Nutritional overload of fat and refined carbohydrates contributes to the development of obesity and insulin resistance. Other medical consequences can be associated with visceral obesity, such as CVD( Reference MohanKumar, King and Shin 1 ), type 2 diabetes( Reference Nikolajczyk, Jagannathan-Bogdan and Shin 2 ), cancer( Reference Roberts, Dive and Renehan 3 ) and cognitive deficiencies( Reference Kerwin, Gaussoin and Chlebowski 4 , Reference Winocur and Greenwood 5 ), making its prevalence in Western countries a serious public health concern. The association of these diseases with obesity is well documented, but the biological connection between them is still under debate. The major experimental studies focusing on the association between diets and these diseases have been carried out using high-fat diets. However, during the last decade, public health campaigns have led to a decline in the intake of fat and there has been an increase in the intake of fructose. This increase in the intake of refined sugars high in fructose appears to be an important factor for the development of obesity and the metabolic syndrome in Western countries( Reference Ferder, Ferder and Inserra 6 , Reference Tappy 7 ). The first objective of the present study was to investigate the impact of a high-fat/high-fructose (HF/HFr) diet on several biological processes that are suspected to play a role in the development of visceral obesity and related diseases, including the innate immune system and the hypothalamic–pituitary–adrenocortical (HPA) axis, in rats.
Some cytokines, TNF-α, IL-1β and IL-6, partly secreted by visceral fat, could be involved in the development of insulin resistance( Reference Bastard, Maachi and Lagathu 8 , Reference Clement and Langin 9 ) and of cognitive deficiencies associated with obesity( Reference Capuron and Miller 10 ). Therefore, we first focused on the components of the innate immune system in the plasma and central structures, hypothalamus and hippocampus, described to respond to immune challenges( Reference Pohl, Woodside and Luheshi 11 , Reference Csolle and Sperlagh 12 ) and to be involved in neurogenesis( Reference Jeon, Jeong and Shin 13 , Reference McNay, Briancon and Kokoeva 14 ). We assessed whether a HF/HFr diet modified the system in basal conditions and its response to a lipopolysaccharide (LPS) challenge as classically described( Reference Pohl, Woodside and Luheshi 11 ).
A large individual variability in HPA axis function( Reference Oitzl, Champagne and van der Veen 15 ), with a high genetic component( Reference Mormede, Foury and Barat 16 ) that influences feeding behaviour, metabolism and energy expenditure( Reference Chrousos and Kino 17 ), has been described. Visceral obesity is usually associated with an overexpression of 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1 in adipose tissue( Reference Draper and Stewart 18 ), an alteration of the HPA axis negative feedback or a blunted circadian rhythm of corticosterone( Reference Rosmond, Dallman and Bjorntorp 19 , Reference Cano, Jimenez-Ortega and Larrad 20 ). Glucocorticoids are known to induce insulin resistance directly by altering insulin signal transduction and indirectly by promoting visceral fat deposition and lean mass loss. Through both the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), glucocorticoid hormones stimulate preadipocyte differentiation, contribute to fat accumulation and drive adipose tissue distribution( Reference Marissal-Arvy, Langlois and Tridon 21 , Reference Devenport, Knehans and Sundstrom 22 ). The interplay between the HPA axis and metabolic disorders may also be mediated by inflammatory processes, since glucocorticoids modulate the secretion and action and cytokines( Reference Morton 23 ) and cytokines down-regulate the function of GR( Reference Pace, Hu and Miller 24 ). Glucocorticoids could also be involved in cognitive deficiencies associated with obesity by their central action, notably in the hypothalamus and hippocampus, as largely described in the literature, for instance, after chronic stress (Str)( Reference Mormede, Foury and Barat 16 ). Therefore, the second objective of the present study was to analyse the effects of a HF/HFr diet on the actors of HPA axis function in basal conditions and in response to acute restraint Str: corticosterone in the plasma and expression of MR, GR, 11β-HSD1 and 11β-HSD2 in two central structures, hypothalamus and hippocampus, that are both components and targets of the HPA axis.
Some nutritional factors, such as polyphenols, may improve insulin resistance through their insulin-potentiating, antioxidant, anti-inflammatory and related properties. Since the beneficial effects of cinnamon (CN) on inflammation( Reference Qin, Dawson and Polansky 25 ) and insulin resistance in humans( Reference Qin, Polansky and Anderson 26 ) and on cognitive functions in an Alzheimer's disease animal model( Reference Frydman-Marom, Levin and Farfara 27 ) have been described, the third objective of the present study was to test whether dietary CN supplementation could counteract the effects of a HF/HFr diet and/or Str on the inflammation status and HPA axis.
