Introduction
Up to one-third of the world's population is currently at risk from vitamin deficiency disorders, which can have a severe impact on metabolism resulting in cumulative damage to health(Reference Farré, Twyman and Zhu1, Reference Yuan, Bassie and Sabalza2). Strategies to address vitamin deficiency include the provision of supplements, the fortification of processed foods and the biofortification of crops to increase the vitamin content at source(Reference Zhu, Naqvi and Gómez-Galera3, Reference Gómez-Galera, Rojas and Sudhakar4). Health authorities in the developed world have established dietary reference intakes (DRI) for vitamins, which are based on recommended daily allowances (RDA) and tolerable upper levels (UL). The RDA is defined as the daily dietary intake level of a nutrient considered sufficient to meet the requirements of 97·5 % of healthy individuals in each life-stage and sex group, and the UL is defined as the highest daily consumption that current data have shown to cause no side effects in humans(5, 6). For example, the RDA for vitamin A is 900 μg/d and the UL is 3000 μg/d. Strategies that address vitamin deficiency must therefore strive to achieve the DRI for each vitamin without exceeding the UL.
Most strategies developed to address vitamin deficiency diseases focus on the total amount of vitamin provided rather than its bioavailability. The latter is important because many different factors affect the efficiency with which vitamins are taken up from food, including the nature of the food matrix, the manner in which the food is prepared and the efficiency with which available free vitamin molecules are absorbed in the human gut. Furthermore, some vitamins are absorbed as pro-vitamins which are converted into their final form in the human body. For example, vitamin A is generally absorbed either as retinol from meat and dairy sources or as β-carotene from plant sources, whereas the functional forms of vitamin A are retinal and retinoic acid. The US Institute of Medicine has therefore recommended that the DRI for vitamin A is expressed as retinol activity equivalents (RAE), which take bioavailability and metabolic conversion into account(7, 8). Each RAE corresponds to 1 μg of pure retinol in oil, 2 μg of β-carotene in oil, 12 μg of dietary β-carotene (accounting for the impact of the food matrix on bioavailability) or 24 μg of dietary α-carotene, γ-carotene or β-cryptoxanthin, since these carotenoids carry only one active retinol group whereas β-carotene carries two(Reference Bai, Twyman and Farré9).
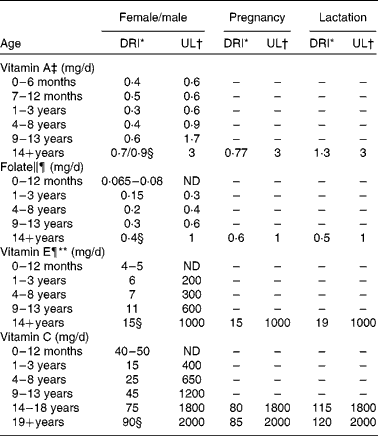
The DRI must also take into account the requirements of different life-stage and sex groups. As stated above, the DRI for vitamin A is 900 μg/d or 900 RAE. However, this is the value for adult males. The DRI for females is 700 RAE, rising to 770 RAE in pregnancy and 1300 RAE when lactating, and the DRI for children is 400–500 RAE depending on age(7). The DRI values for the four vitamins discussed in the present review are listed in Table 1, providing a breakdown by sex and life stage where appropriate. In some cases, the requirements are highly specific; for example, folate requirements increase from 400 μg/d for adult females to 600 μg/d during the first 12 weeks of pregnancy to reduce the risk of neural tube defects(Reference Shaw, Schaffer and Velie10, Reference Berry and Li11). Folate is the form that occurs naturally in food sources, whereas folic acid is the form of the vitamin found in fortified foods and dietary supplements(Reference Bailey, Dodd and Gahche12).
Table 1 Dietary reference intakes (DRI) and tolerable upper intake levels (UL) for the four vitamins discussed in the review

ND, not determinable due to lack of data of adverse effects in this age group and concern with regard to lack of ability to handle excess amounts. Source of intake should be from food only to prevent high levels of intake. EAR, estimated average requirement; DFE, dietary folic acid equivalents.
* The DRI is based on the older recommended daily intake, which is the average daily intake sufficient to meet the requirements of 97·5 % of healthy individuals. It is calculated from an EAR. If sufficient evidence is not available to establish the EAR, an adequate intake may be used instead, which is the mean intake for healthy breastfed infants or that sufficient to cover the needs of all healthy individuals in other life-stage and sex groups, accepting uncertainty in the percentage of individuals covered by this intake. DRI taken from the DRI reports; see Dietary Reference Intakes for Folate(119) and Dietary Reference Intakes for Vitamin C, Vitamin E(7).
† UL is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. Unless otherwise specified, the UL represents total intake from food, water and supplements. In the absence of an UL, extra caution may be warranted in consuming levels above recommended intakes. Members of the general population are advised not to exceed the UL routinely. The UL is not meant to apply to individuals who are treated with the nutrient under medical supervision or to individuals with predisposing conditions that modify their sensitivity to the nutrient. UL is adapted from Institute of Medicine(8).
‡ As preformed vitamin A only.
§ DRI for the four vitamins discussed in the present review.
∥ As dietary DFE. 1 DFE = 1 μg food folic acid = 0·6 μg of folic acid from fortified food or as a supplement consumed with food = 0·5 μg of a supplement taken on an empty stomach.
¶ The UL for vitamin E and UL for folic acid apply to synthetic forms obtained from supplements, fortified foods, or a combination of the two.
