Studies have suggested that vitamin D may increase bone mineral density and reduce fracture risk over a wide range of serum 25-hydroxyvitamin D (25(OH)D) concentrations. The serum concentration of 25(OH)D that would define adequate vitamin D status with respect to bone health is currently estimated to be at least 75 nmol/l( Reference Dawson-Hughes, Heaney and Holick 1 ). Data from observational studies suggest that serum 25(OH)D concentrations of at least 75 nmol/l may also be beneficial for other health outcomes such as colorectal and prostate cancer( Reference Giovannucci 2 , Reference Heaney 3 ), diabetes( Reference Pittas, Lau and Hu 4 ) and possibly gingivitis and periodontitis( Reference Dietrich, Joshipura and Dawson-Hughes 5 , Reference Dietrich, Nunn and Dawson-Hughes 6 ).
Unfortunately, a majority of the US population has vitamin D insufficiency with particular subgroups of the population at an even greater risk (e.g. the elderly and those with dark skin) even among otherwise healthy adults( Reference Newton, Sheltawy and Hay 7 , Reference Nesby-O'Dell, Scanlon and Cogswell 8 ). Moreover, considerable differences in 25(OH)D serum concentration exist between racial/ethnic groups in the USA, most strikingly between non-Hispanic whites and non-Hispanic blacks. In a representative survey of the US population, the third National Health and Nutrition Examination Survey (1988–1994) reported that significantly more non-Hispanic blacks had serum 25(OH)D concentration below 50 nmol/l than did non-Hispanic whites( Reference Looker, Dawson-Hughes and Calvo 9 ). Obesity may also play a key role in the association between vitamin D status and tooth loss. Non-Hispanic blacks and Mexican Americans exhibit a higher prevalence of obesity compared with non-Hispanic whites( Reference Flegal, Carroll and Ogden 10 ) and obesity has been shown to alter the production of 25(OH)D( Reference Wortsman, Matsuoka and Chen 11 ). These marked differences in vitamin D status may, in part, explain the racial/ethnic disparities in prevalence of periodontitis and tooth loss observed in the USA.
We hypothesized that lower serum concentrations of 25(OH)D would be associated with an increased rate of self-reported periodontitis and tooth loss. Therefore, we aimed to evaluate whether vitamin D status was associated with the incidence of tooth loss and self-reported periodontitis, using prospective data from the Health Professionals Follow-up Study (HPFS).
Methods
Study participants
The HPFS consists of 51 529 male health professionals aged 40–75 years, from across all US states and the District of Columbia, who completed a mailed questionnaire in 1986 after providing informed consent to participate( Reference Rimm, Stampfer and Colditz 12 ). The cohort was initiated to explore associations between diet, heart disease and cancer( Reference Rimm, Stampfer and Colditz 12 ). The initial questionnaire collected data regarding diet, lifestyle behaviours, physical characteristics (including height and weight), medication use, and medical and dental history. Follow-up questionnaires have been mailed biennially since 1988 collecting data on various exposures and outcomes and a detailed semi-quantitative FFQ has been mailed every 4 years. Over 90 % of the baseline population has responded to follow-up questionnaires( Reference Rimm, Stampfer and Giovannucci 13 ).
The HPFS was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board of Boston University Henry M. Goldman School of Dental Medicine and were in accordance with institutional guidelines. Written informed consent was obtained from all subjects/patients. The present analysis was approved by the Board for the Protection of Human Subjects at Boston University Henry M. Goldman School of Dental Medicine.
Participants were excluded from the tooth loss analysis if they were missing data on baseline number of teeth, did not wish to participate in the study, were edentulous at baseline, had a previous diagnosis of cancer at baseline, provided poor dietary data or were missing data on region of residence; additionally men were excluded at baseline due to missing data on smoking, BMI and physical activity but were allowed to re-enter analyses in later cycles, resulting in a final sample of 42 730 men. Men were additionally excluded from the periodontitis analysis if they reported a history of periodontitis at baseline, resulting in a final sample of 35 644.