To this end, we measured the effects of a standard v. a HF/HFr diet, supplemented or not supplemented with CN, coupled or not with restraint Str on plasma cytokines and corticosterone and on hypothalamic and hippocampal expression of immune factors, actors of HPA axis function and actors of cerebral plasticity.
Materials and methods
Animals
The present study was approved by the institutional ethics committee for animal care of the Centre de Recherche du Service de Santé des Armées. All the experiments were carried out according to the French (Directive 87/148, Ministère de l'Agriculture et de la Pêche) and international (Directive 86/609, 24 November 1986, European Community) legislation. The procedures followed in the study were approved by Région Aquitaine Veterinary Services (Direction Départementale de la Protection des Animaux, approval ID: A33-063-920). The local ethics committee specifically approved the study. Every effort was made to minimise suffering and the number of animals used. Male Wistar rats (Charles River), 5 weeks old (body weight 194·2 (se 1·7) g), were used for the study. The rats were individually housed in a temperature-controlled room (22°C) under a 12 h light–12 h dark cycle (lights on at 08.00 hours). At the end of the experiments, in the morning (09.00–12.00 hours), the rats were killed under deep anaesthesia (3–4 % fluothane in 100 % O2). Blood samples were collected by cardiac puncture into EDTA-coated tubes (Sarstedt) to avoid haemolysis. Blood samples were centrifuged at 4000 g for 10 min at 4°C. Cerebral tissue samples were dissected on an ice bed, and all the samples were stored at − 80°C until use.
Diets
Diets used in the study were purchased from SAFE. The rats were allowed to acclimatise and fed the control diet for 3 weeks. The control diet (C; 14 633 kJ/kg (3495 kcal/kg)) contained 5 % cellulose, 20 % casein, 25 % maize starch, 25 % potato starch, 16 % maltodextrin, 4 % soyabean oil, 3·5 % American Institute of Nutrition mineral mix, 1 % American Institute of Nutrition vitamin mix, 0·3 % dl-methionine and 0·2 % choline bitartrate. The HF/HFr diet was similar to the C diet, except that maize starch, potato starch and maltodextrin were replaced with 46 % fructose and 20 % lard (19 293 kJ/kg (4608 kcal/kg)). Insulin resistance induced by the HF/HFr diet has been confirmed in a previous study( Reference Couturier, Qin and Batandier 28 ).
Experiment 1
A total of thirty-two male Wistar rats (5 weeks old) were randomly divided into two groups and fed ad libitum for 12 weeks the C diet or the HF/HFr diet. At the end of the dietary period, half the rats in each group were administered a single intraperitoneal injection of LPS, 100 μg/kg (Sigma-Aldrich, Escherichia coli serotype 0127:B8). The control rats were treated with saline, and all the injections were administered in the morning (09.00–12.00 hours). The rats were killed 8 h after injection as described by Pohl et al. ( Reference Pohl, Woodside and Luheshi 11 ), as this time point corresponds to the peak in the response of cytokines in obese rats after the administration of this dose of LPS.
Experiment 2
A total of 120 male Wistar rats (5 weeks old) were randomly divided into four groups of thirty and fed ad libitum for 12 weeks one of the following four diets: C diet, the HF/HFr diet, or the respective diets containing 20 g of CN per kg of diet (C+CN or HF/HFr+CN). The amount of CN used was based on our previous study showing a definite effect of 20 g of CN per kg of diet in rats( Reference Preuss, Echard and Polansky 29 ). The composition of CN has been described by Couturier et al. ( Reference Couturier, Qin and Batandier 28 ). The CN powder (Cinnamomum burmannii) was obtained from McCormick Spice. A water extract of the CN powder contained more than 5 % type A polyphenols with a tetramer with a molecular weight of 1152 and two trimers with a molecular weight of 864( Reference Lu, Zhang and Nam 30 , Reference Anderson, Broadhurst and Polansky 31 ). From each group, ten rats were exposed to restraint Str in a contention tube for 30 min just before being killed. The rats were dissected to evaluate their body composition. The livers and two depots of adipose tissue were carefully removed and weighed: mesenteric (along the mesentery, starting from the lesser curvature of the stomach and ending at the sigmoid colon) and inguinal (subcutaneous fat between the lower part of the rib cage and the thighs).
