoptosis and angiogenesis, are thought to be involved(Reference Zhang, Yao and Li2, Reference Zhang3, Reference Clarke, Dashwood and Ho9, Reference Hecht10).
Following uptake into cells, ITC conjugate rapidly with the free thiol of glutathione(Reference Zhang11, Reference Xu and Thornalley12). Cycles of ITC conjugate efflux, regeneration of ITC by extracellular hydrolysis and reuptake of ITC lead to a very marked accumulation of ITC within cells and concomitant depletion of intracellular GSH. Decreased intracellular GSH leads to increased levels of reactive oxygen species, which may play an important role in suppressing growth and survival of transformed cells(Reference Trachootham, Zhou and Zhang13). In the absence of GSH-mediated defence, intracellular ITC are thought to conjugate with various cellular proteins, predominantly via reactive cysteine thiols(Reference Zhang, Yao and Li2, Reference Mi, Wang and Govind14). One arm of this response, driven by covalent modification of Keap1, leads to the stabilisation of Nrf2, a master regulator of antioxidant gene expression, and induction of protective antioxidant proteins(Reference Dinkova-Kostova, Holtzclaw and Cole15–Reference Xu, Yuan and Pan18). Similar events are thought to contribute to the direct anti-cancer effects of ITC on proliferation, survival and angiogenesis(Reference Zhang, Yao and Li2). Conjugation to α- and β-tubulins may contribute to the mitotic arrest that is frequently observed in ITC-treated cells, although it is likely that ITC exert their biological effects via modulation of multiple downstream effectors(Reference Mi, Wang and Govind14, Reference Mi, Xiao and Hood19–Reference Cross, Foss and Rady22). Metabolites of ITC are also likely to be involved, potentially via effects on chromatin remodelling and gene expression(Reference Myzak, Karplus and Chung23).
We have previously investigated the effects of phenethyl ITC (PEITC) on the response of cancer cells to hypoxia(Reference Wang, Cavell and Syed Alwi24). PEITC is derived from the glucosinolate gluconasturtiin, which is found at particularly high levels in watercress (Nasturtium officinale or Rorippa nasturtium-aquaticum). We demonstrated that PEITC interfered with the ability of hypoxia to activate hypoxia-inducible factor (HIF)(Reference Wang, Cavell and Syed Alwi24), a key transcription factor that mediates cellular responses to low pO2(Reference Rankin and Giaccia25, Reference Weidemann and Johnson26). Activation of HIF leads to increased transcription of a wide range of genes involved in angiogenesis (e.g. vascular endothelial growth factor), apoptosis (e.g. Bcl-2/adenovirus E1B 19-kDa protein-interacting protein 3) and metabolism (e.g. GLUT1). Inhibition of HIF may be important for the anti-cancer effects of PEITC, since PEITC and other ITC have been shown to posses anti-angiogenic activity in vitro and in vivo (Reference Xiao and Singh27–Reference Jackson, Singletary and Venema29), and angiogenesis plays a role in tumourigenesis, enabling growth of nascent tumours beyond a small size limit dictated by the perfusion distance of O2 away from blood vessels(Reference Carmeliet and Jain30).
The mechanism by which PEITC inhibits HIF activity appears to involve inhibition of HIF1α mRNA translation(Reference Wang, Cavell and Syed Alwi24). We(Reference Wang, Cavell and Syed Alwi24) and others(Reference Hu, Straub and Xiao31) have demonstrated that PEITC decreases the levels of phosphorylation of 4E binding protein 1 (4E-BP1), and this may play an important role in the modulation of HIF1α mRNA translation. The translation of HIF1α mRNA is highly dependent on the eIF4E translation factor, which is, in turn, regulated by 4E-BP1(Reference Yee Koh, Spivak-Kroizman and Powis32). Dephosphorylation of 4E-BP1 facilitates its interaction with 4E-BP1, leading to decreased translation of RNA such as HIF1α mRNA. Hu et al. (Reference Hu, Straub and Xiao31) have also demonstrated that PEITC-induced cell death is reversed by overexpression of eIF4E. Thus, 4E-BP1 may be a key target for PEITC-associated anti-cancer effects, leading to the loss of both growth- and angiogenesis-promoting pathways. PEITC decreases the phosphorylation of 4E-BP1 on multiple sites, including Thr70, Ser65 and Thr37/46(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31).
