Introduction
The most south-western département of mainland France, the Pyrénées Atlantiques, is the country's second largest dairy sheep production area after the Roquefort perimeter. It is where the Protected Designation of Origin (PDO) European labelled cheese Ossau Iraty is made, using milk from 3 local breads: Manech Tête Rousse, Manech Tête Noire and Basco Bearnais sheep. Sheep breeding and grazing for cheese making is a vital part of the local culture and economy, so much so that it has been included in the requirements for the production of PDO Ossau Iraty: ‘ewes should graze for at least 240 days per lactation period’ (INAO, 2015). Locally, the climate is oceanic, with mild temperatures year long and is one of the most humid parts of mainland France, receiving about 1300–1600 mm of rain per year (Meteo France). In this setting, sheep are frequently infected with heavy loads of gastrointestinal nematodes (GIN) when grazing, and farmers have to deal with the challenge of trying to control the parasite load almost all year long. Parasite control has relied on benzimidazoles for several decades, but the use of this family of molecules diminished with the increasing appearance of resistant GIN strains (Geurden et al., Reference Geurden, Hoste, Jacquiet, Traversa, Sotiraki, Frangipane di Regalbono, Tzanidakis, Kostopoulou, Gaillac, Privat, Giangaspero, Zanardello, Noé, Vanimisetti and Bartram2014; Rose Vineer et al., Reference Rose Vineer, Morgan, Hertzberg, Bartley, Bosco, Charlier, Chartier, Claerebout, de Waal, Hendrickx, Hinney, Höglund, Ježek, Kašný, Keane, Martínez-Valladares, Mateus, McIntyre, Mickiewicz, Munoz, Phythian, Ploeger, Rataj, Skuce, Simin, Sotiraki, Spinu, Stuen, Thamsborg, Vadlejch, Varady, von Samson-Himmelstjerna and Rinaldi2020) and when the milk withdrawal period changed from zero to at least 4 days in 2014 (Zoetis France, 2009), using these molecules during the lactation period was no longer a financially sound option.
Consequently, the macrocyclic lactone (ML) eprinomectin (EPN) has become the main treatment option during lactation: it has a very low blood to milk partition (Imperiale and Lanusse, Reference Imperiale and Lanusse2021), making it the only available molecule in France with a zero milk withdrawal period. First commercialized for cattle as a topical formulation in 1996, and later on as an injectable formulation in 2015 in France, it was not until 2016 and 2020 that EPN was approved for small ruminants, for the topical (HPRA, 2016) and injectable formula, respectively (HPRA, 2020). Before 2016, the topical formulation was administered to dairy sheep and goats off label. Ineffectiveness of the topical formula was rapidly reported in goats on the basis of fecal egg count reduction tests (FECRT) (Murri et al., Reference Murri, Knubben-Schweizer, Torgerson and Hertzberg2014), which prompted veterinarians to use EPN via other routes of administration that are known to be associated with higher overall exposure in plasma and tissues and potentially higher efficacy than topical administration (Lespine et al., Reference Lespine, Chartier, Hoste and Alvinerie2012). Lack of efficacy of the topical formula has also been recently described in dairy sheep (Bouy et al., Reference Bouy, Fito-Boncompte, Harinck, Lukkes and Heckendorn2021; Bordes et al., Reference Bordes, Ticoulet, Sutra, Lespine and Jacquiet2022).
Routinely, anthelmintic treatments are administered to the whole lactating flock, usually at a fixed time of the year determined by habit, production stage and/or season of the year. Animals are treated against GIN infections 3–4 times a year on average, using mainly molecules from the ML family (Centre Départemental de l'Elevage Ovin, unpublished data). Together with inaccurate animal weight measurements (underdosing), high frequency of treatment has been proven to be one of the main drivers of anthelmintic resistance (AR) (Wolstenholme et al., Reference Wolstenholme, Fairweather, Prichard, von Samson-Himmelstjerna and Sangster2004; Falzon et al., Reference Falzon, O'Neill, Menzies, Peregrine, Jones-Bitton, vanLeeuwen and Mederos2014; Sangster et al., Reference Sangster, Cowling and Woodgate2018) and loss of efficacy of EPN was expected to happen in the Pyrénées Atlantiques sooner or later. From 2018, veterinarians first reported loss of efficacy of avermectins: benzimidazole/ivermectin multi-resistant isolates of Haemonchus contortus have been isolated from an ovine meat production farm in the Hautes Pyrénées (Cazajous et al., Reference Cazajous, Prevot, Kerbiriou, Milhes, Grisez, Tropee, Godart, Aragon and Jacquiet2018) and benzimidazole/EPN multi-resistant isolates of the parasite were identified in a dairy goat herd in the Pyrénées Atlantiques (Bordes et al., Reference Bordes, Dumont, Lespine, Souil, Sutra, Prévot, Grisez, Romanos, Dailledouze and Jacquiet2020). The implication of this resistance motivated the creation of a 3-year long project, ANTHERIN for ANTHelmintic Resistance in dairy sheep farms: survey and INnovative solutions. The results presented in this study are linked to this project.
