Due to the side effects of synthetic remedies, there is growing interest in the use of natural remedies for the treatment of diabetes mellitus in recent years. Several spices have been documented to possess anti-hyperglycaemic action(Reference Patil, Ghadyale and Taklikar1). The seeds of Cuminum cyminum are routinely used in the cuisines of many different cultures from ancient time. Besides being used as a popular spice, cumin seeds are used as an ingredient in many home remedies and Ayurvedic preparations for the treatment of various diseases including diabetes. The anti-hyperglycaemic activity of cumin and its use in secondary complications associated with diabetes mellitus was evaluated in various studies(Reference Roman-Ramos, Flores-Saenz and Alarcon-Aguilar2, Reference Willatgamuwa, Platel and Saraswathi3). The inhibitory activity of C. cyminum seed oil has been reported against lens aldose reductase and α-glucosidase(Reference Lee4). The supplementation of cumin shows delayed progression and maturation of streptozotocin (STZ)-induced cataract in rats(Reference Kumar, Reddy and Srinivas5). The anti-hyperglycaemic activity and the inhibition of advanced glycation end-product formation by the methanolic extract of C. cyminum seeds have been reported in STZ-induced diabetic rats(Reference Jagtap and Patil6). However, despite several studies, the precise mechanism of glucose lowering and the biocomponents responsible for such an action are not identified.
Type 2 diabetes mellitus is often associated with decreased and unregulated insulin secretion(Reference Ashcroft and Rorsman7). A continued state of hyperglycaemia may lead to overstimulation of β-cells(Reference Grill and Bjorklund8). A higher concentration of glucose has been suggested to cause the apoptosis of β-cells through the activation of the Fas pathway(Reference Maedler, Sergeev and Ris9). Insulin secretagogues, such as tolbutamide, glibenclamide and gliclazide, are widely used in the treatment of diabetes mellitus. Although these synthetic remedies are extensively used, they are associated with a number of undesirable side effects such as abnormalities in β-cell function and the progressive loss of β-cell mass(Reference Clark, Jones and Koning10). Moreover, the efficiency of the majority of oral insulin secretagogues decreases with time due to desensitisation(Reference Robertson11).
Inhibition of glucose-induced insulin secretion by the simultaneous infusion of diazoxide (ATP-sensitive K (K+-ATP) channel opener) prevents the overstimulation of β-cells and improved insulin secretion in type 2 diabetic subjects(Reference Greenwood, Mahler and Hales12). These results support the importance of β-cell protection in diabetic patients. Therefore, the protection of β-cells from destruction presents itself as a new therapeutic target.
Thus, the aim of the present study was to identify bioactive components from C. cyminum, illustrate their mechanism of anti-hyperglycaemic action and evaluate their β-cell protective effect.
Materials and methods
All the experiments were carried out according to the guidelines of the Institutional Animal Ethics Committee of Shivaji University, Kolhapur, India (registration no. 233/ CPCSEA) after due submission and approval of the protocols.
Plant material and extraction
Cumin seeds were obtained locally from Kolhapur, Maharashtra, India and identified by the Department of Botany, Shivaji University, Kolhapur, India. Cumin seeds were ground to powder and subjected to steam distillation at 60°C. The distillate was subjected to sequential extraction with petroleum ether (pet ether), chloroform and dichloromethane. Each organic fraction was extracted and the aqueous fraction remaining after solvent extraction was then evaporated in vacuum rotary evaporator. The percentage yield of pet ether, chloroform, dichloromethane and aqueous fractions with respect to cumin powder was 1·01, 0·05, 0·03 and 0·01 % (w/w), respectively. All the fractions were stored in airtight screw-cap glass vials at 4°C. Each fraction was diluted with 0·01 % dimethyl sulphoxide just before use. The purified components cuminaldehyde and cuminol were also diluted with 0·01 % dimethyl sulphoxide. A vehicle control was used in each in vivo and in vitro experiment.