Covariate ascertainment
The baseline and follow-up questionnaires collected detailed information on smoking status, including average number of cigarettes smoked per day, smoking duration and, for former smokers, time since cessation. These data were used to calculate a comprehensive smoking index (CSI) as previously described( Reference Dietrich and Hoffmann 14 , Reference Leffondre, Abrahamowicz and Xiao 15 ). Furthermore, data on dental profession, race and region of residence were collected at baseline and data regarding chewing tobacco use were collected in 1996. Data on routine physical examination in the last 2 years and pipe use were collected on each biennial questionnaire. Alcohol use, multivitamin use, vitamins E and C supplementation and dietary factors were assessed from a quadrennial semi-quantitative FFQ which has been validated against diet records with high validity( Reference Rimm, Giovannucci and Stampfer 16 , Reference Giovannucci, Colditz and Stampfer 17 ). Self-reported binary diabetes status has been collected on all biennial questionnaires and previously validated against medical records in a sub-sample of men demonstrating excellent validity, with 97 % of cases confirmed( Reference Hu, Leitzmann and Stampfer 18 ). Self-reported BMI was calculated from height (collected in 1986) and weight (all biennial questionnaires). Measures of BMI were validated against technician measurements among a subset of participants residing in the Boston area with high validity( Reference Rimm, Stampfer and Colditz 12 ). Physical activity was based on various self-reported activities (collected on all biennial questionnaires) and converted into metabolic equivalents per week (MET/week); it has been validated previously against past-week recalls and activity diaries in a subset of men with reasonable validity for leisure-time activity( Reference Chasan-Taber, Rimm and Stampfer 19 ).
Predicted 25(OH)D score
The derivation and validation of the multivariable prediction score used to estimate predicted 25(OH)D plasma concentration based on known predictors of circulating plasma 25(OH)D( Reference Giovannucci, Liu and Rimm 20 ), hereafter referred to as ‘predicted 25(OH)D score’, has been described in detail previously( Reference Giovannucci, Liu and Rimm 20 ). Briefly, the predicted 25(OH)D score was developed among a sub-sample of 1095 men from the HPFS who had plasma available and were free of cancer at the time of blood collection. Dietary and lifestyle factors were similar among men who provided blood samples and those who did not. Linear regression models were estimated between plasma 25(OH)D (dependent variable) and predictors of plasma 25(OH)D (independent variables), which were determined based on subject matter knowledge. A predicted 25(OH)D score was estimated for each participant based on estimated regression coefficients. The predictor score was estimated from each of the following components: leisure-time physical activity (quintiles of MET/week), BMI (<22·0, 22·0–24·9, 25·0–29·9, 30·0–34·9, ≥35·0 kg/m2), vitamin D from food (<2·50, 2·50–4·99, 5·00–7·49, 7·50–9·99, ≥10·00 μg/d), vitamin D from supplement sources (0, 0·025–4·99, 5·00–9·99, ≥10·00 μg/d), race (white, African American, Asian, other) and UV-B flux (<461, 461–<628, ≥628 J/cm2). Race (proxy for skin pigmentation), UV-B flux (average annual UV-B flux was derived from geographic region of residence at baseline which represents a composite measure of mean UV-B radiation level accounting for latitude, altitude and cloud cover)( Reference Scotto, Fears and Faumeni 21 ), vitamin D intake from diet, BMI and physical activity (proxy for outdoor sunlight exposure) were identified as independent predictors of 25(OH)D( Reference Giovannucci, Liu and Rimm 20 ). The difference in mean plasma 25(OH)D across extreme deciles of the predicted 25(OH)D score was 27·8 nmol/l. The model identified a wide range of predicted 25(OH)D, ranging from a summer high of 91 nmol/l for a man with all favourable characteristics (i.e. highest intake categories, residence in South/Southwest, lowest BMI, highest activity level, low skin pigmentation) to a predicted winter low of 23 nmol/l for the opposite extreme. The predicted 25(OH)D score was then validated in a separate sample of 542 men who had plasma 25(OH)D levels available and were free of cancer at the time of blood collection. The difference in mean plasma 25(OH)D across extreme deciles of the predicted 25(OH)D score was 25·0 nmol/l, which was similar to that observed in the development data set. The associations between the predicted 25(OH)D score and key demographic and dietary characteristics were as expected for plasma 25(OH)D.