A small number of studies have investigated the in vivo effects of watercress consumption, generally, on pathways of carcinogen metabolism and oxidative stress. Pharmacokinetic analysis has demonstrated rapid absorption of PEITC into the blood with a mean maximal plasma concentration (C max) of 928 nm after the ingestion of 100 g watercress(Reference Ji and Morris33). Hecht et al. (Reference Hecht, Carmella and Murphy34, Reference Hecht, Chung and Richie35) have demonstrated that dietary intake of watercress increased urinary metabolites of the tobacco-specific lung carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and cotinine in smokers. Gill et al. (Reference Gill, Haldar and Boyd36) have demonstrated reduced levels of basal and hydrogen peroxide-induced DNA damage in peripheral blood lymphocytes following daily intake of 85 g of watercress for 8 weeks, associated with modest increases in erythrocyte superoxide dismutase and glutathione peroxidase 1 activity in specific cohort individuals carrying the GSTM1*1 allele(Reference Hofmann, Kuhnert and Schubert37). A very recent study(Reference Brown, Blaikie and Smith21) demonstrated reduced immunoreactivity of the proinflammatory cytokine MIF in plasma following the ingestion of a single 50 g portion of watercress.
Since decreased 4E-BP1 modulation has been functionally linked to in vitro anti-cancer effects of PEITC(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31), we investigated the effects of watercress extract on cancer cell growth inhibition and HIF activity. We also performed a small pilot study to determine whether dietary intake of watercress was sufficient to modulate 4E-BP1 phosphorylation in vivo.
Experimental methods
Cell culture
Human MCF7 breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA, USA), and were maintained in Dulbecco's Modified Eagle's medium (Lonza Group Limited, Basel, Switzerland) supplemented with 10 % (v/v) fetal calf serum (PAA Laboratories, Yeovil, UK), 1 mm l-glutamine and penicillin/streptomycin (Lonza group Limited). PEITC and desferrioxamine (DFO) were obtained from Sigma Chemicals (Poole, UK). HIF reporter assays were performed as described previously(Reference Wang, Cavell and Syed Alwi24). Peripheral blood mononuclear cells (PBMC) were isolated using Lymphoprep (Axis-Shield Diagnostics, Dundee, UK) according to the manufacturer's instructions. Growth inhibition assays were performed in duplicate as described previously(Reference Wang, Cavell and Syed Alwi24). LY294002 was obtained from Sigma Chemicals.
Watercress extracts
Watercress samples were snap frozen in liquid N2 before being ground to a fine powder using a pestle and mortar. Ground watercress (1 g) was decanted into a QIAshredder homogeniser (Qiagen, Crawley, UK), and incubated at room temperature for 1 h. Samples were centrifuged at 16 000 g for 6 min to collect the crude watercress extract.
Analysis of 4E binding protein 1 phosphorylation
The analysis of 4E-BP1 phosphorylation was done by single cell flow cytometry. PBMC were washed in ice-cold Roswell Park Memorial Institute 1640 medium (Invitrogen Limited, Paisley, UK) and resuspended in 1 ml of Roswell Park Memorial Institute 1640 medium. Cytofix buffer (BD Biosciences, Oxford, UK; 1 ml) was added, and the cells were incubated for 10 min at 37°C before storage at − 80°C, as per the manufacturer's instructions. On the day of the analysis, samples were thawed, and the cells were washed with flow cytometry buffer (BD Biosciences). Cells were resuspended in 1 ml of Phosflow Permeabilisation buffer III (BD Biosciences), and were incubated on ice for 30 min. Cells were then washed twice with Stain Buffer (BD Biosciences), collected by centrifugation and resuspended in 1 ml of Stain Buffer containing 100 μl of phycoerythrin-conjugated anti-4E-BP1 antibody (Thr37/46 phospho-specific) (BD Biosciences). Unstained cells were analysed as controls. Cells were incubated in the dark at room temperature for 30 min, washed with Stain Buffer and resuspended in 500 μl of the same buffer before flow cytometry. Flow cytometry was performed using the FL2 channel (585 nm) on a BD Biosciences FACSCalibur. An average of 785 000 total events was collected for each sample. For 4E-BP1 fluorescence, we measured the proportion of monocytes with fluorescence values greater than those of unstained controls.