Of the 3 main pathogenic species for sheep and goats, H. contortus is the most pathogenic and prolific (Arsenopoulos et al., Reference Arsenopoulos, Fthenakis, Katsarou and Papadopoulos2021). It has probably spread across the globe thanks to commercial activities, and has been able to adapt to different climates (Sallé et al., Reference Sallé, Doyle, Cortet, Cabaret, Berriman, Holroyd and Cotton2019), yet its development remains conditioned by external temperatures and humidity (O'Connor et al., Reference O'Connor, Walkden-Brown and Kahn2006; Arsenopoulos et al., Reference Arsenopoulos, Fthenakis, Katsarou and Papadopoulos2021). Haemonchus contortus has also been capable of adapting to anthelmintic treatment, and to this day resistance to all major anthelmintic families have been described (Kotze and Prichard, Reference Kotze and Prichard2016). Adult worms being blood-sucking parasites, infection of sheep by H. contortus causes a range of symptoms depending on host susceptibility and parasite load, from loss of milk production and body condition, to anaemia and death (Arsenopoulos et al., Reference Arsenopoulos, Fthenakis, Katsarou and Papadopoulos2021).
This study reports for the first time EPN resistance of H. contortus in 5 dairy sheep farms in France, investigated between June 2020 and April 2021. In addition to anthelmintic efficacy measured by FECRT, concentrations of EPN were determined in sheep sera 2 and 5 days after treatment, to differentiate cases of loss of efficacy due to drug resistance from those linked to underexposure of GIN to EPN.
Materials and methods
Farm selection
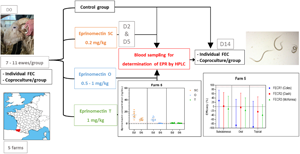
Five farms were included in the study based on suspicion of lack of efficacy of EPN in lactating dairy ewes by the veterinary practitioner. These suspicions emerged in February (farm 5), April (farms 1 and 4), June (farm 2) 2020 and April 2021 (farm 3), following oral or injectable EPN treatment. All farmers observed clinical signs compatible with strongylosis that did not improve after EPN treatment in lactating animals. Of these, 3 flocks had symptoms suggestive of haemonchosis (anaemia and on 2 farms mortality), and the remaining 2 flocks showed milk and weight losses. In all cases, the attending veterinarian did a fecal egg count (FEC) about 2 weeks after treatment that revealed the presence of strongyle eggs. Further investigation into the lack of efficacy was conducted to determine whether it was due to underexposure of the strongyles to EPN, or due to the presence of a resistant strain of worms. The 5 investigated farms had an average of 360 lactating ewes [215–500]. Four out of the 5 farms worked with Basco-Bearnaise sheep, and 1 with Manech Tête Rousse (farm 3). All 5 farms sent their lactating ewes in collective middle (1000 m) to high-altitude (⩾2000 m) summer pastures (at least 1 other sheep herd grazing in the same area).