** As α-tocopherol, which includes RRR-α-tocopherol, the only form of α-tocopherol that occurs naturally in foods, and the 2R-stereoisomeric forms of α-tocopherol (RRR-, RSR-, RRS-, and RSS-α-tocopherol) that occur in fortified foods and supplements. It does not include the 2S-stereoisomeric forms of α-tocopherol (SRR-, SSR-, SRS-, and SSS-α-tocopherol), also found in fortified foods and supplements.
A balanced and varied diet including fresh fruit, vegetables, fish and meat provides the DRI for all vitamins and other nutrients, but such diets are difficult to achieve in many developing-country settings because fresh food is either unavailable or too expensive for the majority of the population and the best that can be provided is a predominantly cereal-based diet(Reference Zhu, Naqvi and Gómez-Galera3, Reference Gómez-Galera, Rojas and Sudhakar4). Although fruits and vegetables are good sources of vitamins, cereals (particularly milled grains) are poor sources of many nutrients, despite providing adequate amounts of carbohydrate and protein. Micronutrient deficiency can be addressed by providing supplements or by fortifying processed foods such as flour with vitamins, but these approaches are generally unsustainable in developing countries because they require continuous investment, efficient government-level infrastructure for production and distribution, and local compliance to achieve adequate doses(Reference Pérez-Massot, Banakar and Gómez-Galera13). An alternative is the biofortification of cereal crops at source by enhancing their intrinsic levels of vitamins, which requires investment only at the development stage and can thereafter rely on existing food processing and distribution networks without specific strategies to ensure local compliance(Reference Christou and Twyman14–Reference Zhu, Sanahuja and Yuan16).
There has been significant recent progress in the biofortification of staple crops with vitamins, including the key examples of Golden Rice producing high levels of β-carotene(Reference Paine, Shipton and Chaggar17) and ‘multivitamin corn’ simultaneously producing high levels of β-carotene, ascorbic acid (vitamin C) and folate(Reference Naqvi, Zhu and Farré18). However, a large majority of the biofortification studies that have been published thus far have measured absolute levels of the enhanced vitamins without considering bioavailability, which will be critical for the success of biofortification programmes. Any biofortified crop deployed in the field will need to provide the DRI of each nutritional component in a reasonable portion, i.e. consistent with a standard daily food intake of 130–150 g of fruits and vegetables, up to 1·6 g of oil and up to 350 g of rice(Reference Dapcich, Salvador Castell and Ribas Barba19). However, the dosage must also take into account the impact of food processing, cooking and co-dietary factors such as the consumption of nutritional promoters and inhibitors. One final significant challenge is that these ideal dosage levels must be achieved for different vitamins simultaneously to avoid merely shifting the challenge of micronutrient deficiency from one set of nutrients to another(Reference Yuan, Bassie and Sabalza2, Reference Naqvi, Zhu and Farré18).
The impact of food processing on vitamin levels and bioavailability in food
Fat-soluble vitamins
The stability of carotenoids differs according to the properties of the food matrix even when the same processing and storage conditions are used(Reference Rodriguez-Amaya20). However, the processing method also has a significant impact. For example, some β-carotene is lost during microwaving, steaming, boiling and sautéing, but much more substantial losses occur during deep-frying, prolonged cooking, and combinations of several preparation or processing methods such as baking and pickling(Reference Rodriguez-Amaya20). Whereas almost all cooking methods reduce the overall content of β-carotene, some simultaneously increase its bioavailability by releasing it from the food matrix(Reference Hart and Scott21). Products that are often consumed raw (for example, carrots, tomatoes and other fruits) may therefore be less suitable sources of β-carotene than cereals, potatoes and rapeseed oil, which are cooked before consumption, even though the overall levels are higher before cooking. For example, although microwave cooking reduces the overall amount of β-carotene it also increases tissue degradation and the amount of β-carotene available for extraction(Reference Howard, Wong and Perry22). These factors explain why only minor changes in β-carotene levels were observed in tomato juice during processing(Reference Lin and Chen23) whereas the levels of bioavailable β-carotene can increase or decrease in carrots and cassava depending on the cooking method(Reference Gayathri, Platel and Prakash24, Reference Carvalho, Oliveira and Godoy25).
Vitamin E is a group of related compounds known as tocochromanols, which are divided into the tocopherols and tocotrienols. Vitamin E levels in cereals are also affected by storage and cooking, but there are conflicting reports in the literature suggesting that the bioavailable levels increase, decrease or do not change(Reference Bernhardt and Schlich26, Reference Mazzeo, N'Dri and Chiavaro27). Minimal losses were recorded when cereal grains were stored under optimal conditions in the absence of insect pests, but processing by milling resulted in significant losses(Reference Pomeranz and Sauer28, Reference Borrelli, De Leonardis and Platani29). Furthermore, the tocochromanol levels in wheat, barley and oats were reported to fall by 30 % during extrusion cooking(Reference Zielinski, Kozlowska and Lewczuk30), whereas cooking in water was shown to increase the total tocochromanol levels in red and white rice by 32 and 37 %, respectively(Reference Finocchiaro, Ferrari and Gianinetti31). Importantly, this increase reflected the 23 % loss of soluble DM during water cooking which resulted in the concentration of lipophilic compounds(Reference Finocchiaro, Ferrari and Gianinetti31). Such reports demonstrate the importance of standardised methods for measuring and recording nutritional components in foods to allow direct and meaningful comparisons.
The processing of plant oils can also reduce the availability of tocochromanols, for example, pre-heating, cooking and screw-pressing can reduce levels by 15 % in olive oil, 25 % in soyabean and rapeseed oils, 28·5 % in high-oleic safflower-seed oil, 32 % in maize oil and 35–40 % in cottonseed and groundnut oil(Reference Kanematsu, Ushigusa and Maruyama32, Reference Ortega-García, Gámez-Meza and Noriega-Rodriguez33).