The analysis of 4E-BP1 phosphorylation was performed on cells gated on the basis of their forward scatter (FSC)/side scatter (SSC) properties. We found considerable variation in the FSC/SSC profiles of isolated PBMC following fixation and immunostaining. The impact of fixatives used for intracellular staining with phospho-specific antibodies on scatter profiles has been described previously(Reference Krutzik, Irish and Nolan38). We separately gated on lymphocytes (abundant population with low FSC and SSC; Fig. 2(a)) and a population with increased FSC/SSC (see oval gate in Figs. 2(a) and 4) that we tentatively described as ‘monocytes’ (see ‘Discussion’). In some analyses, we detected a third population of cells (to the right of the lymphocytes) that we believe is due to variation in fixation. When present, these were excluded from the analysis. Some samples also contained relatively high amounts of dead cells with very low FSC (see Fig. 4). Again, these were excluded from the analysis.
In vivo study
The watercress feeding study was based on the previous work done by Ji et al. (Reference Ji and Morris33), who studied the plasma pharmacokinetics of PEITC following the ingestion of 100 g of watercress in four normal participants. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Southampton and South West Research Ethics Committee (Ref 08/H0504/86). Written informed consent was obtained from all the participants. Potential participants were identified using the Winchester and Andover Breast Unit database of patients, where clinical and diagnostic data are routinely compiled for audit purposes using criteria defined by the British Association for Surgical Oncology. Although the participants had previously been treated for breast cancer, they were considered free of clinically detectable disease for a minimum of 2 years and were otherwise healthy. None of the women was taking any pharmacological medications or herbal supplements.
Participants were requested to avoid the following foods for 3 d before the study day to exclude known sources of glucosinolates from the diet: cabbage, brussels sprouts, broccoli, calabrese, cauliflower, turnip, swede, rutabaga, kohlrabi, kale, chinese kale, sea kale, curly kale, collard, pak choi, radish, horseradish, mustard, mustard greens, mustard leaf, wasabi, salad rocket, cress, watercress, capers, papaya (pawpaw) and nasturtium (Indian cress). The work of McNaughton & Marks(Reference McNaughton and Marks39) informed the compilation of the list of food items to be excluded from the diet. Food and drink diaries were completed to confirm avoidance of glucosinolate-containing food items; dietary histories were also taken to describe participants' usual glucosinolate intakes, although these data are not presented here. The participants fasted from midnight on the day of the study. Approximately, 8 h later the volunteers were cannulated, and a baseline blood sample (5 ml) was obtained. The participants then ate 80 g of watercress, obtained from a local grower and packager (Vitacress Salads Limited, Andover, UK). Eighty grams is the weight of a portion of fruit and vegetables defined by the WHO, and is equivalent to one of the five portions of fruit and vegetables that describes one of UK's nutrition public health messages(40, 41). Blood samples were then obtained at approximately 7·5 min, 15 min, 30 min, 45 min, 1 h, 1·5 h, 2 h, 3 h, 4 h, 6 h and 8 h following the ingestion of the watercress meal. A 24 h blood sample was obtained by venipuncture. Blood samples were collected in heparinised glass tubes, and stored on ice before processing. Participants were free to take water throughout the study, and were allowed to eat and drink freely from 3 h after the ingestion of watercress meal, although glucosinolate-containing foods were eliminated until the end of the study.
We enrolled twelve women into the study, and analysed plasma PEITC concentrations and/or 4E-BP1 phosphorylation in samples from nine of these women (mean age 58 years, median age 56 years and range 48–82). For eight participants, we prepared both PBMC and plasma; samples were overlaid onto Lymphoprep and centrifuged at 800 g for 20 min. The plasma was recovered and stored at − 80°C. For one participant (participant 5 in Table 1), we prepared only plasma; samples were centrifuged at 486 g for 15 min, and the plasma was recovered and stored at − 80°C. Plasma PEITC concentrations were determined as described previously(Reference Ji and Morris33), with the exception that analyses were performed using 0·5 ml of plasma. PBMC were recovered from the interface, washed twice in ice-cold Roswell Park Memorial Institute 1640 medium and fixed using Cytofix (BD Biosciences) as described earlier. Cells were frozen before the analysis, and all the samples from an individual participant were analysed in parallel. The analysis of 4E-BP1 phosphorylation was done as described earlier. The analysis was performed on samples from five participants. However, two were excluded due to technical failures, and another two were excluded because of poor viability of cells.