On-farm protocol
Efficacy of EPN was evaluated using FECRT according to the World Association for the Advancement for Veterinary Parasitology (WAAVP) Guidelines (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992) in lactating dairy ewes. On-farm visits were done rapidly following suspicion, whenever possible. However, for farms 2 and 4, the first Covid-19 lockdown in France caused a 2-month delay between suspicion and visit. On 1 farm (farm 2), the lactating ewes were not available (e.g. they were already grazing in high-altitude summer pastures) and due to the emergency of the situation, FECRT was conducted on ewe lambs. The animals were randomly allocated to 4 groups of 10–11 animals according to their age to compose homogenous groups representative of the herd or the age group. A control group was left untreated, 1 group received injectable EPN (0.2 mg kg−1 of LBW, Eprecis®injectable, CEVA Santé Animale, Libourne, France; further referred to as the ‘SC group’) and another group received a topical ‘Pour-On’ form of EPN (1 mg kg−1 of LBW, Eprinex Multi®, Boehringer Ingelheim, Lyon, France; ‘T group’) according to the manufacturer's indication. The last group received EPN orally, using the topical formula (Eprinex Multi®, Boehringer Ingelheim; ‘O group’) off label and at the dose of 0.5 mg kg−1 of body weight (Badie et al., Reference Badie, Lespine, Devos, Sutra and Chartier2015). All animals were treated with a dose rate of 80 kg, which is heavier than the heaviest animal of the group, the 2 encountered breed weights being on average 55–60 kg for female individuals. For the topical treatment, wool was carefully parted so as to apply the solution as well as possible on the skin. For the oral drench, a graduated single-use syringe was used and the absence of regurgitation was verified after treatment. Groups were marked according to their treatment regimen. Feces was collected individually from all animals, samples were identified using the animals' tag 5 digit number and treatment was administered to the pre-defined groups. Fourteen days after treatment, feces was collected individually from all groups. Animals with no fecal samples collected on day 14 after treatment were excluded from the study.
FECRT
Individual FECs were conducted using the modified McMaster method with a sensitivity of 15 eggs per gram (EpG) (Raynaud et al., Reference Raynaud, William and Brunault1970) within a maximum of 48 h after sampling. Animals for which no strongyle eggs were detected at D0 or animals for which no feces was collected post-treatment were excluded from the study. Once the FEC for the D0 samples were established, the mean FEC was calculated per group, as described by Coles et al. (Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992). Only groups for which the mean FEC was greater than 300 EPG were included in the study (Cabaret and Berrag, Reference Cabaret and Berrag2004).
Fecal egg count reduction (FECR) and 95% confidence intervals (CIs) were calculated using 3 different formulas, as follows (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992; Dash et al., Reference Dash, Hall and Barger1988; McKenna, Reference McKenna2006):
where EpGT1 and EpGT2 are the arithmetic means of FEC in a treated group at D0 and D14, respectively, and EpGC1 and EpGC2 are the arithmetic means of FEC in the control group, at D0 and D14, respectively.
CIs for these 3 formulas were calculated according to the methods described by Coles et al. (FECR1) (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992) and by Lyndal-Murphy et al. (FECR2 and FECR3) (Lyndal-Murphy et al., Reference Lyndal-Murphy, Swain and Pepper2014).
The results were interpreted as described in Table 1.
Table 1. Interpretation guide for FECRT results (COMBAR, 2021)

Larvae collection
After FEC, at D0 and D14, stools were combined by group (control, SC, O, T) for fecal culture. Mixing was done so that when the remaining amount after FEC was sufficient, 3–5 g of feces from each animal was combined into the culture. The composite fecal cultures were then incubated for at least 12 days at 24 ± 1°C, and humidified every 2–3 days with tap water. For larvae collection, pots were filled to the brim with tap water and turned up-side down into Petri dishes, which were in turn filled with water. Larvae were collected twice at a 24 h interval in a volume of 40–45 mL and stored vertically at 4°C until DNA extraction (MAFF, 1986).
Larvae quantification and identification
The supernatant of the tubes stored at 4°C was discarded, and 5 mL of the pellet containing the larvae was kept for further analysis. Furthermore, 500 μL of the pellet was used for the DNA extraction, using the DNeasy PowerSoil kit (QIAGEN, Hilden, Germany). Molecular identification was then performed using a real-time polymerase chain reaction (PCR) according to Milhes et al. (Reference Milhes, Guillerm, Robin, Eichstadt, Roy, Grisez, Prévot, Liénard, Bouhsira, Franc and Jacquiet2017). Experiments were based on real-time PCR reactions, and standard curves for larval DNA quantitation were established for each PCR run and for 3 species H. contortus, Teladorsagia circumcincta and Trichostrongylus colubriformis.