Water-soluble vitamins
Vitamin C is also lost during cooking, but the amount depends on the temperature, the degree of leaching into the cooking medium, the surface area of the food exposed to water, oxygen levels, pH, and the presence of pro-oxidation factors(Reference Lešková, Kubíková and Kováčiková34). Vitamin C in potatoes is rapidly degraded during boiling and frying, but is less susceptible during braising, sautéing and pressure-cooking, and only minimal losses occur during baking and microwaving(Reference Han, Kozukue and Young35). In addition, boiling in salted water can offer partial protection from degradation(Reference Han, Kozukue and Young35).
The levels of plasma vitamin C were measured in human subjects after the consumption of either fresh strawberries or strawberries stored in an open container at 4°C for 4 d. Although the levels of ascorbic acid in the fruits were the same, the plasma ascorbic acid levels were higher in subjects who had consumed the stored strawberries, which was attributed to the modification of bioactive compounds that influence the bioavailability of ascorbic acid(Reference Azzini, Vitaglione and Intorre36).
Folate is also lost during cooking and food preparation, reflecting a combination of thermal degradation and leaching into the cooking water(Reference Bassett and Sammán37). Leaching was found to be the major factor; for example, boiling in water reduced the folate content of broccoli and peas more than blanching and microwaving, whereas minimal losses were recorded after steaming, which involves the least contact with water(Reference Stea, Johansson and Jägerstad38). The boiling of peeled and unpeeled potatoes resulted in folate losses of 52 and 37 %, respectively, suggesting that the skin protects against folate loss(Reference Stea, Johansson and Jägerstad38). This also indicated the greater role of leaching compared with thermal degradation in the loss of folate(Reference Dang, Arcot and Shrestha39, Reference Scott, Rébeille and Fletcher40).
Vitamin biofortification to improve nutrition
As discussed above, the biofortification of crops at source provides a sustainable approach to improve the nutritional quality of food without the continual need for a specialised distribution infrastructure or compliance monitoring(Reference Pérez-Massot, Banakar and Gómez-Galera13, Reference Farré, Ramessar and Twyman15). Two strategies have been developed to increase the vitamin levels in plants: one involving conventional breeding and the other involving genetic engineering.
Numerous studies have shown that so far only genetic engineering has provided the means to produce nutritionally enhanced crops that can meet DRI values. For example, different genetic engineering strategies have yielded tomato fruits producing (in separate plants individually) up to 205 μg of β-carotene/g fresh weight(Reference D'Ambrosio, Giorio and Marino41), 1159 μg of ascorbic acid/g fresh weight(Reference Haroldsen, Chi-Ham and Kulkarni42), 10 μg of folate/g fresh weight(Reference Díaz de la Garza, Gregory and Handson43) or 4·57 μg of α-tocopherol/g fresh weight(Reference Seo, Kim and Harn44). In contrast, conventional breeding in different tomato varieties has achieved maximum levels of 20 μg of β-carotene/g fresh weight(Reference Lenucci, Cadinu and Taurino45), 180 μg of ascorbic acid/g fresh weight(Reference Proteggente, Pannala and Paganga46) or 0·29 μg of folate/g fresh weight(Reference Bekaert, Storozhenko and Mehrshahi47). Focusing on β-carotene, the difference in achievement therefore equates to 28 % of the DRI in conventionally bred tomatoes(Reference Lenucci, Cadinu and Taurino45) compared with 285 % of the DRI in tomato lines produced by genetic engineering(Reference D'Ambrosio, Giorio and Marino41). This means that the genetically engineered tomatoes would be required as minor food additives rather than whole fruit in order to achieve the DRI. We provide a breakdown of the nutritional values of other conventional and genetically engineered crops in online Supplementary Table S1.
Most of the nutritionally enhanced crops reported thus far produce higher levels of a single vitamin, and can therefore be regarded as targeted intervention crops suitable for populations with particular nutrient deficiencies(Reference Pérez-Massot, Banakar and Gómez-Galera13, Reference Berman, Zhu and Pérez-Massot48). However, many populations in developing country suffer from multiple nutrient deficiencies simultaneously, and in the future it will be necessary to develop staple crops providing the DRI for several vitamins and other nutrients in one variety (see the Multivitamin-enhanced crops section). Again, this is beyond the abilities of conventional breeding, since each additional nutritional trait would require individual selection followed by introgression into existing nutritionally enhanced locally adapted varieties. In contrast, it is possible to introduce multiple genes directly into local cultivars by genetic engineering in order to enhance different nutritional components simultaneously(Reference Naqvi, Zhu and Farré18). Only one such report has been published thus far, describing a genetically engineered maize line producing higher levels of ascorbic acid, β-carotene and folate(Reference Naqvi, Zhu and Farré18). This was achieved by combinatorial nuclear transformation with genes representing the three separate metabolic pathways(Reference Zhu, Naqvi and Breitenbach49). The adoption of staple crops producing higher levels of multiple nutrients will help to improve the health and well-being of the world's poorest individuals.
Hypervitaminosis
Tolerable upper limits have been established for most vitamins, including the four discussed in the present review (Table 1).