Measurement of plasma concentrations of cytokines
The plasma concentrations of IL-1β, IL-6, TNF-α, IL-2 and IL-10 were measured using a rat cytokine LINCOplex 5-plex kit (Linco research, Inc.) on a Bioplex-200 apparatus (Bio-Rad) following the manufacturer's instructions as described previously( Reference Moreau, Andre and O'Connor 32 ). The intra-assay CV were 4, 4, 5, 4 and 4% and inter-assay CV were 2, 3, 7, 3 and 6%, respectively, for IL-1β, IL-6, TNF-α, IL-2 and IL-10.
Measurement of plasma concentrations of corticosterone
The concentrations of plasma corticosterone were measured with an in-house RIA( Reference Richard, Helbling and Tridon 33 ) using a highly specific antibody provided by H. Vaudry (University of Rouen, France). Briefly, after the extraction of steroids from plasma samples with absolute ethanol, we measured the concentrations of total corticosterone by competition between cold corticosterone and 3H-corticosterone using our specific antibody anti-corticosterone.
Measurement of the central expression of genes
The expression levels of mRNA were measured by real-time RT PCR using SYBR® Green I (Life Technologies) as the fluorescent marker(
Reference Holland, Abramson and Watson
34
). Total RNA was extracted from the hypothalamus and hippocampus using a TRIzol® extraction kit (Invitrogen) according to the manufacturer's instructions. Samples in a final volume of 30 μl were treated with DNAse (Turbo DNA-free Kit, Ambion, Invitrogen). RNA were quantified on the NanoDrop 1000 (Thermo Scientific), and their quality was estimated on Agilent RNA 6000 nano chips using the Agilent 2100 Bioanalyzer (Agilent Technologies). RT were carried out using aliquots of total RNA (2 μg) using a Superscript III Invitrogen kit (Invitrogen, Life Technologies). The RT reactions were carried out in 20 μl of reaction buffer at 50°C for 55 min and terminated by heating at 85°C for 5 min followed by cooling at 4°C. The primers were designed using the Primer Express® program (Applied Biosystems). We measured the hypothalamic and hippocampal expression of immune factors (IL-1β, IL-1 receptor antagonist, IL-1 receptor (IL-1R)1, IL-6, IL-6R, glycoprotein 130 (Gp130), TNF-α, TNF receptor (TNF-R)1, TNF-R2, NF-κB, inhibitor of κB, cyclo-oxygenase 2 (COX2), integrin-αM (ITGAM) (or Cdllb), transforming growth factor (TGF)-β1, TGF-β2, TGF-β receptor 1 and TGF-β receptor 2), actors of HPA axis function (corticotrophin-releasing hormone, MR, GR, 11β-HSD1 and 11β-HSD2) and actors of cerebral plasticity (neurogenesis with brain-dervied neurotrophic factor (BDNF) and its receptor tyrosine kinase receptor (TrK) B and nerve growth factor (NGF) and its receptor TrkA and synaptogenesis with synaptophysin (SYP), synaptotagmin, neurogranin, growth-associated protein 43 (GAP43) and discs, large (Drosophila) homolog-associated protein 2 (DlGap2). The sequences of primers used and the description of the abbreviations are given in Table S1 (available online). We tested the primers by quantitative PCR (qPCR) with a standard curve of concentrations obtained with a pool of our complementary DNA to evaluate their efficacy (which should be between 95 and 105 %) and specificity. We also tested them on agarose gel for their ability to produce a single product of correct size. Each sample of complementary DNA was randomly measured in duplicates of 4 ng for the target and housekeeping genes. Real-time PCR were carried out in ninety-six-well plates with the Opticon 2 BioRad Software (MJ Research) using the DynamoTM HS SYBR® Green qPCR Kit Finnzymes (Ozyme). The reactions were carried out at 95°C for 15 min, followed by forty cycles of 95°C for 20 s and 61°C for 35 s. The fluorescence of the SYBR Green dye was determined as a function of the PCR cycle number, giving the threshold cycle (C
t) number at which the amplification reached a significant threshold. The C
t values were used to estimate the amount of PCR products according to the C
t of the housekeeping gene. ΔC
t was calculated by subtracting the C
t of the control gene (enolase 2) from the C
t of the target gene, and the expression level of the target gene was calculated by considering the C
t of enolase 2 to be about 24·06 cycles. The relative expression level of the target gene was expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group. The ΔC
t values were compared using ANOVA (three-way: diet, CN and Str) and were considered significant when P< 0·05. The expression levels are reported as
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group. The ΔC
t values were compared using ANOVA (three-way: diet, CN and Str) and were considered significant when P< 0·05. The expression levels are reported as
![]() $$2^{ - \Delta \Delta C _{t}} $$
(fold change, means with their standard errors).
$$2^{ - \Delta \Delta C _{t}} $$
(fold change, means with their standard errors).