EPN analysis in sheep serum
Blood samples were collected 2 and 5 days post-EPN treatment, in dry tubes from the jugular vein of all treated animals. Blood samples were centrifuged at 3500 rpm for 10 min. Serum was collected and stored at −20°C until further analysis.
After extraction from serum with acetonitrile, EPN concentration was measured using high-performance liquid chromatography with fluorescent detection, as previously described by Sutra et al. (Reference Sutra, Galtier, Alvinerie and Chartier1998). The quantification limit of the method was 0.07 ng mL−1, and the inter-assay coefficient of variation was lower than 5%.
Statistical analysis
Graphs were executed using GraphPad Prism version for Windows, GraphPad Software, San Diego, California, USA. Statistical analyses were conducted using R [version 4.1.1 (2021-08-10)] and RStudio version 1.4.1106 (RStudio Team, 2021). Mean EPN concentrations were compared for different treatment regimens within farms using a non-parametric Wilcoxon test.
Results
FECRT
Average FEC on D0 in every group and on each farm were above 300 EpG. On farms 2–5, after withdrawing animals not responding to inclusion criteria detailed in ‘FECRT’ section of Materials and methods, 7–11 animals remained per group. At the moment of testing, farm 1 had started the transfer of some animals to summer pastures. The number of animals remaining on the farm was sufficient for 3 groups of 8 lactating ewes (control, treated by subcutaneous and with the topical route) (Table 2).
Table 2. Mean FEC results, with minimal and maximal individual value and final number of animals included, per group and per farm

In bold, values of mean and minimum–maximum FEC on day 0, for an easier quick reading.
1 On farm 2, FECRT were conducted on ewe lambs.
Calculated FECR are presented in Fig. 1. Depending on the farm, the group and the formula used, FECR results varied widely. All values of FECR were lower than 95%, except for FECR3 for group O of farm 4 and all lower level CIs were inferior to 90%. With the exception of FECR3 of group O for farm 4 (97%), these criteria indicate reduced efficacy for all 5 farms. Regarding group O of farm 4, interpretation would have been that efficacy of EPN was doubtful. However, given results obtained with 2 other formulas, including FECR1 recommended by the WAAVP guidelines, efficacy for group O of farm 4 is clearly reduced.

Fig. 1. FECR results and confidence intervals for the 5 farms, calculated according to 3 different formulas and for all treatment types.
Different FECR formulas yield different results, depending mainly on the value of the mean FEC of different groups. FECR1 and FECR3 yield similar results when the mean FEC of the control group on D14 is close to the mean FEC of treated groups on D0, as is the case on farm 2. On this farm, the FEC on D14 for the control group (3756 EpG) is not significantly different from FECs for the SC, O and T groups (respectively 4036, 1445 and 5072 EpG) on D0 (P value < 0.05). FECR1 formula that compares the FEC of treated and control groups on D14 yields similar percentages to FECR3, which compares FEC of treated group on D14 and D0, in this case (e.g. 87 and 88% reduction for the SC group with FECR1 and FECR3, respectively). On farm 4, percentages of fecal egg reduction differ between FECR1 and FECR3. There is a significant difference between the FEC of the control group on D0 (194 EpG) and of the treated groups on D14 (1813, 1986 and 1338 EpG for SC, O and T groups, respectively, P < 0.05). For example, considering the SC group on this farm, post-treatment fecal egg reduction is −25% using FECR1, yet it is 83% using FECR3.
Mean EPN concentrations in serum 2 days after administration were significantly higher after subcutaneous injection than after oral administration in 3 out of the 4 farms where both these routes were tested (Fig. 2; farms 2, 3 and 5; P value < 0.01). On farm 4, there was no significant difference between both routes. Topical administration of EPN resulted in dramatically low drug concentrations in the serum of ewes in all 5 farms (Fig. 2; P value < 0.01). Mean values were between 14.35 [s.d.: 4.43] and 27.84 ng mL−1 [s.d.: 6.37] for the SC group; 5.06 [s.d.: 5.71] and 25.14 ng mL−1 [s.d.: 9.74] for the O group and between 0.97 [s.d.: 0.54] and 6.92 ng mL−1 [s.d.: 4.2] for the T group, 2 days after treatment (Fig. 2). Five days after treatment, EPN serological concentrations were significantly higher after subcutaneous injection than after either of the other routes.