Water-soluble vitamins must be ingested daily because they cannot be stored in the body. Therefore, although tolerable upper limits have been proposed for ascorbic acid and folate, it is unusual to see clinical effects unless there is contraindication with metabolically related drugs. For example, the tolerable upper limit for ascorbic acid is 2000 mg/d, but an intake of >100 mg results in rapid excretion in the urine without harmful effects(Reference Cho, Ko and Cheong50, Reference Levine, Conry-Cantilena and Wang51). Similarly the tolerable upper limit for folate is 1 mg/d for adults, but there is no consensus about what blood concentrations of folate might cause harm(Reference Smith, Kim and Refsum52). In contrast, fat-soluble vitamins are stored in the body and overdosing can cause harmful effects. In adults, the tolerable upper limit for vitamin A is 3 mg/d, and clinical studies have shown that acute doses above this limit or chronic doses of 7·5–15 mg/d can induce anorexia, headaches, nausea and muscle weakness in the short term(Reference Hathcock, Hattan and Jenkins53) and osteoporosis in the long term(Reference Penniston and Tanumihardjo54). The tolerable upper limit for vitamin E in adults is 1 g/d, and although no harmful effects have been observed from acute doses of up to 3·2 g/d, overdosing can exacerbate the blood coagulation defect of vitamin K deficiency caused by malabsorption or anticoagulant therapy. High levels of vitamin E are therefore contraindicated in these subjects(Reference Bramley, Elmadfa and Kafatos55, Reference Kappus and Diplock56).
The concept of a tolerable upper level for vitamins is pertinent only for vitamin supplements because it is difficult to ingest toxic levels of vitamins with a natural diet, although excess levels of the fat-soluble vitamins can be reached through the overconsumption of highly fortified processed food(Reference Sizer, Sienkiewicz, Whitney, Sizer and Whitney57). However, nutritionally enhanced crops are subject to appropriate nutritional and feeding studies before commercialisation. All genetically engineered crops undergo rigorous risk assessment to ascertain their potential impact on human health and the environment before they receive market authorisation(58). In addition, genetically engineered crops must undergo toxicological analysis to demonstrate the absence of unintended effects using animal models (usually rodents) and this may be extended from acute tests to multigenerational chronic toxicity/reproductive tests on a case-by-case basis(59). The best-known example of a nutritionally enhanced crop is Golden Rice with the successful completion of multiple safety assessment in silico, in vitro, in animals and in human trials(Reference Tang, Qin and Dolnikowski60). Another more recent example is ‘multivitamin corn’, which was tested in animal feeding studies for sub-chronic toxicity. No indications of toxicity were evident compared with animals fed on control diets. The study thus confirmed that diets enriched with ‘multivitamin corn’ did not have any adverse effects on mice, did not induce any clinical signs of toxicity and did not contain known allergens(Reference Arjó, Capell and Matias-Guiu61).
Multivitamin-enhanced crops
The biofortification of local elite crop varieties with multiple vitamins will require a combination of breeding and genetic engineering. Breeding alone cannot be used to produce nutritionally complete crops because the selection process is too complex(Reference Naqvi, Zhu and Farré18, Reference Naqvi, Farré and Sanahuja62, Reference Naqvi, Zhu and Farré63). Thus far, one genetically engineered ‘multivitamin corn’ variety has been described(Reference Naqvi, Zhu and Farré18) and this was simultaneously enhanced for three different vitamins (ascorbic acid, β-carotene and folate). Online Supplementary Table S2 shows the percentage of DRI provided by the best-performing transgenic crops enhanced with the vitamins discussed in the present review. Ideally, it would be useful to combine these traits so that a single portion could meet the DRI for all vitamins, for example, a transgenic tomato providing 950 μg of β-carotene/g fresh weight, 1159 μg of ascorbic acid/g fresh weight, 11·04 μg of folate/g fresh weight and 4·57 μg of α-tocopherol/g fresh weight (see online Supplementary Table S2). In maize, the best-performing transgenic lines developed thus far yield 59·3 μg of β-carotene/g dry weight, 344·75 μg of α-tocopherol/g dry weight, 1·94 μg of folate/g dry weight and 168 μg of ascorbic acid/g dry weight.
Processing of biofortified crops
Few studies have considered the bioavailability of β-carotene in genetically engineered staple crops after processing. In the case of Golden Rice, which accumulates 31 μg/g dry weight of grain by overexpressing the enzymes phytoene synthase (PSY1) and carotene desaturase to remove an early bottleneck in the carotenoid biosynthesis pathway(Reference Paine, Shipton and Chaggar17), the total amount of β-carotene was found to be the same before and after cooking(Reference Tang, Qin and Dolnikowski60). A small-scale study with Golden Rice fed to US adults showed efficient bioconversion of β-carotene to vitamin A(Reference Tang, Qin and Dolnikowski60). The study indicated that 3·8 μg β-carotene delivered as Golden Rice provides 1 μg retinol. The amount of β-carotene in Golden Rice is 20–30 μg/g uncooked rice; consequently 100 g uncooked rice provides 500–800 μg retinol(Reference Tang, Qin and Dolnikowski60). Another trial compared the vitamin A value of Golden Rice with that of spinach and pure β-carotene in oil, in schoolchildren of marginal or normal vitamin A status(Reference Tang, Hu and Yin64). The study concluded that β-carotene in Golden Rice is as effective as pure β-carotene in oil and better than that in spinach at providing vitamin A to children(Reference Tang, Hu and Yin64).
In transgenic potatoes expressing the Orange (Or) gene, which leads to the induction of chromoplast development thus creating a metabolic sink for carotenoids, the levels of β-carotene were five-fold higher than in normal potatoes at harvest, i.e. 5 μg β-carotene/g dry tuber weight(Reference López, Van Eck and Conlin65), but this increased to 10-fold higher when the tubers were stored at 5°C for 6 months(Reference Li, Yang and Xu66).
