INTRODUCTION
Meningococcal disease (McD) has been epidemic in Western Norway since 1976 [Reference B⊘vre and Gedde-Dahl1]. The annual incidence has varied between 5 and 8/100 000 inhabitants and the case fatality rate (CFR) has been around 10% [Reference Lystad and Aasen2, Reference Iversen and Aavitsland3]. The meningococcal strain B:15:P1.7,16 has dominated this epidemic, and mainly young children and teenagers have been affected. During 1976–1984, a clinical study of 211 McD patients from Western Norway, admitted to Haukeland University Hospital, Bergen, showed that admission during the morning hours and severe septicaemia correlated strongly with a poor prognosis [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4]. After this study, the focus changed from meningitis to septicaemia, and health personnel and the general public were repeatedly given information about septicaemia up to 1991, including colour prints of meningococcal skin rash and recommendations to look for skin rash in patients with acute fever during the first day and night of illness. Furthermore, the Norwegian Meningococcal B Vaccine trial during 1988–1991 provided relevant information to the vaccinees and their families, and achieved substantial media attention in national and local TV channels and newspapers [Reference Bjune, Hoiby and Gronnesby5, Reference Bjune and Sundelin6].
The aims of the present study were to study the fatality of McD patients in Western Norway during 1985–2002, to examine the influence of any epidemiological changes on fatality during this period and to evaluate the measures taken after the previous study to reduce fatality [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4].
MATERIAL AND METHODS
Patients and diagnosis
Haukeland University Hospital, Bergen serves as an emergency hospital for ∼300 000 inhabitants living in the city of Bergen and the surrounding areas. A total of 293 McD patients hospitalized during 1985–2002 were included in this study. The inclusion criteria were based on typical clinical signs of meningitis, fever and petechial rash and bacteriological confirmation by isolation of N. meningitidis from a normally sterile site [blood, cerebrospinal fluid (CSF), synovial fluid] or by microscopy of Gram-negative diplococci in CSF (Table 1). The patients were routinely screened for possible underlying conditions including complement deficiencies, and no patient was detected with any underlying condition that could have contributed to their outcome.
Table 1. Inclusion criteria for the meningococcal disease patientFootnote *

* No fatal patients were excluded due to these criteria.
† One case was confirmed by agglutination test.
‡ Three patients lacked meningococcal skin rash.
Septicaemia was defined as fever with signs of serious infectious disease such as petechial rash. Petechiae are intracutaneous blood spots <5 mm in diameter, whereas ecchymoses are >5 mm. Children and teenagers with severe septicaemia (septicaemia with hypotension and/or ecchymoses) but no systemic isolate were also included, so as not to underestimate the CFR. Patients who did not fulfil the inclusion criteria (n=5) or who were transferred from other hospitals (n=11) were excluded.
Data collection, serogrouping and disease categories
This study was a 9+9-year follow-up of the previous study from the 9-year period 1976–1984. The patients were identified continuously and retrospectively. Epidemiological, clinical and laboratory data from the patients' hospital records were recorded retrospectively on a data collection form, in use since 1983. Missing data due to incomplete hospital records resulted in exclusion of some variables from the multivariate analyses.
The patients were classified into four disease categories based on clinical findings on admission to the hospital [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4, Reference Gedde-Dahl, H⊘iby, Schillinger, Lystad and B⊘vre7];
I. Meningitis (⩾100 cells/μl CSF or back rigidity) with no hypotension or ecchymoses.
II. Severe septicaemia with hypotension [systolic blood pressure (SBP) ⩽70 mmHg in ⩽12 year olds and SBP ⩽100 mmHg in >12 year olds] and/or ecchymoses, but with no signs of meningitis.
III. Severe septicaemia and meningitis, as category II but with signs of meningitis.
IV. Bacteraemia/septicaemia, with or without signs of meningitis but with no hypotension or ecchymoses.
Serogrouping was performed on systemic isolates from CSF and/or blood (and from synovial fluid for one patient). The results of further strain characterizations, including serotype, serosubtype and molecular typing will be presented in a follow-up publication. Tonsillopharyngeal isolates were not used as inclusion criteria, but were used to classify 26 patients as serogroup B or C disease. Thrombocytopenia was defined as thrombocytes ⩽100×109 cells/l blood and leukopenia was defined as leukocytes ⩽5×109 cells/l blood, both measured on admission.