Fig. 2. Serological concentrations of EPN by farm, and by administration route. Horizontal lines indicate mean concentrations per treatment type and per day post-treatment. Black triangle: SC group; medium grey diamond: O group; light grey dot: T group. Red dotted line is at 2 ng mL−1. D2: 2 days after treatment; D5: 5 days after treatment. Individual concentrations and mean concentrations per group ± s.d. (7–11 animals per group).
For the T groups, the individual concentrations of EPN varied from being all below 2 ng mL−1 (farm 5) to all above (farm 1). On farms 2, 3 and 4, respectively, 44, 60 and 50% of individual EPN concentration values in the T group were below this concentration threshold (Fig. 2 and Supplementary data).
Strongyle species present pre- and post treatment
Before treatment, at D0, the strongyle species most present in the cultured feces of every farm was H. contortus. On farm 1, it was the only species of the 3 the qPCR could identify. On the 4 other farms, T. colubriformis and T. circumcincta were present in small proportions: T. colubriformis larvae composed at most 16.4% (on farm 3) of the larval culture yield (Table 3).
Table 3. Proportions (%) of the 3 main pathogenic species in bulk fecal cultures before (D0) and after (D14) for every group of the 5 farms

Hc, Haemonchus contortus; Te, Teladorsagia circumcincta; Tr, Trichostrongylus colubriformis; SC, subcutaneous EPN; O, oral drench EPN; T, topical EPN
aOn farm 2, FECRT were conducted on ewe lambs.
After treatment, H. contortus was the main species identified on the 5 farms (Table 3). For 4 farms out of 5, H. contortus was the only species remaining for the groups treated by sub-cutaneous injection. On farm 3, T. colubriformis larvae were also present in the cultures post injectable EPN treatment (Table 3). On farm 2, T. colubriformis larvae were the only species present post oral treatment, however in small quantities (Supplementary data, Table S8). Haemonchus contortus and T. colubriformis larvae were present in the post-treatment fecal cultures of the ewes treated with a topical solution, except in farm 1 where the collected larvae were 100% H. contortus.
Discussion
In this field study, EPN was found to have a reduced efficacy whatever the formula tested (SC, O, T) in 5 commercial dairy sheep farms where veterinarians and farmers suspected a lack of efficacy based on the persistence of clinical signs following oral or injectable treatment. Low drug efficacy was observed even in the SC administration group, which showed the highest drug levels in the hosts' serum.
We confirmed that EPN serum levels are highly dependent upon the route of administration. EPN concentration measured in the serum of treated lactating and pre-lactating ewes 2 days after treatment was well above the 2 ng mL−1 minimal efficacy concentration for all the animals treated with a subcutaneous or oral formula, which supports the idea that these ewes received a dose of the molecule that can be considered sufficient to kill strongyle adults. The poor FECRT performance obtained in these animals strongly supports the presence of strongyles resistant to EPN. Although no pharmacokinetic and pharmodynamic (PK/PD) study has been conducted to specifically identify the minimal EPN therapeutic dose, the minimal active dose for this drug family (e.g. ivermectin) has been shown to be above 2 ng mL−1 (Bousquet-Mélou et al., Reference Bousquet-Mélou, Jacquiet, Hoste, Clément, Bergeaud, Alvinerie and Toutain2011). This concentration is therefore considered a threshold that guaranties efficacy of EPN in small ruminants (Hoste et al., Reference Hoste, Lespine, Lemercier, Alvinerie, Jacquiet and Dorchies2004; Rostang et al., Reference Rostang, Devos and Chartier2020). The time at which EPN concentration reaches its highest averages between 1.2(±0.4) and 3.13(±2.99) days depending on dosage rate and physiology of the animals was considered (Imperiale et al., Reference Imperiale, Pis, Sallovitz, Lisfchitz, Busetti, Suárez and Lanusse2006; Hodošček et al., Reference Hodošček, Grabnar, Milčinski, Süssinger, Eržen, Zadnik, Pogačnik and Cerkvenik-Flajs2008; Hamel et al., Reference Hamel, Bosco, Rinaldi, Cringoli, Kaulfuß, Kellermann, Fischer, Wang, Kley, Mayr, Rauh, Visser, Wiefel, Fankhauser and Rehbein2017). Average serum EPN concentrations in the groups treated with a topical solution of EPN were low in most of the farms on D2. Differences in the breed and physiology of the animals could explain values below those found by Hamel et al. (Reference Hamel, Bosco, Rinaldi, Cringoli, Kaulfuß, Kellermann, Fischer, Wang, Kley, Mayr, Rauh, Visser, Wiefel, Fankhauser and Rehbein2017) in dry merino crossed sheep. In the present study, on all farms except farm 2, FECR tests were conducted on lactating ewes and in all farms animals bore a substantial worm burden, and both lactation and body condition have been shown to influence ML pharmacokinetic parameters (Lespine et al., Reference Lespine, Sutra, Dupuy, Alvinerie and Aumont2004, Reference Lespine, Chartier, Hoste and Alvinerie2012; Rostang et al., Reference Rostang, Devos and Chartier2020).