Table 1 Analysis of plasma phenethyl isothiocyanates concentrations following the consumption of watercress
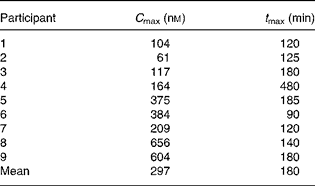
C max, maximal plasma concentration; t max, time to reach C max.
Results
Effect of watercress extract on cancer cell growth and hypoxia-inducible factor activity
PEITC has been shown to decrease cancer cell growth and to inhibit HIF activity, actions which have been linked to decreased 4E-BP1 phosphorylation(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31). Watercress is a particularly rich source of the PEITC precursor glucosinolate, gluconasturtiin, and we therefore investigated whether watercress extract could exert similar effects. Extracts were prepared by grinding watercress leaf, which releases myrosinase, the enzyme which catalyses the conversion of gluconasturtiin to PEITC. Watercress extract inhibited the growth of MCF7 breast cancer cells with an half maximal inhibitory concentration (IC50) of 24·6 (sd 12·3) μl/ml (Fig. 1(a)).
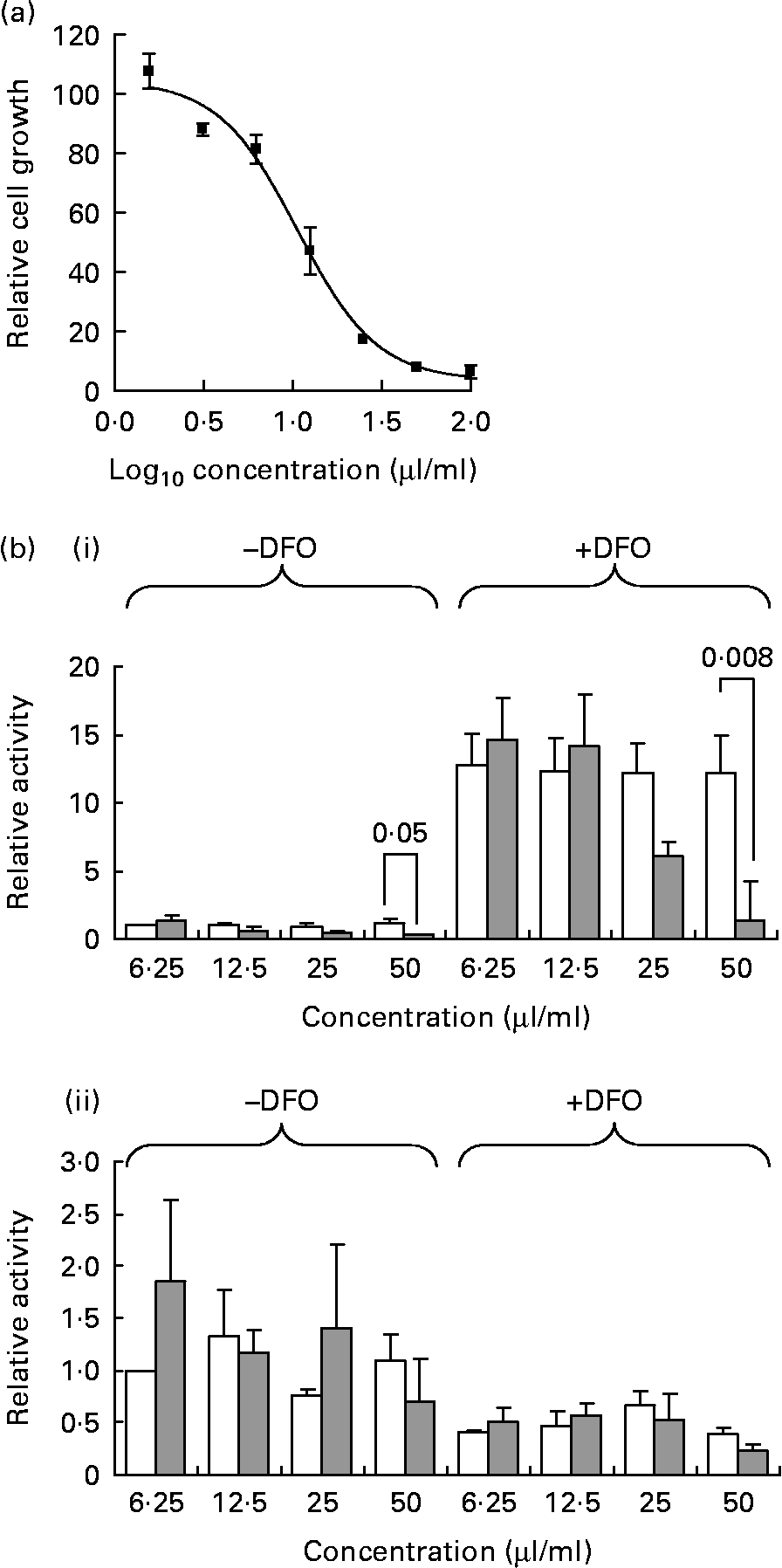
Fig. 1 Inhibition of cancer cell growth and hypoxia-inducible factor (HIF)-dependent transcription by watercress extract. (a) Representative growth inhibition experiment. MCF7 cells were incubated with the indicated concentrations of watercress extract. After 6 d, relative cell numbers were determined using the CellTiter 96® AQueous One Solution reagent. Results are derived from means of duplicate wells. (b) MCF7 cells were transfected with (i) pGL2-9TK-HRE or (ii) control pGL3-promoter reporter constructs, and were treated with the indicated concentrations of watercress extract (![]() ) or with equivalent amounts of water (□). HIF activity was induced by treating the cells with desferrioxamine (DFO) (100 μm), and luciferase activity was analysed after 24 h. Data indicate mean with their standard errors for luciferase activity relative to control cells (i.e. cell in the absence of DFO and with water equivalent to the lowest concentration of watercress extract tested was set to 1·0) derived from three independent experiments, each performed in triplicate. Statistically significant differences (Student's t test) between dimethyl sulphoxide- and watercress-treated cells are shown. All other differences were not statistically significant.