Statistical analysis
Results are expressed as means with their standard errors. For experiment 1, data were analysed by a two-way ANOVA (diets and LPS treatment). For experiment 2, data were first analysed by a three-way ANOVA with diets (C v. HF/HFr), with or without CN, and with Str v. no Str. Subsequently, when the results of the three-way ANOVA was significant, given the number of experimental groups, two-way ANOVA were carried out separately for the C and HF/HFr diets. Newman–Keuls post hoc analyses were conducted when the results of the ANOVA were significant (P< 0·05). They focused on (1) the diet effect upon the inflammation status and the HPA axis, (2) the Str effect and (3) the CN effect.
Results
Inflammation status
Experiment 1
The plasma concentrations of IL-1β increased in response to the LPS treatment (Fig. 1(A)), but to a lesser extent under the HF/HFr diet than under the C diet (significant diet × treatment interaction P< 0·05). The same pattern of increase in response to the LPS treatment was observed for the plasma concentrations of IL-6, TNF-α and IL-10 according to the diet (Table S2, available online).

Fig. 1 Effect of lipopolysaccharide (LPS) under the control (C) diet or the high-fat/high-fructose (HF/HFr) diet on the plasma concentrations of (A) IL-1β and (B) hypothalamic and (C) hippocampal expression of IL-1β and integrin-αM (ITGAM). a,b,cValues with unlike letters were significantly different (P< 0·05).
Fig. 1(B) and (C) shows the results for the expression of IL-1β and ITGAM in the hypothalamus and hippocampus, respectively, in response to the HF/HFr diet and/or LPS treatment. The LPS treatment induced a strong increase in the expression of IL-1β and ITGAM in the hypothalamus (treatment effect P< 0·001). This effect was less remarkable for the expression of ITGAM in the hippocampus. In both the structures, the LPS treatment induced a greater increase in the expression of IL-1β under the HF/HFr diet than under the C diet (diet × treatment interaction P< 0·05). The same results were obtained for the expression of IL-6 and TNF-α (Table S2, available online).
Experiment 2
Among the cytokines, the plasma concentrations of only IL-6 were increased by the HF/HFr diet (P< 0·05), and this effect was reversed by CN supplementation (Table 1).
Table 1 Plasma concentrations of cytokines (pg/ml) according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition in experiment 2 (Mean values with their standard errors)
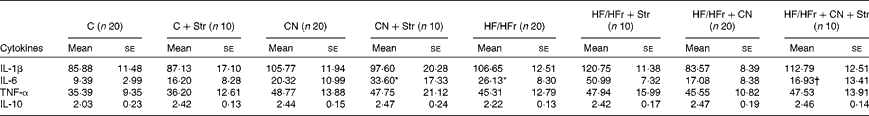
* Diet effect, with reference to its control (P< 0·05).
† Cinnamon effect, with reference to its control (P< 0·05).
The body composition of the rats is summarised in Table S3 (available online). No significant effect was revealed by ANOVA for body weight or fat weight. A strong diet effect was revealed by ANOVA for liver weight. The HF/HFr diet globally increased the liver weight of the rats (P< 0·001).
Fig. 2 and Tables S4 and S5 (available online) show the main results for the expression of cytokines in the hypothalamus and hippocampus. Results are reported only when ANOVA revealed at least a diet × CN × Str interaction (P< 0·05). Under the C diet, restraint Str decreased the expression of IL-1β, IL-1R1, IL-6 and Gp130 in the hypothalamus and hippocampus. Under the HF/HFr diet, restraint Str either exerted no effect or induced an increase in the expression of IL-1R1, IL-6 and Gp130 in both the structures.