The main weaknesses of the study were that the examination and treatment of patients were not performed by the same individuals due to mobility among the health-care workers during the long study period, some missing data and low numbers in some subgroups of patients.
Hospital routines and initial treatment
The initial treatment of McD at our hospital has changed little since 1976. It has included prompt intravenous administration of benzyl penicillin and chloramphenicol, and correction of fluid and electrolyte disturbances. Early respiratory support and vasopressive therapy were given to patients suffering from severe septicaemia. Chloramphenicol was discontinued as soon as the meningococcal aetiology was confirmed. Seven of the 293 patients in the study received cephalosporins instead of benzyl penicillin and chloramphenicol as diagnosis was unclear on admission. Systemic therapy with mannitol was given to reduce intracranial pressure if symptoms of meningitis were present.
Statistical analysis
The association between risk factors and fatality was analysed by univariate analyses using t tests for continuous and exact χ 2 tests for categorical variables (Table 2). Variables that were significantly associated with fatality were entered into forward stepwise multivariate logistic regression analyses using likelihood ratio tests. A separate analysis was performed for fatality risk factors before and on admission to the hospital (Table 3).
Table 2. Number of patients, deaths and case fatality rate (CFR) by clinical, laboratory and time variables as recorded on admission of 293 meningococcal disease patients
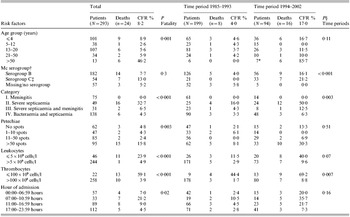
* All five patients aged >70 years hospitalized during 1994–2002.
† Tonsillopharyngeal isolates included (n=26).
‡ One isolate from synovial fluid.
§ P=variable variations between two time periods.
Table 3. Multivariate logistic regression analyses of the association between fatality and risk factors before and on admission in 293 meningococcal disease patients
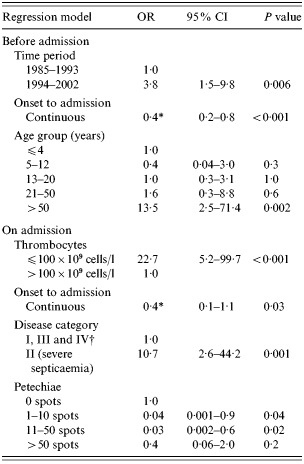
OR, Odds ratio; CI, confidence interval.
* OR for a 12 h increase.
† Differences in the CFRs for categories I, III and IV were n.s. (P=0·15).
The distribution of each risk factor in the two 9-year periods 1985–1993 and 1994–2002 was compared using t tests for continuous and exact χ 2 tests for categorical variables (Table 2). These periods were also compared descriptively with published summaries for the previous 9-year period 1976–1984 [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4]. SPSS version 11.5 (SPSS Inc., Chicago, IL, USA) was used to process the data. A P value of ⩽0·05 was considered significant.
RESULTS
Age and gender
This study included 293 hospitalized patients (144 males, 149 females; aged 0–98 years) (Table 2). The age distribution peaked in children ⩽4 years old (n=101) and in teenagers (n=107); these two age groups accounted for 71% of the patients. The sex distribution was similar in the younger age groups, whereas all the 13 patients aged >50 years were females.
The overall CFR was 8·2% (Table 2). The highest CFR was found in patients >50 years old (46%) (P=0·001). None of the 26 infants <1 year old died. No patient was dead on admission. The CFR was higher for females (10%) than for males (6%), but the difference was not significant.
Bacteriology and laboratory values
Of 215 systemic meningococcal isolates, 165 (77%) were serogroup B meningococci, 46 (21%) were serogroup C meningococci and four (2%) were non-groupable (NG) meningococci (Table 2). Serogroup C disease was more common in patients >12 years old than in those ⩽12 years old (P=0·03). In the remaining 78 patients, 27 had Gram-negative diplococci by microscopy of CSF, of which 16 also had tonsillopharyngeal isolates.
Systemic isolates were found in 21 of the 24 patients who died, 14 patients had serogroup B and seven serogroup C disease (Table 2). The CFR was higher for serogroup C (13%) than serogroup B (8%), although not significantly different (P=0·3). However, patients with serogroup C disease (30%) were more often classified as disease category II (severe septicaemia) than patients with serogroup B disease (14%) (P=0·007).