) or with equivalent amounts of water (□). HIF activity was induced by treating the cells with desferrioxamine (DFO) (100 μm), and luciferase activity was analysed after 24 h. Data indicate mean with their standard errors for luciferase activity relative to control cells (i.e. cell in the absence of DFO and with water equivalent to the lowest concentration of watercress extract tested was set to 1·0) derived from three independent experiments, each performed in triplicate. Statistically significant differences (Student's t test) between dimethyl sulphoxide- and watercress-treated cells are shown. All other differences were not statistically significant.
To analyse the transcriptional activity of HIF, MCF7 cells were transfected with a HIF reporter construct and treated with the hypoxia mimetic DFO (Fig. 1(b)). DFO displaces Fe from prolyl hydroxylases leading to the stabilisation of HIFα proteins. DFO caused a strong induction of the HIF reporter construct, and this was inhibited in a dose-dependent manner by watercress extract, although this was statistically significant only at the highest concentration. Watercress extract also decreased the basal activity of the HIF reporter in the absence of DFO. As a control, we also analysed the effects of DFO and watercress extract on the activity of the SV40-promoter-based reporter plasmid pGL3-promoter. The activity of the control promoter was reduced following treatment with DFO, and was not affected by watercress extract under any condition. Therefore, similar to PEITC(Reference Wang, Cavell and Syed Alwi24), watercress extract inhibits HIF activity in MCF7 breast cancer cells.
Phenethyl isothiocyanates decreases 4E binding protein 1 phosphorylation in peripheral blood mononuclear cells
Since PEITC decreases 4E-BP1 phosphorylation and this has been linked to HIF inhibition(Reference Wang, Cavell and Syed Alwi24) and growth inhibition(Reference Hu, Straub and Xiao31), we selected 4E-BP1 phosphorylation as a potential biomarker to monitor in vivo exposure to PEITC. To explore the potential utility of 4E-BP1 phosphorylation as a biomarker, we first examined the levels of 4E-BP1 phosphorylation in PBMC and its modulation by PEITC.