Fig. 2 (A) Hypothalamic and (B) hippocampal expression of pro-inflammatory cytokine IL-1β, its antagonist IL-1 receptor antagonist (IL-1ra) and IL-1 receptor 1 (IL-1R1) according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition. The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, ** P< 0·01, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, †† P< 0·01, ††† P< 0·001. Stress effect, with reference to its control: ‡ P< 0·05, ‡‡‡ P< 0·001. IL-1β: (a) ΔC
t about − 8·30, (b) ΔC
t about − 9·82; IL-1ra: (a) ΔC
t about − 9·17, (b) ΔC
t about − 10·28; IL-1R1: (a) ΔC
t about − 3·51, (b) ΔC
t about − 6·33.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, ** P< 0·01, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, †† P< 0·01, ††† P< 0·001. Stress effect, with reference to its control: ‡ P< 0·05, ‡‡‡ P< 0·001. IL-1β: (a) ΔC
t about − 8·30, (b) ΔC
t about − 9·82; IL-1ra: (a) ΔC
t about − 9·17, (b) ΔC
t about − 10·28; IL-1R1: (a) ΔC
t about − 3·51, (b) ΔC
t about − 6·33.
Under the C diet, CN supplementation strongly increased the expression of IL-1 receptor antagonist (P< 0·001) in the hypothalamus and hippocampus, did not modify (IL-1R1, IL-6R, TNF-α and receptors) or counteract the effect of Str (IL-1β and IL-6) in the hypothalamus, and did not change (IL-1β) or counteract the effect of Str (IL-1R1, IL-6, Gp130, TNF-α and TNF-R1) in the hippocampus. Under the HF/HFr diet, CN supplementation also inhibited the effect of Str on the expression of IL-1R1 and IL-6 in the hypothalamus and on that of IL-1R1 and IL-6 and TNF-R1 in the hippocampus.
Results for the expression of intermediates of cytokine transduction, NF-κB, inhibitor of κB, targets such as COX2, ITGAM, and anti-inflammatory factors TGF-β1 and TGF-β2 and their two receptors in the hypothalamus and hippocampus, are given in Tables S6 and S7 (available online).
Hypothalamic–pituitary–adrenocortical axis function
A strong Str effect (P< 0·001), a diet effect (P< 0·01), and diet × CN and diet × Str interactions (P< 0·05) were revealed by ANOVA for the plasma concentrations of corticosterone. Str strongly increased the plasma concentrations of corticosterone under the C diet than under the HF/HFr diet (P< 0·01; Fig. 3) or CN supplementation (P< 0·05).

Fig. 3 Post-stress plasma concentrations of corticosterone according to the diet ((A) control (C) v. (B) high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition. Values are means, with their standard errors represented by vertical bars. ** Diet effect, with reference to its control (P< 0·01). † Cinnamon effect, with reference to its control (P< 0·05). ‡‡‡ Stress effect, with reference to its control (P< 0·001).
A Str effect was revealed by ANOVA for the expression of genes related to HPA axis function in the central structures (P< 0·05). Under the C diet, Str induced a decrease in the hypothalamic expression of corticotrophin-releasing hormone and in the hypothalamic and hippocampal expression of MR and GR (Fig. 4; Tables 2 and 3). Under the HF/HFr diet, Str either exerted no effect or induced an increase in (GR) mRNA levels. CN supplementation reduced or suppressed the effects of Str irrespective of the diet. Results for the hypothalamic and hippocampal expression of 11β-HSD1 and 11β-HSD2 are shown in Fig. S8 (available online).

Fig. 4 (A) Hypothalamic and (B) hippocampal expression of the glucocorticoid receptor according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition. The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, ††† P< 0·001. Stress effect, with reference to its control: ‡‡ P< 0·01, ‡‡‡ P< 0·001. (A) ΔC
t about − 1·81; (B) ΔC
t about − 1·49.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, ††† P< 0·001. Stress effect, with reference to its control: ‡‡ P< 0·01, ‡‡‡ P< 0·001. (A) ΔC
t about − 1·81; (B) ΔC
t about − 1·49.
Table 2 Hypothalamic expression of the mineralocorticoid receptor and corticotrophin-releasing hormone according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition§ (Mean values with their standard errors)

HPA, hypothalamic–pituitary–adrenocortical axis; MR, mineralocorticoid receptors; CRH, corticotrophin-releasing hormone.
Diet effect, with reference to its control: * P< 0·05, ** P< 0·01.
Cinnamon effect, with reference to its control: † P< 0·05.
Stress effect, with reference to its control: ‡‡ P< 0·01; ‡‡‡ P< 0·001.
§ The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
Table 3 Hippocampal expression of the mineralocorticoid receptor according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition§ (Mean values with their standard errors)

MR, mineralocorticoid receptors
Diet effect, with reference to its control: ** P< 0·01.
Cinnamon effect, with reference to its control: †† P< 0·01.