The purpose of this study was to provide reliable information about AR status in farms. We set up a feasible protocol to monitor drug efficacy through combining FECR and drug concentration monitoring in treated animals. Measuring concentrations at 2 critical times 2 days (close to maximal concentration) and 5 days (elimination phase), these data points allow simulation of the complete drugs' pharmacokinetics.
FECR values indicate a reduced efficacy with all 3 formulas and the low FECR after a topical treatment is due to the presence of resistant strongyle. However, cases of underexposure of GIN to EPN when using a topical formula have previously been reported by veterinary practitioners and confirmed by 2 recent studies in France. Bouy et al. and Bordes et al. described cases where FECR after EPN treatment were below 95% when using the topical solution and above 95% when animals of the same flock were treated with a subcutaneous solution (Bouy et al., Reference Bouy, Fito-Boncompte, Harinck, Lukkes and Heckendorn2021; Bordes et al., Reference Bordes, Ticoulet, Sutra, Lespine and Jacquiet2022). In the study by Bordes et al. (Reference Bordes, Ticoulet, Sutra, Lespine and Jacquiet2022) serum concentrations of EPN were below 2 ng mL−1 for all animals treated with a topical formula. These findings are in line with others (Hoste et al., Reference Hoste, Lespine, Lemercier, Alvinerie, Jacquiet and Dorchies2004; Hodošček et al., Reference Hodošček, Grabnar, Milčinski, Süssinger, Eržen, Zadnik, Pogačnik and Cerkvenik-Flajs2008) that underline the highly variable bioavailability of the topical formula of EPN. The use of topical route for EPN is therefore not recommended.
The significant difference between mean concentrations of EPN in SC and T groups is observed although the dose rate for the topical solution is 5 times higher than for the injection solution (1 mg kg−1 of LBW for the topical solution and 0.2 mg kg−1 LBW for the injection solution). Given the impact of ML on non-target species such as dung beetles, elimination of the molecule, through direct contact or by the fecal matters, could have an impact on pasture quality (Sands and Wall, Reference Sands and Wall2018; Verdú et al., Reference Verdú, Lobo, Sánchez-Piñero, Gallego, Numa, Lumaret, Cortez, Ortiz, Tonelli, García-Teba, Rey, Rodríguez and Durán2018; Weaving et al., Reference Weaving, Sands and Wall2020).
Upon communication of these results back to farmers and veterinarians, one of the challenges was explaining the discrepancy between the reduction calculated for the SC group and the one calculated for the oral drench group on the same farm at the same date, therefore with the same control group. Questions arose for these 2 treatment regimens although not for the topical formula group for the reasons explained above. Much has already been said about FECRT and their limits, and the debate remains open as summarized very recently by Morgan et al. (Reference Morgan, Lanusse, Rinaldi, Charlier and Vercruysse2022). However, our field experience teaches us that such differences need explanations, in order to be accepted by farmers and veterinarians. In our study, FECRT variations could hardly be explained by pharmacological factors, as EPN concentrations were well above 2 ng mL−1, a concentration at which the molecule is considered to be efficient (Guillot et al., Reference Guillot, Wright and Oehler1986; Guyonnet et al., Reference Guyonnet, Karembe, Magnier and Menudier2017). Although ewes of similar ages were evenly distributed between the SC and O group, differences in mean EPG partly explain the differences observed between the FECR of these 2 treatment formulas. Host factors, such as consistency of feces could contribute to variations in egg outputs and contribute to variations from one group to another, and host immunity could also be of importance for the inter-individual variations. Haemonchus contortus being the main strongyle species before and after treatment in all 5 farms, variations in FECR due to initial diversity of species is limited. Parasite fitness, including its fecundity, could add to the observed variations, although this should be limited by the fact that it is highly probable all sheep of the same flock harbour the same resistant isolate.