PBMC were obtained from healthy donors and analysed by single cell flow cytometry. For these studies, we used an antibody that selectively reacted with 4E-BP1 phosphorylated on Thr37/46, since it gave stronger staining compared with a 4E-BP1 Thr70 phospho-specific antibody (data not shown). 4E-BP1 phosphorylation varied among different cell populations in peripheral blood based on FSC/SCC properties (see ‘Experimental methods’ for gating strategy). We readily detected phosphorylated 4E-BP1 in a population of cells that we tentatively described as ‘monocytes’, whereas the levels of phosphorylation were much lower in lymphocytes (Fig. 2). To confirm that immunostaining was specific for phosphorylated 4E-BP1, cells were treated with LY294002. LY294002 decreases 4E-BP1 phosphorylation via inhibition of Phosphoinositide 3 kinase, a key upstream positive regulator of 4E-BP1 phosphorylation via the phosphoinositide 3 kinase-AKT-mammalian target of rapamycin pathway(Reference Proud42). Treatment of cells with LY294002 reduced 4E-BP1 phosphorylation by 63 ± 13 % (mean ± range of two experiments; Fig. 2). Consistent with previous data(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31), there was also a significant reduction in 4E-BP1 phosphorylation following in vitro treatment with PEITC (Fig. 2). Thus, 4E-BP1 phosphorylation appears to be a suitable biomarker to monitor in vivo responses to PEITC in PBMC.

Fig. 2 Analysis of 4E binding protein 1 (4E-BP1) phosphorylation in peripheral blood mononuclear cells (PBMC). PBMC were isolated from healthy individuals and analysed by flow cytometry using a Thr37/46 4E-BP1 phosphorylation-specific antibody. (a) Forward scatter (FSC)/side scatter (SSC) plot showing gating of lymphocytes (lower left) and a population of cells with increased FSC and SSC, which we tentatively classed as monocytes (oval gate). (b–e) Fluorescence intensity of ‘monocytes’ (b)–(d) and lymphocytes (e). In (b) and (e), black line indicates unstained control, and the grey line indicates antibody-stained cells. In (c), the grey line indicates antibody-stained cells, and the black line indicates cells treated with LY294002 (100 μm) for 2 h before staining with the 4E-BP1 antibody. In (d), the grey line indicates antibody-stained cells, and the black line indicates antibody-stained cells treated with phenethyl isothiocyanates (20 μm) for 2 h before staining with the 4E-BP1 antibody. Data are representative of at least two independent experiments. FL2-H, fluorescence pulse height.
Watercress consumption down-regulates 4E binding protein 1 phosphorylation in vivo
We performed a small feeding study to determine whether the ingestion of watercress was sufficient to modulate 4E-BP1 phosphorylation in vivo. We first analysed plasma concentrations of PEITC (Table 1). Similar to a previous study(Reference Ji and Morris33), there was a rapid increase in plasma PEITC, which, on average, reached a maximal concentration at 3 h (Fig. 3). The mean C max was 297 nm, although there was a wide interindividual variation (range 61–656 nm). Background PEITC concentrations before the ingestion of watercress were very low (generally < 1 nm).

Fig. 3 Analysis of plasma phenethyl isothiocyanates (PEITC) concentrations following the consumption of watercress. Plasma concentration of PEITC was determined at various time points following the consumption of 80 g watercress. Data obtained from four representative subjects are shown.
The analysis of 4E-BP1 phosphorylation was done in four participants (Fig. 4). All the participants showed a marked reduction in 4E-BP1 phosphorylation at 6 and 8 h following the ingestion of watercress compared with pre-watercress meal values. Twenty-four hour data were available for three participants; in one participant, 4E-BP1 phosphorylation was maintained at a low level until this time point, whereas recovery of 4E-BP1 phosphorylation was observed in the other two participants. The decreases in 4E-BP1 phosphorylation at 6 and 8 h were highly statistically significant (P = 0·001 and 0·002, respectively; Student's t test compared to pre-watercress samples). There was a considerable variation in the levels of 4E-BP1 phosphorylation before 6 h within individual subjects. It is possible that this is due to technical variation in transport and sample processing, and the average level of 4E-BP1 phosphorylation was not significantly altered at these time points. Thus, the analysis of 4E-BP1 Thr37/46 phosphorylation suggests a significant decrease at 6 and 8 h after the ingestion of watercress.

Fig. 4 Analysis of 4E binding protein 1 (4E-BP1) phosphorylation following the consumption of watercress. 4E-BP1 phosphorylation was analysed by flow cytometry in peripheral blood-derived monocytes at various time points following the consumption of 80 g watercress. (a) Representative data obtained from two participants showing forward scatter (FSC)/side scatter (SSC) plots with ‘monocyte’ gate (a) and fluorescence intensity (b) of unstained control cells and stained cells before (T 0) and 8 h (T 480) after the consumption of watercress. (b) Graphs showing (i and ii) the levels of 4E-BP1 phosphorylation in two representative participants (1 and 4) and (iii) the mean with their levels of 4E-BP1 phosphorylation in all four subjects following the consumption of watercress. In (iii), the level of 4E-BP1 phosphorylation at T 0 was set to 1·0 to allow comparison between individuals. FL2-H, fluorescence pulse height. Mean values were significantly different compared to T 0 are indicated (Student's t test): * P = 0·001, ** P = 0·002.