Stress effect, with reference to its control: ‡‡‡ P< 0·001.
§ The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
Cerebral plasticity
Neurogenesis
A Str effect (P< 0·05) and a Str × CN interaction (P< 0·05) were revealed by ANOVA for the expression of BDNF and NGF under the C diet in both the hypothalamus and hippocampus. A profile the same as that for immune factors and HPA axis function was observed for BDNF and NGF, as shown in Tables 4 and 5, i.e. under the C diet, a decrease in the expression induced by Str and an inhibition of this effect by CN supplementation in both the hypothalamus and hippocampus. The HF/HFr diet once again reversed or blunted these effects (diet × CN interaction P< 0·05).
Table 4 Hypothalamic expression of some actors of neurogenesis and synaptogenesis according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition§ (Mean values with their standard errors)

BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B; NGF, nerve growth factor; TrkA, tyrosine kinase receptor A; SYP, synaptophysin; DLGAP2, discs, large (Drosophila) homolog-associated protein 2.
Diet effect, with reference to its control: * P< 0·05, ** P< 0·01, *** P< 0·001.
Cinnamon effect, with reference to its control: † P< 0·05, †† P< 0·01, ††† P< 0·001.
Stress effect, with reference to its control: ‡ P< 0·05, ‡‡ P< 0·01, ‡‡‡ P< 0·001.
§ The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
Table 5 Hippocampal expression of some actors of neurogenesis according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition§ (Mean values with their standard errors)
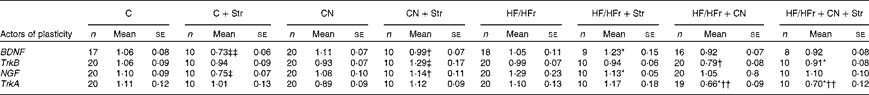
BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B; NGF, nerve growth factor; TrkA, tyrosine kinase receptor A.
Diet effect, with reference to its control: * P< 0·05.
Cinnamon effect, with reference to its control: † P< 0·05; †† P< 0·01.
Stress effect, with reference to its control: ‡ P< 0·05; ‡‡ P< 0·01.
§ The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t of the control group.
Synaptogenesis
The most interesting results were obtained for the hippocampal expression of SYP and DlGap2 (Fig. 5; other data given in Tables S9 and S10 (available online)). A diet effect (P< 0·01) was revealed by ANOVA for the expression of SYP in the hippocampus and a diet × CN × Str interaction (P< 0·01) was revealed for the expression of SYP in the hypothalamus and hippocampus and for that of DlGap2 in the hippocampus. The histograms showed a profile the same as those described above: a decrease in the expression by Str under the C diet and a reversion of this effect by the HF/HFr diet. CN supplementation counteracted the effect of Str, irrespective of the diet.

Fig. 5 Hippocampal expression of some actors of synaptogenesis according to the diet (control (C) v. high-fat/high-fructose (HF/HFr)), the cinnamon (CN) supplementation or the stress (Str) condition. (A) Synaptophysin, (B) discs, large (Drosophila) homolog-associated protein 2 (DlGap2). The relative expression level of the target gene (fold change) is expressed as
![]() $$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, ††† P< 0·001. ‡‡‡ Stress effect, with reference to its control (P< 0·001). (A) ΔC
t about 2·19; (B) ΔC
t about − 1·11.
$$2^{ - \Delta \Delta C _{t}} $$
, when compared with the mean ΔC
t (threshold cycle) of the control group. Values are means, with their standard errors represented by vertical bars. Diet effect, with reference to its control: * P< 0·05, *** P< 0·001. Cinnamon effect, with reference to its control: † P< 0·05, ††† P< 0·001. ‡‡‡ Stress effect, with reference to its control (P< 0·001). (A) ΔC
t about 2·19; (B) ΔC
t about − 1·11.
Discussion
The present study aimed to determine the effects of a HF/HFr diet, restraint Str and/or dietary CN supplementation on plasma cytokines and corticosterone and hypothalamic and hippocampal expression of actors of immune function, HPA axis function and cerebral plasticity. Protein data would also be very interesting in this context, but considering the low levels of variations expected, we chose to quantitatively determine the variations in the expression of genes by qPCR.