Outcome
Incident tooth loss, the primary outcome of interest, was measured by self-report from biennial questionnaires beginning in 1986 through 2006. Self-reported tooth loss measures have been validated in other populations and found to be highly valid( Reference Douglass, Berlin and Tennstedt 22 ). Furthermore, the HPFS population comprised health professionals with over 50 % being dentists; hence we expect tooth loss to be reported with high validity.
Data on periodontitis were collected on each biennial questionnaire. The question administered was ‘Have you been professionally diagnosed with periodontal disease with bone loss?’ Men who responded ‘Yes’ were considered positive for periodontitis. Self-reported periodontitis has been validated in this population and shown good validity against bitewing radiographs among both dentists and non-dentists( Reference Joshipura, Pitiphat and Douglass 23 , Reference Joshipura, Douglass and Garcia 24 ). The self-reported binary measure of periodontitis has been validated against bitewing radiographs, using a case definition based on alveolar bone loss which reflects history of periodontitis. Bitewing radiographs were obtained from 352 men and classified as periodontally diseased for bone loss >2 mm and/or complete loss of the crestal lamina dura( Reference Joshipura, Pitiphat and Douglass 23 , Reference Joshipura, Douglass and Garcia 24 ). Radiographs were reviewed by three examiners blinded to the respondents’ self-reported periodontal disease status. Among dentists self-reported periodontitis status showed a positive predictive value (PV+) of 0·76 and a negative predictive value (PV−) of 0·74, and among non-dentists the corresponding values were PV+ = 0·83 and PV− = 0·69. Hence, the self-reported measure shows good validity in representing periodontitis compared with radiographic bone loss, which is a well-accepted clinical measure of the cumulative effects of periodontitis( Reference Reddy 25 ).
Statistical methods
The analysis consisted of examining two relationships: (i) the association between predicted 25(OH)D score over follow-up and incident tooth loss; and (ii) the association between predicted 25(OH)D score over follow-up and incident periodontitis. Cox models( Reference Cox 26 ) were used to estimate the association between time-varying predicted 25(OH)D score and incident tooth loss and periodontitis, separately, using age- and multivariable-adjusted models. The predicted 25(OH)D score was updated at each follow-up cycle based on updated values for each of the components as follows: physical activity and BMI were updated biennially, vitamin D from foods and supplements were updated every 4 years, whereas race and UV-B radiation were based on a single measurement at baseline in 1986. The effect estimates presented are hazard ratios (HR) with corresponding 95 % confidence intervals. Person-time was calculated from the date of return of the baseline questionnaire in 1986 to the date of first self-reported tooth loss/periodontitis, date of death, diagnosis of cancer or 31 January 2006, whichever came first. To address missing information, data were carried forward no more than one questionnaire cycle, the median value was substituted for missing values on the CSI variable and the complete case method was utilized thereafter (<5 % of observations were missing data on any particular covariate).