Discussion
Numerous epidemiological studies have suggested that high dietary intake of cruciferous vegetables is associated with reduced cancer risk, and in vitro studies have indicated that reduced 4E-BP1 phosphorylation may be an important mechanism contributing to anti-cancer effects of PEITC(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31). In this work, we have performed a pilot study to determine whether the ingestion of watercress, a rich dietary source of PEITC, is sufficient to modulate 4E-BP1 phosphorylation levels in vivo. The mechanisms by which PEITC decreases 4E-BP1 phosphorylation are not known. Modulation of upstream regulators, including mammalian target of rapamycin and phosphatase and tensin homologue (PTEN), both of which contain redox-sensitive cysteine residues, may play a role(Reference Dames, Mulet and Rathgeb-Szabo43, Reference Salmeen and Barford44).
We selected 4E-BP1 phosphorylation as a potential molecular biomarker for several reasons. First, modulation of 4E-BP1 phosphorylation by PEITC has been demonstrated in multiple cell types(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31). Secondly, modulation of 4E-BP1 phosphorylation has been mechanistically linked to both growth-inhibitory and anti-angiogenic effects of PEITC(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31). Thirdly, it was possible to measure 4E-BP1 phosphorylation on a single cell basis using a quantitative flow cytometry assay. Finally, we were able to measure 4E-BP1 phosphorylation in PBMC, a relatively accessible tissue source suitable for repeat sampling. However, it is possible that other watercress-derived ITC, PEITC metabolites or unrelated bioactives may also modulate 4E-BP1 phosphorylation. We therefore cannot exclude the possibility that other compounds are involved in the in vitro and in vivo effects of watercress. Although we consider it unlikely, all the participants ate the watercress at the same time of day, and we therefore also cannot exclude the possibility that decreased 4E-BP1 phosphorylation is unrelated to watercress consumption, and may reflect diurnal modulation of activity.
Although flow cytometric analysis of 4E-BP1 phosphorylation appeared to be a promising approach for the evaluation of in vivo responses to ITC, this pilot study identified a number of key technical challenges that should be addressed in future studies. First, intracellular antibody staining requires cell fixation, and this resulted in a large variation in the scatter properties of the cells. The difficulties caused by variation in scatter properties, specifically in the context of staining with phospho-specific antibodies, have been discussed previously(Reference Krutzik, Irish and Nolan38). Our gating strategy excluded lymphocytes and encompassed a population that we tentatively identified as monocytes. However, in future studies, it would be important to combine intracellular staining with specific surface markers to unambiguously identify specific cell subpopulations, and to improve the flow cytometric analyses. Secondly, we observed significant levels of cell death in some samples. The clinical and laboratory sites were geographically separate, and transportation between these sites may have resulted in increased cell death. For technically demanding analyses, such as phospho-specific flow cytometry, we recommend that samples be processed with the minimum of delay.
Flow cytometric analysis demonstrated a statistically significant reduction in 4E-BP1 Thr37/46 phosphorylation at 6 and 8 h following the consumption of watercress in 4/4 participants studied. Caution is required in interpreting our data, since there was significant variability in the levels of 4E-BP1 phosphorylation within individual samples before this time point. However, these differences disappeared when results from the four individuals were combined, whereas the consistent down-regulation of 4E-BP1 phosphorylation at 6 and 8 h was highly statistically significant. This suggests that dietary intake of a single 80 g portion of watercress is sufficient to modulate this potential anti-cancer pathway. These results are consistent with those reported previously from studies investigating the effects of watercress consumption on carcinogen metabolism/oxidative stress, although it should be noted that these studies involved repeated ingestion of watercress over a period of 3 d–8 weeks(Reference Hecht, Carmella and Murphy34–Reference Hofmann, Kuhnert and Schubert37). Recently, Brown et al. (Reference Cross, Rady and Foss20, Reference Brown, Blaikie and Smith21) have shown that the ingestion of a single 50 g portion of watercress is associated with reduced plasma immunoreactivity of the proinflammatory cytokine macrophage migration inhibitory factor, which is a direct target for covalent modification by PEITC.