A low-level inflammatory state is usually associated with visceral obesity( Reference Tordjman, Guerre-Millo and Clement 35 ), but is still controversial and not reproducible in rodents. In the present study, only plasma concentrations of IL-6 were increased significantly by the HF/HFr diet. The plasma concentrations or central expression of other cytokines was not modified by the high-energy diet, perhaps due to there being no enough fat to induce such a reaction( Reference Pohl, Woodside and Luheshi 11 , Reference Terra, Pallares and Ardevol 36 , Reference Cano, Cardinali and Rios-Lugo 37 ). We studied the response of Wistar rats to a challenge with LPS at a moderate dose, as has been already done earlier in this context( Reference Pohl, Woodside and Luheshi 11 ). Indeed, a recent study has shown a sensitisation of the plasma concentrations and hypothalamic expression of pro-inflammatory cytokines in rats subjected to LPS treatment at the same dose( Reference Pohl, Woodside and Luheshi 11 ). In the present study, the plasma concentrations of cytokines were less increased by the LPS treatment under the HF/HFr diet than under the C diet. On the other hand, a stronger response of the hypothalamus and hippocampus to the LPS treatment was observed, inflammatory message perhaps due to an increase in the levels of between the periphery and the cerebral structures in HF/HFr rats. For the inflammatory state in the periphery, we did not obtain results the same as those reported by Pohl et al. ( Reference Pohl, Woodside and Luheshi 11 ); that is, we found no increase, perhaps because of a different diet or a higher body weight of the obese rats in their study. However, in agreement with the findings of their study, we observed an induction of the central inflammatory state. In case of obesity, such a hypersensitisation of the hypothalamic and hippocampal inflammatory processes could have, in the long term, a deleterious effect on normal cognitive functions( Reference Capuron and Miller 10 ).
Str did not alter the plasma concentrations of cytokines, whereas it decreased the central expression of IL-1 and its receptor, IL-6 and TNF-α. Globally, central cytokines seem to be more sensitive to environmental challenges than peripheral cytokines. The HF/HFr diet abolished or even reversed this effect of Str. Such an effect of a high-energy diet illustrates the comfort food theory( Reference Dallman, Pecoraro and la Fleur 38 ), but at the molecular level. According to this theory, a high-fat diet would act directly on the brain and by an intermediate process, which could, for instance, arise from the visceral fat, to counteract the effect of Str.
The HF/HFr diet that we used did not contain enough fat to induce visceral obesity, but it induced fatty livers and insulin resistance as has been shown previously( Reference Couturier, Qin and Batandier 28 ). Str strongly increased the plasma concentrations of corticosterone, but in each group, Str increased the plasma concentrations of corticosterone to a lesser extent under the HF/HFr diet than under the C diet. This is also in line with the comfort food theory and a lower effect of Str under a high-energy diet. The HF/HFr diet did not affect the central expression of HPA components in standard conditions. Str strongly decreased the central expression of MR and GR in rats fed the C diet, in agreement with the classical autoregulation of corticosteroid receptors( Reference Schmidt and Meyer 39 ). Once again, this effect was either blunted or reversed by the HF/HFr diet.
Under the C diet, the expression of actors of neurogenesis and synaptogenesis was decreased by Str, which corresponds to the classical deleterious effect of glucocorticoids at a high dose at the central level( Reference Oitzl, Champagne and van der Veen 15 , Reference De Kloet, Vreugdenhil and Oitzl 40 ). Under the HF/HFr diet, this effect was reversed for the expression of SYP and DlGap2. In this case, the HF/HFr diet could be protective against the central effects of Str, in accordance with the comfort food theory. The modulation of the effect of glucocorticoids by the composition of a diet could involve numerous factors of the cellular machinery and needs to be investigated further.
CN exerted different effects according to the diet. CN has been described to exert numerous beneficial effects under a standard diet( Reference Qin, Polansky and Anderson 26 ). The active components of CN have been known to exert several pharmacological effects such as anti-inflammatory, antioxidant, anti-tumour, anti-obesity and anti-diabetic effects. We confirmed its anti-inflammatory actions in some cases. For instance, the plasma concentrations of IL-6 that were increased by the HF/HF diet were restored to basal levels by CN supplementation. In both the hypothalamus and hippocampus, under the C diet, CN supplementation increased the expression of IL-1 receptor antagonist, an antagonist of IL-1β, which could contribute to the beneficial anti-inflammatory and antioxidant effects of CN. On the other hand, this effect was not observed under the HF/HFr diet. Along this line of discussion, it is worth noting that the beneficial effects of CN under a control diet disappear under an unbalanced diet.