Potential confounders and risk factors were selected based on a priori subject matter knowledge, the underlying causal structure of the association between vitamin D, tooth loss and periodontitis, and examination of a ≥10 % change in main effect estimates; however, covariates deemed clinically significant were retained irrespective of the 10 % change in estimate criterion. Two multivariable analyses were conducted for each outcome. The primary analyses are provided in Model 1 adjusted for smoking history (CSI), pipe or chewing tobacco use (yes/no), multivitamin use (yes/no), vitamin E supplementation (quintiles), vitamin C supplementation (quintiles), dental professional (yes/no), alcohol use (0, 0·1–4·9, 5·0–14·9, 15·0–29·9, ≥30·0 g/d), routine physical examination (yes/no) and diabetes status (yes/no). Model 2 presents sensitivity analyses further adjusted for components of the predicted 25(OH)D score, including BMI (<22·0, 22·0–24·9, 25·0–29·9, 30·0–34·9, ≥35·0 kg/m2), race (white, African American, Asian, other) and physical activity (quintiles). We additionally evaluated the association between the components used to create the predicted 25(OH)D score and tooth loss in a model without the predicted 25(OH)D score while adjusting for all other confounders described in multivariable Model 1.
A priori we proposed to evaluate whether the association between the predicted 25(OH)D score and tooth loss or periodontitis varied by age, BMI, dental occupation, physical activity and alcohol intake, based on subject matter knowledge. The variation of these associations across the a priori identified covariates was evaluated by the introduction of interaction terms into a model with continuous main effects (multivariable Model 1). Significance of the interaction was evaluated by a likelihood ratio test. We evaluated the influence of misclassification of the outcome on effect estimates using methods proposed by Duffy et al.( Reference Duffy, Warwick and Williams 27 ). Predictive values were obtained from the prior validation studies conducted by Joshipura et al.( Reference Joshipura, Pitiphat and Douglass 23 , Reference Joshipura, Douglass and Garcia 24 ) separately among independent samples of dentists and non-dentists from this cohort. A weighted average of these independent samples was calculated to obtain mean predictive values (PVm+ and PVm−). The adjusted HR was estimated by taking the exponential of the log of the uncorrected HR divided by the sum of (PVm+, PVm− and –1). All P values were two-sided at α = 0·05. Analyses were conducted with the SAS for UNIX statistical software package version 9·1·3.
Results
Over 20 years of follow-up 13 581 men reported incident tooth loss and 3158 men reported incident periodontitis. Men within the highest quintile of the predicted 25(OH)D score were more likely to be Caucasian, physically active, live in the South/Southwest and less likely to be diabetic or report periodontitis compared with those in the lowest quintile of the predicted 25(OH)D score (Table 1). As expected, men in the highest quintile of the predicted 25(OH)D score consumed more vitamin D from food and supplements than those in the lowest quintile.
Table 1 Age-standardized baseline characteristics by quintile of the predicted 25(OH)D scoreFootnote * among 42 730 men participating in the Health Professionals Follow-up Study, USA, 1986–2006
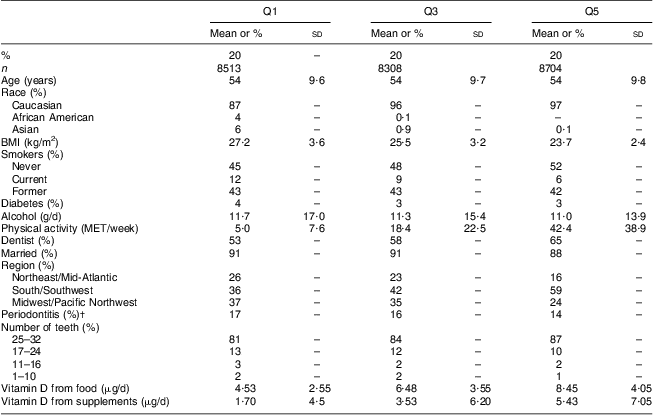
25(OH)D, 25-hydroxyvitamin D, Q1, lowest quintile; Q3, middle quintile; Q5, highest quintile; MET, metabolic equivalents.
* Values are standardized to the age distribution of the study population. Percentages may not sum to 100 % due to rounding.
† Men with periodontitis at baseline were excluded from the incident periodontitis analysis.