Identification of the 3 main pathogenic strongyle species for small ruminants was done using fecal cultures and qPCR. In all 5 farms, H. contortus was the main species present after treatment. Furthermore, resistance to EPR of isolates from farms 1 and 4 was confirmed by infestation and EPN challenge in experimental sheep (G. Salle, unpublished data). Interestingly, before treatment, H. contortus was predominant in the fecal culture yields of all 5 farms, and on farm 1 it was the only species present. These farms were investigated because EPN treatments were not resolving the observed symptoms, although the farmers had sometimes drenched the animals with EPN several times before calling their veterinarians to alert them to the problem. Our hypothesis is that these repeated treatments have exerted an important selective pressure upon the present worms, and cleared all susceptible populations. Farms 1, 4 and 5 were included in this study because during the spring of 2020 they were facing dire situations. On farms 2 and 3, health issues were sub-acute. Veterinarians knew the history of these 5 farms and that they are prone to facing GIN issues. This first report of EPN resistance in dairy sheep in this dairy region is a description of how resistance can manifest itself and how it has been investigated. This study was conducted to investigate issues raised by some farmers and veterinarians, and the aim was not to determine the prevalence of EPN resistance. Hence, to this date, it is not known if these cases are the tip of the iceberg regarding EPN resistance in the Pyrénées Atlantiques, and if they are, how big the iceberg is.
In post topical treatment coprocultures from farms 2 to 5, T. colubriformis and T. circumcincta (on farm 3) were also identified. Considering the EPR serum concentration for this treatment formula, we hypothesize these worms are still present because they have been under-exposed to the molecule. Furthermore, the intestinal species T. colubriformis has already been described as dose-limiting for EPN, its presence after topical treatment is not surprising (Chartier et al., Reference Chartier, Etter, Pors and Alvinerie1999; Hoste et al., Reference Hoste, Lespine, Lemercier, Alvinerie, Jacquiet and Dorchies2004).
In this southwestern area of France, resistance of H. contortus to EPN had previously been described in lactating goats (Bordes et al., Reference Bordes, Dumont, Lespine, Souil, Sutra, Prévot, Grisez, Romanos, Dailledouze and Jacquiet2020) and in sheep raised for meat (Cazajous et al., Reference Cazajous, Prevot, Kerbiriou, Milhes, Grisez, Tropee, Godart, Aragon and Jacquiet2018), but this is the first report of the presence of resistant strongyle strains in dairy sheep in the Pyrénées Atlantiques. Special attention has been brought to EPN resistance in this area, as well as in the Roquefort area, being the only molecule with a zero milk withdrawal period. Hence, EPN resistance means treating ewes against GIN during their lactation will inevitably come at the cost of, at least, throwing away milk during the withdrawal period. Farmers have to find other ways of controlling parasitism than solely relying on drugs, in order to keep their grazing flocks healthy while maintaining a decent level of production. The presence of resistant strains is also of concern here because of the use of collective pastures that could allow for the dissemination of resistant strains.