Under the C diet, CN supplementation decreased the response of plasma corticosterone to restraint Str. Under this diet, Str decreased the expression of numerous factors involved in immune system, HPA axis function or cerebral plasticity, and this effect was reversed by CN supplementation. Under the HF/HFr diet, the effect of CN supplementation on plasma corticosterone and central GR expression in response to Str disappeared. Perhaps, there exists an interaction between compounds of the HF/HFr diet and CN to blunt this effect. The HF/HFr diet either blunted or reversed the effects of both Str and CN supplementation. In the case of actors of cerebral plasticity, the effect of CN supplementation in reversing the Str-induced increase of the expression of SYP and DlGap2 under the HF/HFr diet could be seen as deleterious.
Co-activators and co-repressors are integral components of the signal transduction pathways of steroid hormones. GR enhances or represses the transcription of target genes by directly binding to the glucocorticoid responsive element, by interacting with other transcription factors apart from binding to DNA or, in a composite manner, by both directly binding to glucocorticoid responsive element and interacting with other transcription factors bound to neighbouring sites( Reference Oakley and Cidlowski 41 ). The c-AMP response element-binding protein (CREB)-binding protein could be a good candidate for the effect of CN or diet on GR action, because it may function not only as a co-activator but also as a repressor, depending on the local concentration of other co-activators( Reference Kino, Nordeen and Chrousos 42 ). Nevertheless, no interaction between CREB-binding protein and polyphenols has been described in the literature so far. On the other hand, recent data suggest that polyphenols can function as modifiers of signal transduction pathways to exert their beneficial effects, mainly by acting through NF-κB, activator protein 1 (AP-1) and mitogen-activated protein kinases signalling, which also interacts with GR( Reference Santangelo, Vari and Scazzocchio 43 , Reference Trzeciakiewicz, Habauzit and Horcajada 44 ). The explorations of the actions of CN on cellular machinery are still limited. CN extract has recently been shown to inhibit the activation of p38, c-Jun N-terminal kinase, extracellular-signal-regulated kinase 1/2 and signal transducer and activator of transcription 4 in vitro ( Reference Lee, Kim and Cho 45 ). CN extract induces tumour cell death through the inhibition of NF-κB, via three signal transduction pathways, NF-κB-inducing kinase/IκB kinase (NIK/IKK), extracellular-signal-regulated kinase and p38 mitogen-activated protein kinases( Reference Kim, Kim and Kim 46 ), and through AP-1( Reference Kwon, Hwang and So 47 ). Another means to change GR transactivation and signalling would be its phosphorylation state( Reference Oakley and Cidlowski 41 ). CN has recently been found to inhibit PKA activity in vitro ( Reference Moskaug, Borge and Fagervoll 48 ). Cinnamaldehyde, a major active component of CN, has also recently been shown to increase the phosphorylation levels of the insulin-like growth factor-1 receptor and its downstream signalling molecules( Reference Takasao, Tsuji-Naito and Ishikura 49 ).
Conclusions
In conclusion, we showed that in rats fed a HF/HFr diet there is an increase in the plasma concentrations of IL-6 and that the central structures of their immune system exhibit a high sensitisation to LPS. We also showed that the HF/HFr diet is sufficient to reverse the negative effects of Str on central gene expression. These data confirm the comfort food theory at the molecular level. CN exerted beneficial effects under the C diet (notably via an increase in the expression of IL-1 receptor antagonist and by counteracting the effects of Str), but its effects were blunted or even reversed under the HF/HFr diet. Numerous processes are candidate to these effects of inversion of Str responses by the diet or by CN, and need to be further investigated.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513003577
Acknowledgements
The authors thank Thierry Leste-Lassere and Guillaume Drutel for their useful advice on qPCR. They thank Nadine Fidier and Renaud Maury for their help during dissections. They also thank Martine Schuler for her careful reading of the manuscript and her corrections of the language of the article.
The present study was supported by the ANR (Agence Nationale de la Recherche) ‘CERVIRMIT’ project (ANR-07-PNRA-003-02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors' contributions are as follows: N. M.-A. wrote the manuscript and was responsible for Bioplex and qPCR measurements; C. B., F. C. and L. P. took care of the animals, carried out the LPS challenge test and were responsible for animal killing and dissections; J. D. was involved in mRNA extraction and qPCR measurements; K. C. and I. H.-F. were responsible for animal killing and dissections in experiment 2; M.-P. M. was in charge of reading the manuscript; A.-M. R. and P. M. supervised this work.
None of the authors has any conflicts of interest to declare.