The predicted 25(OH)D score was associated with a lower risk of incident tooth loss among men in age- and multivariable-adjusted models (Table 2). Men with a predicted 25(OH)D score in the highest quintile exhibited a significantly lower risk of tooth loss compared with men in the lowest quintile after adjustment for covariates in multivariable Model 1 (smoking, pipe, chewing tobacco, multivitamin use, vitamin E, vitamin C, dental profession, alcohol consumption, routine physical examination and diabetes status). When additionally adjusted for race, BMI and physical activity (Model 2), the association was slightly attenuated. Each 10 nmol/l increase in the predicted 25(OH)D score was associated with a 10 % significantly lower risk of tooth loss when adjusted for confounders in Model 1; the results were similar when additionally adjusted for race, BMI and physical activity (Model 2). We separately evaluated the influence of Ca intake on multivariable association between quintiles of the predicted 25(OH)D score and tooth loss (Model 1); however, estimates were materially unchanged (results not shown).
Table 2 Age- and multivariate-adjusted hazard ratios and 95 % confidence intervals for the predicted 25(OH)D score and incident tooth loss and periodontitis among men participating in the Health Professionals Follow-up Study, USA, 1986–2006
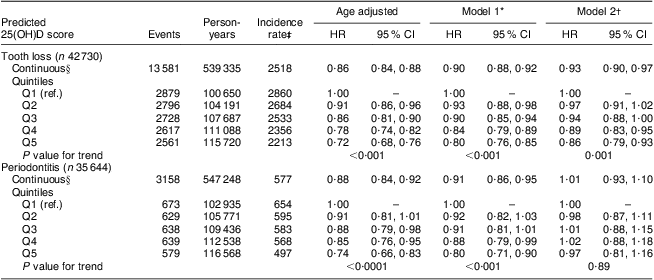
25(OH)D, 25-hydroxyvitamin D; HR, hazard ratio; Q1–Q5, quintile 1–quintile 5; ref., reference category; MET, metabolic equivalents.
*Model 1: age, smoking (CSI, comprehensive smoking index), pipe (yes/no), chewing tobacco (yes/no), multivitamin use (yes/no), vitamin E (quintiles), vitamin C (quintiles), dental profession (dentist/non-dentist), alcohol consumption (0, 0·1–4·9, 5·0–14·9, 15·0–29·9, ≥30·0 g/d), routine physical examination (yes/no) and diabetes (yes/no).
†Model 2: Model 1 + BMI (<22·0 (ref.), 22·0–24·9, 25·0–29·9, 30·0–34·9, ≥35·0 kg/m2), race (white, African American, Asian, other), and physical activity (MET/week; quintiles).
‡Incidence rate per 100 000 person-years.
§Per 10 nmol/l.
There was evidence to suggest that the association between the predicted 25(OH)D score and tooth loss varied by age and dental profession (Fig. 1). The association between the predicted 25(OH)D score and tooth loss was stronger among younger men compared with the association observed among men aged ≥65 years (P value for interaction = 0·03). Furthermore, the association between the predicted 25(OH)D score and tooth loss among dentists was stronger than the association observed among non-dentists (P value for interaction = 0·01). We additionally modelled quintiles of the predicted 25(OH)D score among dentists and observed that when compared with the lowest quintile, all higher quintiles of the predicted 25(OH)D score were significantly associated with a lower risk of tooth loss (reference = Q1; Q2: HR = 0·89 (95 % CI 0·82, 0·96); Q3: HR = 0·86 (95 % CI 0·80, 0·93); Q4: HR = 0·80 (95 % CI 0·74, 0·87); Q5: HR = 0·73 (95 % CI 0·68, 0·79)). There were no significant interactions observed for the predicted 25(OH)D score and periodontitis with any of the covariates in Fig. 1 (results not shown).