The impact of processing on nutritionally enhanced foods can be predicted by extrapolating the effect of processing on normal foods, i.e. the percentage loss or gain of bioavailable vitamin resulting from different processing methods. For example, baking tomatoes at 160°C for 20 min increases the bioavailable β-carotene content by 81 %(Reference Hwang, Stacewicz-Sapuntzakis and Bowen67) whereas canned tomato juice contains 20 % less β-carotene than fresh tomatoes(Reference Dietz and Gould68). Therefore, if we take the best-performing genetically enhanced tomato line, which produces 205 μg of β-carotene/g in the fresh tomato fruits(Reference D'Ambrosio, Giorio and Marino41), we can predict that a reasonable portion of tomatoes (150 g/d) would provide 285 % of the DRI for vitamin A when consumed as fresh fruit, 516 % when consumed after baking and 222 % as processed tomato juice (see online Supplementary Table S1).
Similarly, β-carotene levels in carrots fall by 16 and 59 % after conventional cooking for 10 and 60 min, respectively(Reference Gayathri, Platel and Prakash24, Reference Nagra and Khan69), and by 27 % after pressure-cooking for 10 min(Reference Howard, Wong and Perry22). Puréeing reduces β-carotene levels by 56 %(Reference Olunlesi and Lee70) and carrot juice contains 23 % less β-carotene than fresh carrots(Reference Kim and Gerber71). Therefore, the best transgenic carrot variety, which produces 39 μg of β-carotene/g fresh weight(Reference Jayaraj, Devlin and Punja72), would achieve 54 % of the DRI for vitamin A if 150 g/d were consumed as raw vegetables and 22 % if cooked for 60 min, compared with conventional carrots which achieve 0·2 or 0·09 % if consumed raw or cooked for 60 min, respectively (see online Supplementary Table S1).
The highest level of vitamin E produced thus far in transgenic tomatoes is 4·57 μg/g fresh weight, which is equivalent to 5 % of the DRI assuming a portion of 150 g/d(Reference Seo, Kim and Harn44). Transgenic lettuce plants have been produced yielding up to 64·55 μg of vitamin E/g fresh weight, which assuming the same portion size would be equivalent to 64 % of the DRI(Reference Li, Wang and Hou73). Food processing has a small but significant impact on vitamin E levels; for example, microwaving reduces overall vitamin E levels in soyabean oil by 10 %(Reference Yoshida, Hirooka and Kajimoto74) and cooking for 165 min reduces vitamin E levels by 8 %(Reference Masková, Rysová and Fiedlerová75).
The impact of processing on water-soluble vitamins is more potent. Transgenic tomatoes, lettuce and strawberries have been developed containing up to 1159, 792 and 1303 μg of vitamin C/g fresh weight, respectively(Reference Haroldsen, Chi-Ham and Kulkarni42, Reference Jain and Nessler76, Reference Bulley, Wright and Rommens77). These values represent 193, 133 and 217 % of the DRI for vitamin C assuming a daily portion of 150 g (see online Supplementary Table S1). However, up to 89 % of the vitamin C in tomatoes is lost by cooking, reducing its nutritional value to 21 % of the DRI(Reference Alvi, Khan and Sheikh78). Transgenic potatoes have been produced yielding 915 μg of vitamin C/g fresh weight, which is equivalent to 133 % of the DRI for vitamin C assuming a daily portion of 130 g(Reference Qin, Shi and Yu79). However, vitamin C is particularly susceptible during wet cooking methods and 20–40 % is lost; even dry cooking methods such as microwaving reduce vitamin C levels by 8–17 %(Reference Golaszewska and Zalewski80). Therefore, if the transgenic potatoes described above were prepared by conventional cooking, pressure-cooking or microwaving, the vitamin C content of a daily portion would be reduced to 80, 90 and 110 % of the DRI, respectively (see online Supplementary Table S1).
Transgenic tomato and lettuce plants have been produced yielding 10 and 1·89 μg of folate/g fresh weight, respectively(Reference Díaz de la Garza, Gregory and Handson43, Reference Nunes, Kalkmann and Aragão81). Although, as discussed above, folate is lost by leaching during processing, both tomatoes and lettuce tend to be eaten fresh and portions of 150 g/d would achieve 380 and 71 % of the DRI, respectively (see online Supplementary Table S1).
Maize is a particularly interesting example to investigate because in this case it is possible to predict the nutritional values of three different vitamins in one meal of 100–200 g of grain (a typical portion). The carotenoid content of maize is reduced by more than 50 % by lime-cooking(Reference De la Parra, Serna Saldivar and Hai Liu82), which means that a portion of ‘multivitamin corn’(Reference Naqvi, Zhu and Farré18) would provide 35 % of the DRI for vitamin A when prepared in this manner. ‘Multivitamin corn’ also yields 106 μg of vitamin C/g dry weight and 1·94 μg of folate/g dry weight(Reference Naqvi, Zhu and Farré18) but there are no data in the literature to show how these levels would be affected by storage, processing and cooking.
Efficiency of vitamin absorption
Fat-soluble vitamins
The absorption, transport and distribution of vitamins A and E within the body are linked to the intake of dietary fat(Reference Li and Tso83, Reference Jeanes, Hall and Ellard84). The amount of fat ingested in the meal plays a crucial role because it may promote carotenoid absorption by stimulating bile secretion and increasing the luminal concentration of bile salts, which act as surfactants in the formation of mixed micelles(Reference Hofmann85). The bioavailability of vitamin E also depends on the amount of fat ingested with the meal(Reference Lodge86). There is no specific recommended dose of fat to promote vitamin adsorption(Reference Jeanes, Hall and Ellard84) and dietary compounds that inhibit fat absorption and facilitate its excretion from the body such as plant fibres and sterols have the opposite effect on vitamin bioavailability(Reference Yonekura and Nagao87).