Resistance of GIN to ML molecules in France appeared relatively late, compared to other countries: Geurden et al. (Reference Geurden, Hoste, Jacquiet, Traversa, Sotiraki, Frangipane di Regalbono, Tzanidakis, Kostopoulou, Gaillac, Privat, Giangaspero, Zanardello, Noé, Vanimisetti and Bartram2014) and Paraud et al. (Reference Paraud, Pors, Rehby and Chartier2010) reported no resistance to ML in the 2 main dairy sheep regions and in dairy goats, respectively, and the first case of ivermectin resistance was described for T. circumcincta in meat sheep production in central France in 2014 (Paraud et al., Reference Paraud, Marcotty, Lespine, Sutra, Pors and Devos2016). These observations are in line with the general trend in Europe of increasing AR throughout anthelmintic families and GIN species (Rose Vineer et al., Reference Rose Vineer, Morgan, Hertzberg, Bartley, Bosco, Charlier, Chartier, Claerebout, de Waal, Hendrickx, Hinney, Höglund, Ježek, Kašný, Keane, Martínez-Valladares, Mateus, McIntyre, Mickiewicz, Munoz, Phythian, Ploeger, Rataj, Skuce, Simin, Sotiraki, Spinu, Stuen, Thamsborg, Vadlejch, Varady, von Samson-Himmelstjerna and Rinaldi2020). Of the 3 main pathogenic GIN species for sheep, H. contortus, originally a parasite of warm and humid climates (Sallé et al., Reference Sallé, Doyle, Cortet, Cabaret, Berriman, Holroyd and Cotton2019; Arsenopoulos et al., Reference Arsenopoulos, Fthenakis, Katsarou and Papadopoulos2021), profits from global warming and is reaching farther north in Europe and infection pressure expands during the year (Rose et al., Reference Rose, Caminade, Bolajoko, Phelan, Van Dijk, Baylis, Williams and Morgan2016). Although located in southern France, the area of interest for the present study harbours an oceanic climate with normally mild temperatures. In the last few years, it seems to be hosting more frequent cases of severe haemonchosis (P. Jacquiet, T. Cazajous, personal communication), in line with the rise of average temperatures. Four of the 5 cases of haemonchosis presented here happened after a particularly mild winter (MeteoFrance, 2020), which could have allowed an increased survival of H. contortus larvae on pastures. As well as adapting to climate, H. contortus has also been adapting to ML treatments, and in addition to the present study, resistance of the Barber Pole Worm has also been recently described in sheep in southwest England (Bull et al., Reference Bull, Glover, Rose Vineer and Morgan2022) and in goats in Austria (Hinney et al., Reference Hinney, Wiedermann, Kaiser, Krücken and Joachim2022).
Conclusion
The present study reports for the first time in France 5 cases of EPN-resistant H. contortus in dairy ewes in the south western département of the Pyrénées Atlantiques, the country's second largest sheep milk and cheese production area. These isolated cases are particularly worrying for the dairy sheep production in this area, where an important percentage of farms rely on summer pastures, usually shared, as part of their forage resource. However, they reflect what is happening elsewhere in Europe, in meat but also dairy sheep production. For milk-producing farms, EPN resistance in GIN does not come as a complete surprise, as only a very small pool of molecules is used for treatment. This study further confirms the variability of EPN serum concentration depending on administration route, with the injectable formula yielding the highest concentration post-treatment. The topical solution yields highly variable and sub-therapeutic EPN concentrations. Therefore, the use of topical EPN should be discouraged so as not to exacerbate the problem of resistance to ML and to better maintain good animal health. Dairy production adds an extra challenge to field management, yet some farmers have already found some encouraging, and hopefully durable, improvements to their system. For the farms not yet facing resistance of GIN to EPN, a simple and robust protocol to target and selectively treat lactating ewes to maintain a refuge population is in trial.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000069.
Data availability
Data supporting results are provided within the article and in the supplementary materials.
Acknowledgements
The authors are very grateful to the farmers who took part in this study, for their cooperation and for granting them access to their flocks.
Author's contributions
P. J. and A. L. conceived and designed the study. L. B., P. J., S. J., T. C., J. L., N. D., M. C., C. V.-N., M. D., M. A. and C. D. carried out the fieldwork. L. B., C. G., S. J., J. F. S., M. D., M. A. and C. D. carried out laboratory analysis. S. J., L. B., N. D., M. D., D. A., H. K., A. L. and P. J. wrote the article.
Financial support
Research presented in this study has received financial support from the Nouvelle Aquitaine Region ‘Paralut’ project, from the France Futur Elevage (F2E Institut-Carnot Santé Animale) ‘Antherin’ project and CEVA Santé Animale.
Conflict of interest
S. J., D. A. and H. K. are employees of CEVA Santé Animale. All work herein was conducted in academic settings, and the authors declare no conflict of interest regarding the present results.
Ethical standards
Stool and blood collection and anthelminthic treatments are a part of routine veterinary procedures without any traumatic method. Such procedures are not qualified as animal experimentation involving vertebrates according to French laws, so no specific ethical clearance was required.