Fig. 1 Stratified multivariable-adjusted hazard ratios (HR; ▪) and 95 % confidence intervals (represented by horizontal bars) for a 10 nmol/l increment in predicted 25(OH)D score* and tooth loss, according to categories of age, dental profession, BMI, physical activity and alcohol intake, among 42 730 men participating in the Health Professionals Follow-up Study, USA, 1986–2006. P values shown are for interaction based on likelihood ratio test; – – – shows null effect (25(OH)D, 25-hydroxyvitamin D)
We additionally evaluated the association between the components of the predicted 25(OH)D score and tooth loss without including the predicted 25(OH)D score while adjusting for all potential confounders in Model 1 (Table 3). We found a significantly lower risk of tooth loss associated with residence in an area with UV-B flux ≥628 J/cm2 when compared with the UV-B flux <461 J/cm2; and an increased risk of tooth loss with BMI ≥ 35·0 kg/m2 compared with BMI<22·0 kg/m2. There was no association observed between tooth loss and vitamin D intake from food (<2·50 μg/d v. ≥10·00 μg/d) or supplements (0 μg/d v. ≥10·00 μg/d). An elevated but non-significant association was observed between African American race and tooth loss compared with Caucasians.
Table 3 Multivariable association between predicted 25(OH)D score components and tooth lossFootnote * among 42 730 men participating in the Health Professionals Follow-up Study, USA, 1986–2006
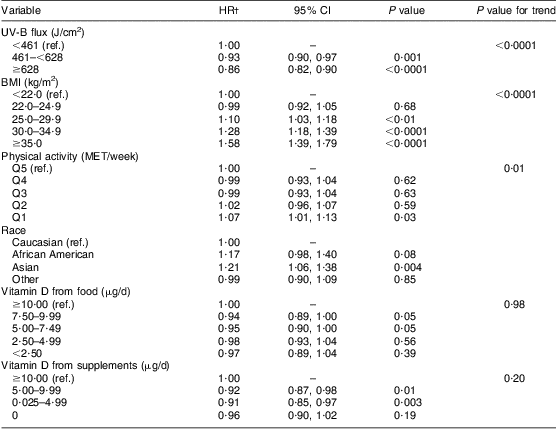
25(OH)D, 25-hydroxyvitamin D; HR, hazard ratio; Q5–Q1, quintile 5–quintile 1; ref., reference category; MET, metabolic equivalents.
* Predicted 25(OH)D score not included in model.
† Model adjusted for all predictor score covariates presented in the model including smoking (CSI, comprehensive smoking index), pipe (yes/no), chewing tobacco (yes/no), multivitamin use (yes/no), vitamin E (quintiles), vitamin C (quintiles), dental profession (dentist/non-dentist), alcohol consumption (0, 0·1–4·9, 5·0–14·9, 15·0–29·9, ≥30·0 g/d), routine physical examination (yes/no) and diabetes (yes/no).
Overall, results were similar for periodontitis (Table 2). When adjusting for covariates in Model 1, the highest quintile of the predicted 25(OH)D score was associated with a lower risk of periodontitis compared with the lowest quintile. Additionally adjusting for components of the predictor score (Model 2) resulted in substantial attenuation of the association towards the null. When evaluated as a continuous exposure, the risk of periodontitis decreased significantly by 9 % for each 10 nmol/l increase in the predicted 25(OH)D score, when adjusted for potential confounders in Model 1.
Discussion
The present study is the longest prospective observational study to evaluate the association between predictors of 25(OH)D and incidence of tooth loss and periodontitis. In multivariable analyses, adjusted for key confounders and risk factors, the risk of tooth loss and periodontitis was significantly lower with higher levels of the predicted 25(OH)D score. Furthermore, the association between the predicted 25(OH)D score and tooth loss may vary by age and dental profession.