During absorption, the first step is the release of vitamins from the food matrix, which is strongly influenced by different food processing techniques as discussed earlier. The type of food and the subcellular compartment containing the vitamin also has an impact, particularly in the case of β-carotene: plasma retinol levels are up to six times higher following the consumption of fruits compared with leafy vegetables containing the same overall amount of β-carotene, suggesting that it is released more easily from chromoplasts than chloroplasts(Reference De Pee, West and Permaesih88). The molecular structure of each type of vitamin influences its absorption. Hydrocarbon-rich carotenoids such as β-carotene are less bioavailable than oxygenated carotenoids (for example, lutein) because the latter are easily incorporated into the outer portions of lipid micelles within the gastrointestinal tract and by enterocyte membranes(Reference Van het Hof, Brouwer and West89). In the case of tocopherols, all isomers can be absorbed equally during digestion(Reference Traber and Sies90) but once chylomicrons reach the liver, the hepatic α-tocopherol transfer protein preferentially retains α-tocopherol, making it the most important isomer in terms of vitamin E activity(Reference Traber and Arai91).
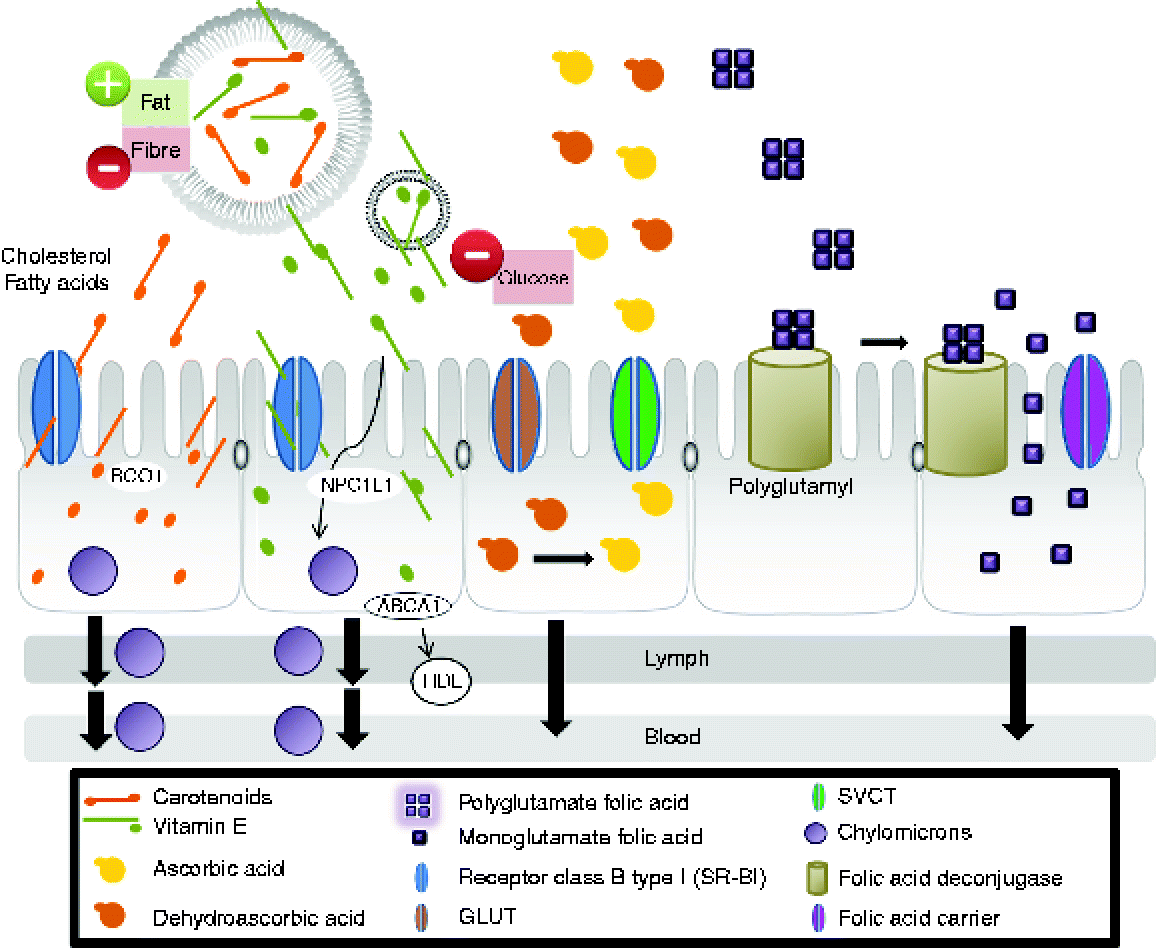
Vitamin A activity in the diet derives from two sources: preformed vitamin A as retinyl esters in foods of animal origin and provitamin A carotenoids, such as β-carotene, α-carotene and β-cryptoxanthin, found in plant-derived foods(Reference Harrison92). In vivo studies showed that intestinal absorption of carotenoids involves several crucial steps: release from the food matrix, solubilisation into mixed lipid micelles in the lumen, cellular uptake by intestinal mucosal cells(Reference Harrison92–Reference During and Harrison97), incorporation into chylomicrons and secretion of carotenoids and their metabolites associated with chylomicrons into the lymph(Reference Harrison92) (Fig. 1). Intestinal cells take up carotenoids by facilitated processes involving scavenger receptor class B (SR-BI)(Reference Harrison92, Reference Lobo, Hessel and Eichinger93). This receptor facilitates the intestinal absorption not only of β-carotene(Reference Van Bennekum, Werder and Thuahnai94), but also of fatty acids, cholesterol, vitamin E and non-provitamin A carotenoids(Reference Reboul, Klein and Bietrix95–Reference During and Harrison97). Studies in mice have shown that elevated intestinal SR-BI levels accompany enhanced absorption of cholesterol and fatty acids(Reference Bietrix, Yan and Nauze96).