The CFRs were 24% (11/46) and 59% (13/22) for patients with leukopenia and thrombocytopenia on admission respectively (both P<0·001) (Table 2).
Clinical symptoms and prognosis on admission to hospital
Of 24 fatalities, 16 belonged to disease category II (severe septicaemia). The CFR for this category was 33%, which was significantly higher than for the other disease categories (P<0·001) (Table 2). Among the remaining eight patients who died, six belonged to disease category IV (bacteraemia/septicaemia), and only two to disease category III (severe septicaemia and meningitis) (CFR=6·5%). None of the 75 patients in disease category I (meningitis) died.
Petechiae and/or ecchymoses were found in 227 (78%) patients on admission. Fatality was associated with multiple (>50) petechiae (P=0·003) and ecchymoses (P<0·001) (Table 2). However, five patients who died had either no petechiae (aged 1, 89 and 90 years) or ⩽10 petechiae (aged 1 and 8 years). The three children had an onset to admission time of ⩽8 h, belonged to disease category IV (septicaemia/bacteraemia) and developed multiple (>50) petechiae within 1, 1 and 5 h after admission to hospital.
Hypotension was found in 67 patients on admission and was associated with fatality (P<0·001). Impaired consciousness and a temperature of ⩾40 °C were not significantly associated with fatality.
Among the 63 patients who received pre-admission antibiotics, seven (11%) died compared to 17 out of 230 (7%) non-treated patients (P=0·34). More patients received pre-admission antibiotics in the age groups 0–4 or 13–20 years (P=0·04), who were in disease category I (meningitis) (P=0·0001), who had an onset to admission time of 13–24 hours (P=0·04) and/or who were fully conscious (P=0·03) (univariate analyses). Stratification by time of admission did not give significant results possibly due to small numbers.
Onset to admission time, hour, weekday and season of admission
The 23 fatalities with a known onset time had an onset to admission time of ⩽24 h, and 13 of them had been ill for ⩽12 h. The onset to admission time was significantly shorter for those who died [mean 14 h, 95% confidence interval (CI) 11·0–16·4] than for the survivors (mean 22 h, 95% CI 20·3–24·4) (P<0·001).
Patients hospitalized between 07:00 and 11:00 hours had a poorer prognosis than those admitted during other hours of the day (P=0·02) (Table 2). The proportion of fatal patients admitted in the morning hours was not significantly different during 1976–1984 and 1985–2002 (P=0·24).
The fatality was not significantly associated with weekday of admission. The proportion of fatal patients admitted from May to August 1985–2002 was not significantly different from that of the former study 1976–1984 (P=0·08), nor was it significant for admissions during the rest of the year (P=0·48).
Time periods 1985–1993 and 1994–2002
The CFR varied substantially during the study period, from 4% during 1985–1993 to 17% during 1994–2002 (P<0·001) (Fig. 1, Table 2). From 1985–1993 to 1994–2002 the CFR increased in all age groups except for the 5–12 years group. The greatest increase was found in patients >50 years, from 0% (0/6) to 86% (6/7) (P=0·005). During 1985–1993, the annual incidence was 7/100 000 inhabitants and the CFR was low (Fig. 2). From October 1990 to August 1993 onwards, there were no fatalities among the 79 hospitalized patients. There were also no deaths in 1988, 2000 and 2001.
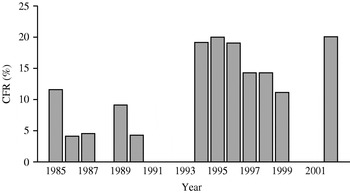
Fig. 1. Annual case fatality rate for meningococcal disease during 1985–2002. CFR, Case fatality rate.
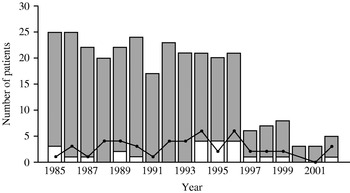
Fig. 2. Patients with meningococcal disease, severe septicaemia and fatal outcome per year during 1985–2002. ![]() , Survivors; □, deaths; —•—, severe septicaemia without meningitis.
, Survivors; □, deaths; —•—, severe septicaemia without meningitis.