Tooth loss is most commonly due to caries and/or periodontitis. There is a paucity of data evaluating the associations between 25(OH)D concentrations, tooth loss and periodontitis. Furthermore, most studies have been cross-sectional, obfuscating the temporal sequence. One study evaluated the association between measures of vitamin D, periodontitis and tooth loss and four others examined vitamin D status and periodontitis exclusively; results have been inconsistent. Krall et al.( Reference Krall, Wehler and Garcia 28 ) reported that randomization to Ca and vitamin D supplements resulted in a 60 % lower odds of tooth loss compared with placebo (OR = 0·40; 95 % CI 0·20, 0·90), yet no association was observed with history of periodontal treatment; however, individual effects could not be established. On the other hand, exposure to sunlight, rather than diet, constitutes the primary contribution to serum vitamin D among non-institutionalized individuals( Reference Newton, Sheltawy and Hay 7 , Reference Lips, van Ginkel and Jongen 29 ). Three other studies( Reference Dietrich, Joshipura and Dawson-Hughes 5 , Reference Miley, Garcia and Hildebolt 30 , Reference Garcia, Hildebolt and Miley 31 ) reported low 25(OH)D concentrations were associated with higher prevalence of periodontitis, while another study observed higher concentrations were associated with higher prevalence of disease( Reference Liu, Meng and Tang 32 ).
There is a paucity of data available with respect to the association between vitamin D and risk of caries. Dunning( Reference Dunning 33 ) used geographical variation/latitude as a surrogate marker of 25(OH)D due to UV-B exposure among World War I to World War II Army and Navy recruits( Reference Britten and Perott 34 – Reference Nizel and Bibby 36 ). Results were suggestive of a higher prevalence of caries associated with greater latitude. Vitamin D may influence caries risk through its effects on innate antimicrobial peptides such as cathelicidin; however, data are limited( Reference Park, Elias and Oda 37 ).
Vitamin D may influence tooth loss through periodontitis via immunomodulatory or antimicrobial effects, Ca absorption and/or effects on bone metabolism. Various cell types express vitamin D receptors, including immune cells, and are capable of converting 25(OH)D to the metabolically active 1,25-dihydroxyvitamin D (1,25(OH)2D)( Reference Zittermann 38 ). When ample plasma 25(OH)D substrate is available for 1,25(OH)2D production, local production of 1,25(OH)2D by various immune cells may play a key role in regulating the innate or acquired immune response at local sites of inflammation( Reference Adams, Liu and Chun 39 ). The pro-inflammatory cytokines IL-1β and TNF-α both play central roles in the pathogenesis of periodontitis by impairing wound healing and inducing bone resorption( Reference Kornman, Page and Tonetti 40 ). 1,25(OH)2D has been shown to inhibit monocyte production of IL-1β and TNF-α both in vitro and in vivo ( Reference Almerighi, Sinistro and Cavazza 41 – Reference Muller, Haahr and Diamant 43 ), supporting a role for locally produced 1,25(OH)2D in the regulation of periodontal health.
The observed association between the predicted 25(OH)D score and tooth loss could be mediated through periodontitis, caries or both. If the association was primarily mediated through periodontitis, one would expect a stronger association between the predicted 25(OH)D score and periodontitis than with tooth loss. Hence, similar associations observed with both outcomes may be suggestive of alternative pathways in addition to periodontitis. While self-reported periodontitis has previously shown good validity in this population, it may be subject to greater misclassification than the tooth loss measure, potentially explaining the observed attenuation of periodontitis estimates, although this could not be assessed. Furthermore, we cannot account for severity of disease which may attenuate results. To quantify misclassification of the outcome, an adjusted HR was estimated from the log of the uncorrected effect estimate divided by the sum of the mean predictive values among dentists and non-dentists and −1( Reference Duffy, Warwick and Williams 27 ). The multivariable-adjusted HR estimate for the highest quintile of the predicted 25(OH)D score compared with the lowest from Table 2 (Model 1) was 0·65 v. an uncorrected estimate of 0·80. Additionally, although information on periodontal treatment is unavailable, the level of care is expected to be high.