Fig. 1 Mechanisms for the absorption of carotenoids, vitamin E, vitamin C and folate in the human body (modified substantially from Yonekura & Nagao(Reference Yonekura and Nagao103)). The first step is the release of vitamins from the food matrix. Dietary provitamin A carotenoids (for example, β-carotene) and other carotenoids are taken up by intestinal cells by processes involving scavenger receptor class B type I (SR-BI)(Reference Harrison92, Reference Lobo, Hessel and Eichinger93). SR-BI facilitates the intestinal absorption not only of β-carotene(Reference Van Bennekum, Werder and Thuahnai94), but also of fatty acids, cholesterol, vitamin E and non-provitamin A carotenoids(Reference Reboul, Klein and Bietrix95–Reference During and Harrison97). Thus, β-carotene is partially converted to retinol by β-carotene oxygenase 1 (BCO1) and retinal reductase(Reference Lobo, Hessel and Eichinger93). The retinol so formed is then metabolised in the same manner as that originating from preformed vitamin A. The intact carotenoids are incorporated into nascent chylomicrons. Chylomicrons containing newly absorbed retinyl esters (from animal origin) and carotenoids (from plant-derived food) are then secreted into the lymph. Vitamin E is solubilised in micelles and also is distributed between different lipid structures (vesicles). Absorption mechanisms are affected by the structure with which vitamin E is associated. Intestinal absorption of vitamin E was assumed to occur by passive diffusion: the transport of vitamin E was found to be non-ATP-dependent and also involve a membrane protein in the cellular uptake of vitamin E, SR-BI(Reference Voolstra, Kiefer and Hoehne99). More recent studies demonstrated that Niemann-Pick C1-like 1 (NPC1L1) protein is involved in vitamin E uptake at the apical membrane of the intestinal cell. Another protein, ATP binding cassette, subfamily A (ABCA1), is involved in vitamin E secretion at the basolateral side(Reference Takada and Suzuki100–Reference Oram, Vaughan and Stocker102). α-Tocopherol is secreted by ABCA1 that transports cellular cholesterol and phospholipids to lipid-poor HDL into the cells(Reference Oram, Vaughan and Stocker102). In the intestinal cells, the main fraction is secreted into chylomicrons which are released from enterocytes into the lymphatic system and later the circulation en route to the liver(Reference Takada and Suzuki100). Both forms of vitamin C are absorbed by enterocytes and transported to target cells(Reference Malo and Wilson106). Ascorbic acid is absorbed via Na-dependent vitamin C transporter (SVCT) while dehydroascorbic acid is absorbed via facilitative GLUT transporters. Polyglutamyl folates in the diet must be deconjugated by enzymes tethered to the intestinal epithelium before absorption(Reference Gregory, Williamson and Liao113). (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr).
Vitamin E is not only solubilised in micelles but it is also distributed among different lipid structures (vesicles) in the intestinal lumen during digestion(Reference Borel, Preveraud and Desmarchelier98). Absorption mechanisms seem to be affected by the cellular structure(s) with which vitamin E is associated: micelles may interact with proteins located in membranes, while vesicles might have a different transport pathway(Reference Borel, Preveraud and Desmarchelier98). Intestinal absorption of vitamin E was assumed to occur by passive diffusion involving different proteins (Fig. 1)(Reference Voolstra, Kiefer and Hoehne99–Reference Oram, Vaughan and Stocker102). The efficiency of vitamin E absorption is maximal where the intestinal transporters of vitamin E are most highly expressed. In the intestinal cells, the main fraction is secreted into chylomicrons which are released from enterocytes into the lymphatic system and later the circulation en route to the liver(Reference Yonekura and Nagao103) (Fig. 1). Fat-soluble vitamins become associated with fat-transport proteins such as the various lipoprotein receptors that facilitate their distribution around the body(Reference Harrison92, Reference Borel, Preveraud and Desmarchelier98).
Water-soluble vitamins
Unlike fat-soluble vitamins, water-soluble vitamins must be ingested daily as they cannot be stored in the body. Although ascorbic acid is the principal active form of vitamin C, dehydroascorbic acid also has some activity and the two can easily be interconverted so it is important to consider them both in bioavailability studies(Reference Lee and Kader104). The different forms of vitamin C are absorbed by enterocytes via Na-dependent transporters (reflecting the differential concentration of Na ions across the plasma membrane, maintained by Na+/K+-ATPases) or via facilitated diffusion mediated by GLUT transporters, although high intracellular glucose levels can inhibit this process(Reference Corti, Casini and Pompella105). Within the enterocyte, dehydroascorbic acid is reduced to ascorbic acid (Fig. 1), which leaves the cell at the basal membrane by an unknown mechanism(Reference Malo and Wilson106). Ascorbic acid is taken up into target cells via specific transporters(Reference Malo and Wilson106). Ascorbic acid can also be reabsorbed by renal tube cells(Reference Carlsson, Wiklund and Engstrand107).