During 1994–2002, six of the 16 deaths occurred in patients >50 years old and there was significantly more serogroup C disease (P<0·001), thrombocytopenia (P<0·001), severe septicaemia (disease category II) and less meningitis (disease category I) (P=0·003) in this period. From 1997, the incidence dropped to ⩽2/100 000, which was the lowest level since 1976. The greatest decline was in teenagers. There was no significant change in the age distribution during the study period (P=0·11).
Multivariate analyses
Two separate logistic regression analyses included risk factors related to fatality (Table 3). In the first analysis with risk factors on admission, a fatal outcome was associated with severe septicaemia, thrombocytopenia, multiple (>50) petechiae, and a short onset to admission time. In the second analysis with risk factors before admission, time period, age >50 years and a short onset to admission time were significantly associated with fatality.
DISCUSSION
The annual incidence in Western Norway has been around 7/100 000 inhabitants per year until 1997, when it dropped (Fig. 2). Nationally, the incidence has also declined suggesting that the Norwegian epidemic may be at its tail [Reference Iversen and Aavitsland3, Reference Connolly and Noah8]. The overall average CFR was 8·2%, which was in accordance with our previous study (8·5%) and national data [Reference Lystad and Aasen2–Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4]. However, the annual CFRs have varied substantially during the 27 years of registration, i.e. 8, 4 and 17% during the three 9-year periods from 1976 (Fig. 1), corresponding with variations observed in Denmark and the United States as opposed to the constant CFR in Sweden [Reference Havens, Garland, Brook, Dewitz, Stremski and Troshynski9–Reference Berg, Trollfors, Alestig and Jodal11].
During 1985–1993 the CFR was low, only 4%. Most patients had symptoms of meningitis (disease category I) or bacteraemia without hypotension (disease category IV) on admission, and both disease categories had low CFRs. During 1986–1991, the public frequently received information about the early clinical features of McD with pictures of meningococcal skin rash, which together with the attention surrounding the Norwegian meningococcal B vaccine trial increased the awareness of McD in the population and led to earlier hospitalization and improved outcomes [Reference Bjune, Hoiby and Gronnesby5, Reference Bjune and Sundelin6, Reference Halstensen, Aase, Bj⊘rnevik, Smith, Halstensen, Bj⊘rnevik and Smith12–Reference Cartwright14]. Since only 4% of the population was vaccinated and the vaccine efficacy was 56%, it is unlikely that increased immunity in the population due to the vaccination trial can account for the low overall CFR [Reference Iversen and Aavitsland3].
The CFR increased substantially during 1994–2002. Since the incidence was high until 1997, it is unlikely that reduced awareness of McD among health personnel explained the increased CFR. During this period, significantly more patients had serogroup C disease, were admitted with thrombocytopenia and leukocytopenia, and were classified as disease category II (severe septicaemia). This shift towards more severe disease suggests more virulent circulating meningococcus, although other factors may be involved [Reference Stephens15]. The only strain characteristic recorded was serogrouping, and the fatality was not significantly higher for either of the serogroups. However, a new serogroup C strain, C:15:P1.7,16, caused a local outbreak just north of Bergen with a CFR of 20%, suggesting that specific strains may have contributed to the high CFR observed during 1994–2002 [Reference Jensen, Sch⊘nheyder, Lind, Berthelsen, N⊘rgard and S⊘rensen10, Reference Smith, Lehmann and Lie16–Reference Whalen, Hockin, Ryan and Ashton18]. The association between meningococcal strains and fatality will be examined in a subsequent publication.
Corresponding with the Norwegian epidemic, the age distribution peaked in children ⩽4 years old and in teenagers [Reference Lystad and Aasen2–Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4]. No infant <12 months of age died, which was unexpected since CFR is reported to be high in this age group [Reference Lystad and Aasen2, Reference Jones and Cartwright19]. The highest CFR was found in patients >50 years old, as reported by others [Reference Connolly and Noah8, Reference Jones and Cartwright19–Reference Rosenstein, Perkins and Stephens21]. Interestingly, all the patients in this age group were female, possibly attributable to sex-specific risk factors [Reference Rosenstein, Perkins and Stephens21]. McD is rare in elderly patients but it may be under-diagnosed, since the elderly often have atypical symptoms [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4, Reference Stephens, Hajjeh, Baughman, Harvey, Wenger and Farley22]. In this study, two of the six elderly fatalities had neither signs of meningitis nor petechial rash on admission, which probably delayed the diagnosis and treatment.