We additionally evaluated the impact of the predicted 25(OH)D score components on risk of tooth loss and periodontitis. First, the association between the predicted 25(OH)D score and periodontitis was substantially attenuated upon adjustment for three key components (BMI, race and physical activity), unlike the association for tooth loss, which was attenuated only by including BMI. Second, when modelling the associations between the predicted 25(OH)D score components and tooth loss without the predicted 25(OH)D score itself, dietary sources of vitamin D were not associated with tooth loss, while the strongest associations were observed for exposure to UV-B radiation and BMI; similar results were observed for periodontitis. These results may suggest non-25(OH)D related pathways or alternative exposure windows, which are not well understood (UV-B in childhood v. exposure proximate to the pathogenesis of disease). Further, confounding by socio-cultural factors related to region and oral disease may be intractable. Additionally, increased adiposity may influence periodontitis by inducing low-grade inflammation and/or immune cell dysfunction related to dyslipidaemia; data for caries are equivocal( Reference Chaffee and Weston 44 ). Adjustment for Ca did not change the estimates, suggesting associations independent of Ca absorption.
We conducted several a priori sub-analyses and observed that the association between the predicted 25(OH)D score and tooth loss varied by age and dental profession. While plausible that these observations were due to chance, one may consider alternative explanations. Age-related tooth loss is multifactorial, hence tooth loss at younger ages may reflect specific pathology potentially mediated in part by 25(OH)D-impaired immune response. Although highly speculative, the variation by dental profession may reflect different underlying susceptibilities to tooth loss, periodontitis and/or caries between dental and non-dental professionals due to oral health behaviours. Oral disease-related tooth loss among non-dental health professionals may largely reflect the effects of plaque and other localized risk factors; whereas, among those with a higher level of dental care (e.g. dental professionals), the pathogenesis may reflect a greater susceptibility to oral disease by systemic factors including but not limited to plasma 25(OH)D.
Although the present study consisted primarily of white men, similar results have been reported in diverse populations. It is unlikely that residual confounding or an unmeasured factor would entirely account for the observed associations given the longitudinal assessment of potential confounders and modest attenuation towards the null between the age- and multivariable-adjusted models.
Important strengths of the study include the use of a novel prediction score which does not replace plasma measurements, but has been shown to predict approximately a 10 ng/ml difference in true 25(OH)D at extremes, representing a physiologically important increment( Reference Bertrand, Giovannucci and Liu 45 ). However, measurement error in the predicted 25(OH)D score may mask any underlying threshold effect in the exposure disease association as linear. Furthermore, study power was increased by use of the full cohort irrespective of biomarker samples. Additional strengths include the longitudinal design with 20 years of follow-up including updated values of covariates and a homogeneous population minimizing confounding by socio-economic status and health-oriented behaviours.
Conclusion
The present results suggest that vitamin D itself and/or components associated with vitamin D status including UV-B may be associated with lower risk of tooth loss and periodontitis. These results warrant further investigation by epidemiological and clinical studies. Since vitamin D can be obtained free or at low cost, the implications of these associations in primary prevention of periodontitis and tooth loss are large.
Acknowledgements
Sources of funding: The study was supported by grant R03DE017948 from the National Institute of Dental and Craniofacial Research of the National Institutes of Health. The authors wish to acknowledge institutional support from the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School. Conflicts of interest: The authors declare they have no conflicts of interest. Authors’ contributions: T.D., E.G. and E.K.K. designed the research; K.J.J. contributed to designing the oral health data collection; M.J., T.D. and E.G. conducted the research; M.J. analysed the data; M.J., T.D., E.G. and E.K.K. interpreted findings; M.J., K.J.J., E.G. and T.D. contributed to writing the paper; and T.D. had primary responsibility for final content. All authors read and approved the final manuscript.