Folate is generally presented in the diet as polyglutamyl folate, which must be deconjugated by enzymes tethered to the intestinal epithelium before absorption by passive diffusion (if present at concentrations greater than 5–10 mmol/l) or otherwise by a concentration-dependent specific transport process(Reference Gregory108) (Fig. 1). The monoglutamyl:polyglutamyl folate ratio in the diet appears to have no influence on the efficiency of absorption, suggesting that the enzymic deconjugation is not a rate-limiting step(Reference McKillop, McNulty and Scott109). However, the entrapment of folate in food matrices can influence bioavailability by rendering it inaccessible to the epithelial enzymes(Reference Storozhenko, Navarrete and Ravanel110). In this context, the consumption of a folate tracer with a light breakfast meal reduced bioavailability by 15 % compared with the folate tracer alone(Reference Pteiffer, Rogers and Bailey111). Folate bioavailability can be increased by enhancing the activity of γ-glutamyl hydrolase in plants, as this would be released from the vacuole following maceration and would promote the release of unconjugated folate from the food matrix before ingestion(Reference Storozhenko, Navarrete and Ravanel110).
Folate is also absorbed in the colon, which is a site of folate synthesis by gut bacteria(Reference Aufreiter, Gregory and Pfeiffer112). Absorbed folate is transported to the liver, which contains approximately half the folate pool in the body(Reference Gregory, Williamson and Liao113) and retains 10–20 % of absorbed folate due to the first-pass effect(Reference Gregory and Bailey114). The remainder is transported through the vasculature to the rest of the body. Some liver folate participates in the enterohepatic circulation and is secreted into the bile(Reference Herbert115).
Conclusions and perspectives
Vitamins are essential nutrients that play specific roles in metabolism and a DRI level has been established to promote nutritional health and reduce the likelihood of micronutrient deficiency(Reference Lešková, Kubíková and Kováčiková34). Fruits and vegetables provide adequate sources of vitamins, but large parts of the developing world have insufficient access to such foods and rely instead on a monotonous cereal-based diet(Reference Farré, Ramessar and Twyman15). Although nutritional health can be maintained by the provision of supplements or fortified processed foods, these are expensive and unsustainable practices that require government-level intervention and continuous funding. Biofortification is an alternative strategy that is sustainable because the vitamins are produced at source by the crops, thus allowing the distribution of nutritionally enhanced staple foods via established food distribution networks(Reference Gómez-Galera, Rojas and Sudhakar4, Reference Farré, Sanahuja and Naqvi116).
Although there has been recent progress in the development of genetically engineered biofortified crops, most studies have focused on the total vitamin content of the crops and have overlooked the impact of bioavailability which determines whether such crops could achieve the DRI for each vitamin in a reasonable portion. The bioavailable amount of vitamins depends on the food matrix, the processing and cooking methods, and co-dietary factors. Different food preparation methods can reduce the total levels of vitamins by thermal degradation and/or leaching, but may also increase vitamin bioavailability by partially degrading the food matrix and therefore improving accessibility(Reference Lešková, Kubíková and Kováčiková34).
We have considered the overall improvement in vitamin levels achieved by genetic engineering and compared these with DRI values for fresh produce and portions prepared using different cooking methods. This is an important element in the development of nutritionally enhanced staple crops targeting specific developing country populations because the value of such products would depend on the inclusion of traditional cooking methods when calculating the impact on DRI. Our data suggest that most of the nutritionally enhanced crops developed thus far provide an improvement over conventional crops in terms of DRI even after food processing (see online Supplementary Table S2; see the Multivitamin-enhanced crops section) but their efficacy (biological impact under controlled conditions) and effectiveness (biological impact in real life) need to be evaluated further.
From an economic perspective, biofortified crops are likely to be more cost-effective than supplementation or conventional fortification(Reference Gómez-Galera, Rojas and Sudhakar4, Reference Qaim117). However, this will only be the case if such crops achieve the DRI for target vitamins in a reasonable portion, otherwise supplementation and conventional fortification will need to be implemented alongside in order to address concurrent micronutrient deficiencies. If these conditions can be met, then nutritionally enhanced crops will provide a sustainable and economically viable approach to address the challenge of micronutrient deficiency in developing countries(Reference Stein, Sachdev and Qaim118).
Further studies will be required to determine whether biofortified crops maintain their enhanced nutritional value in local agricultural environments. The varieties must also perform well in terms of yield and pest resistance in order to perform well economically and therefore gain acceptance from farmers and consumers. The introduction of new varieties must take into account the potential impact on local markets and on the local agricultural system, because biofortified varieties may attract a market premium that could encourage farmers to adopt the biofortified variety as a marketable commodity. The development of a viable and sustainable humanitarian product must not be derailed by poor economic performance and negative public perception.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0954422413000176
Acknowledgements
Research at the Universitat de Lleida is supported by MICINN (Ministerio de Ciencia e Innovacion), Spain (BIO2011-23324; BIO02011-22525; PIM2010PKB-00746); European Union Framework 7 Program-SmartCell Integrated Project 222716; European Union Framework 7 European Research Council IDEAS Advanced Grant (to P. C.) Program-BIOFORCE; COST Action FA0804: Molecular farming: plants as a production platform for high value proteins; Centre CONSOLIDER on Agrigenomics funded by MICINN, Spain and RecerCaixa.
P. C., T. C. and C. Z. conceived of and designed the review. All authors wrote the paper.
The authors declare that there are no conflicts of interest.