The simple clinical disease categorization applied on admission has proved valuable for a rapid selection of the seriously ill patients in need of immediate intensive medical treatment. No patient categorized as meningitis (disease category I) died. In contrast, one-third of the patients with severe septicaemia without meningitis (disease category II) died [Reference Cartwright14, Reference Shigematsu, Davison, Charlett and Crowcroft23]. Previously, no patient in disease category IV (bacteraemia/septicaemia) had died [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4, Reference Gedde-Dahl, H⊘iby, Schillinger, Lystad and B⊘vre7]. In our study, six patients (4%) in this category died, five of them were admitted <10 h after onset of disease and six developed hypotension within 10 h of admission. Thus, the applied disease categorization does not seem sensitive enough to predict fatality in patients admitted early with rapidly progressing fulminant disease.
According to the official Norwegian recommendations, pre-admission antibiotics should be given to patients with suspected McD [Reference Moe24, Reference Lystad25]. The fact that most of our patients can reach the hospital within 1 h may explain why only 22% of the patients received pre-admission antibiotics. A valid evaluation of such treatment could not be performed due to these low numbers. The importance of pre-admission antibiotics in reducing fatality is not conclusively supported by the available data [Reference Cartwright14]. However, recent reports have shown that the improved initial management of McD, including pre-admission antibiotics and pre-admission oxygen and intravenous fluid therapy, reduces fatality [Reference Booy, Habibi and Nadel26, Reference Thorburn, Baines, Thomson and Hart27]. In this study, few patients received such basic hospital treatment before admission and no records were made of possible changes in the quality of the pre-admission and in-hospital treatment during the study period. However, our opinion is that more patients in our region with signs of septicaemia without meningitis should be offered such basic treatment before admission.
The onset to admission time has been found to correlate with the disease categories [Reference Cartwright14, Reference Niklasson, Lundbergh and Strandell28–Reference Goldacre, Roberts and Yeates30]. All the fatalities had an onset to admission time of ⩽24 h, but those who died from severe septicaemia (disease category II) had the lowest onset to admission times, which underlines the explosive nature of fatal McD and the importance of early diagnosis, admission and treatment of these patients [Reference Cartwright14, Reference Booy, Habibi and Nadel26].
Patients admitted during the morning hours were at high risk of a fatal outcome. The proportion of fatal patients admitted in the morning hours declined from 1976–1984 to 1985–2002, although the difference was not significant. Nonetheless, the repeated recommendations given during 1985–1991 to examine patients with acute fever for meningococcal skin rash during the first night have probably resulted in earlier hospitalization [Reference Halstensen, Aase, Bj⊘rnevik, Smith, Halstensen, Bj⊘rnevik and Smith12].
The winter months have been associated with a higher CFR of McD. In our study the CFR was independent of season [Reference B⊘vre and Gedde-Dahl1–Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4]. Among the patients admitted during May–August who died, there was a significant increase in the number of patients >50 years old, which contributed to the unexpectedly high CFR found in these months.
In conclusion, the CFR of McD increased substantially in Western Norway over the last decade. During this period serogroup C disease increased, which calls for more use of serogroup C vaccines. Fatality was significantly associated with a short onset to admission time, severe septicaemia with hypotension and/or ecchymoses (disease category II), thrombocytopenia and multiple petechiae on admission [Reference Halstensen, Pedersen, Haneberg, Bjorvatn and Solberg4, Reference Niklasson, Lundbergh and Strandell28]. Early diagnosis, hospitalization and treatment are crucial to reduce fatality [Reference Cartwright14, Reference Booy, Habibi and Nadel26, Reference Goldacre, Roberts and Yeates30]. To achieve this, health personnel and the general public regularly need adequate information about McD, including the initial symptoms of septicaemia, colour prints of skin rash and recommendations to examine all patients with acute fever for skin rash during the first day and night. The relation between meningococcal strain and fatality will be examined in a subsequent publication.
ACKNOWLEDGEMENTS
We thank statistician Geir Egil Eide at Centre for Clinical Research, Haukeland University Hospital, for valuable statistical advice and senior consultant Asbj⊘rn Digranes, the Department of Microbiology and Immunology, the Gade Institute, Haukeland University Hospital for serogrouping of meningococci.