As part of the present study, we also analysed the plasma concentrations of PEITC. These results are generally in line with those reported previously by Ji et al. (Reference Ji and Morris33), who analysed the plasma concentrations of PEITC in four participants following the consumption of 100 g of watercress. Both the studies showed a rapid increase in plasma PEITC concentrations, peaking at 2–3 h, followed by a decline to near background levels at 24 h. However, the C max values in this study were generally lower (297 v. 929 nm on average), and showed much more interindividual variation in the present study. Multiple variables are likely to contribute to these differences.
The lower average C max at least partly reflects the smaller portion size selected for the present study (80 v. 100 g), and although the difference in intake is small, it is not clear whether PEITC accumulation in the plasma is proportionate to dose. It is also possible that differences in glucosinolate content of the crop will have contributed to the variation in mean C max concentrations between the studies, since it is known that differences in sunlight exposure, temperature and added fertilisers can all influence gluconasturtiin production in watercress(Reference Engelen-Eigles, Holden and Cohen45, Reference Kopsell, Barickman and Sams46). The average age of the participants in the present study was relatively high, and age-related changes in absorption may have also impacted on the overall C max and contributed to interindividual variation. Finally, glutathione-S-transferase variants have previously been demonstrated to modulate ITC metabolism and potential chemopreventive effects, and it is also possible that genetic variation may have contributed to some of the differences. Such age-, genetic- and crop-related differences are all ‘real world’ variables that are likely to interact to determine exposure, and thus complicate the analysis of biological effects of plant-derived agents.
A key question is why dietary intake of watercress may be sufficient to modulate 4E-BP1 phosphorylation, although plasma concentrations may not achieve the concentrations that are required to effect this pathway in vitro (typically 1–5 μM(Reference Wang, Cavell and Syed Alwi24, Reference Hu, Straub and Xiao31)). One possible explanation lies in the interaction of PEITC with GSH. Efflux of ITC conjugates, extracellular hydrolysis and reuptake of ITC lead to marked accumulation of intracellular ITC(Reference Zhang11, Reference Xu and Thornalley12). Thus, the intracellular concentration of PEITC in monocytes may be much higher than what is predicted from the plasma concentrations. Similar interactions may account for the time lag between peak plasma concentration and inhibition of 4E-BP1 phosphorylation. It will be important to investigate further how differences in glutathione levels and metabolism alter cellular accumulation of ITC and their metabolites, since GSH levels are altered in many cancer cells(Reference Estrela, Ortega and Obrador47). As discussed earlier, it is also important to bear in mind the alternate possibilities. For example, in vivo modulation of 4E-BP1 phosphorylation may be unrelated to or only partially dependent on PEITC, and may be due to other bioactives derived from watercress. For example, Brown et al. (Reference Brown, Blaikie and Smith21) recently reported that the mean plasma concentration of total ITC and dithiocarbamate metabolites reached approximately 1·5 μm at 2 h following the ingestion of a 50 g portion of watercress. At present, it is not known whether dithiocarbamate metabolites may contribute to the modulation of 4E-BP1 phosphorylation, either directly or following conversion to ITC.
In summary, we have performed a small pilot study to investigate the feasibility of measuring 4E-BP1 phosphorylation as a biomarker to monitor in vivo effects of PEITC. We tentatively conclude that flow cytometry may be a suitable approach to measure changes in 4E-BP1 phosphorylation following the ingestion of watercress. However, further studies are required with larger sample sizes to test this more rigorously.
Acknowledgements
We thank the medical assistants and ward staff at the Royal Hampshire County Hospital for their assistance and expertise. We particularly thank the women who volunteered for the in vivo study. This work was supported by the Watercress Alliance and BBSRC. The authors declare no conflicts of interest. G. P. and B. M. P. were the principal investigators of the study, and were responsible for the study design and supervision. M. E. M. was responsible for the design and supervision of LC/MS/MS analysis. S. S. A. A. was responsible for the analysis of 4E-BP1 phosphorylation. B. E. C. was responsible for the analysis of HIF activity and cell growth inhibition assays. U. T. was responsible for LC/MS/MS analysis. G. P. wrote the manuscript, which was reviewed by all the authors.