Induction of diabetes
Normal male rats weighing 200 (sd 10) g were fasted overnight and diabetes was induced by intraperitoneal administration of freshly prepared STZ (65 mg/kg body weight (bw)) in 0·1 m-citrate buffer (pH 4·5). After 14 d, the animals showing stable glucose values above 2000 mg/l were considered as diabetic and selected for further experimentation.
In vivo prolonged treatment
For prolonged experiments, a total of thirty male rats (six normal and twenty-four diabetic) were used and divided into five groups as normal control-, diabetic control-, positive control glibenclamide- (2·5 mg/kg bw) and pet ether fraction-treated groups (5 and 10 mg/kg). The animals from the treatment groups were fed orally with the respective drug twice per d for a period of 45 d, while the animals from the control group were administered with a vehicle (0·1 % dimethyl sulphoxide).
Estimation of biochemical parameters
Fasting blood glucose levels of all groups were estimated by ACCU-CHECK glucometer every 10 d. At the end of 45 d, all the rats were fasted overnight and killed by cervical dislocation. Blood was withdrawn immediately and serum was collected for biochemical assays. Serum insulin was measured using an insulin ELISA kit (CalBiotech). Glycated Hb (GHb), serum total cholesterol and HDL-cholesterol (HDL) were estimated using a biochemical kit (Crest Biosystems), while total TAG were estimated using a liquid gold kit. Serum LDL and VLDL were calculated using the Friedewald formula as:
Oral glucose tolerance test
A total of forty-two rats (six normal and thirty-six diabetic) were fasted overnight and given free access to water. They were divided into seven groups (n 6) as normal control, diabetic control, positive control (glibenclamide 2·5 mg/kg bw) and rats from the remaining four groups were administered with 10 mg/kg of the respective solvent fraction. All rats were fed orally with a glucose load of 3 mg/g bw. Rats from each group were administered orally with a vehicle or respective test component 10 min before glucose administration. Blood glucose was measured using ACCU-CHECK at 0, 30, 60 and 120 min.
Isolation of rat pancreatic islets
The isolation of islets was carried out using the collagenase digestion method modified in our laboratory(Reference Patil, Ghadyale and Taklikar1). The isolated islets were cultured overnight in Roswell Park Memorial Institute-1640 medium supplemented with 2·8 mm-glucose, penicillin (100 units/ml), streptomycin (100 μg/ml), gentamycin (25 mg/ml) and 10 % fetal calf serum in a CO2 incubator (Thermo Scientific) at 37°C in a 5 % CO2 atmosphere. After 24 h, islets were collected and washed twice with PBS.
Insulin release assay
Groups of ten islets were placed in wells containing 1 ml Hank's balanced salt solution (pH 7·4) supplemented with 10 mm-HEPES and 2 mg/ml of bovine serum albumin, and incubated for 1 h with glucose in the respective test condition. Glibenclamide (10 μg/ml) was used as the positive control. To illustrate the probable mechanism of insulin secretion, islets were incubated in the presence or absence of the isolated compounds under the following conditions, in the presence of varying glucose concentrations: 11·8 mm-glucose and 300 μm-diazoxide or 20 μm-nifedipine(Reference Baptista-Saidemberg, Saidemberg and Ribeiro13); 5·6 mm-glucose and 1 mm-3-isobutyl-1-methylxanthine (IBMX); 2·8 mm-glucose and 10 mm-alanine(Reference Hannan, Marenah and Ali14). After 1 h incubation, the supernatant from each well was collected and stored at 4°C. The insulin content in all the stored samples was determined using the ELISA kit (CalBiotech) and quantified with an ELISA microplate reader (Multiskan EX; Thermo Scientific) at 450 nm.
Viability assessment by 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan conversion
Viability of the isolated islets after the experimental treatment was assessed by the 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) assay(Reference Mosman15). Cell viability was expressed as a percentage of viability (% viability) relative to islets in the vehicle control group which were considered as 100 % viable.
Isolation and characterisation of bioactive compounds
The pet ether fraction was subjected to silica gel column chromatography. The fraction was loaded onto a silica gel column (60–120 mesh size, 1·2 cm inner diameter × 50 cm) and successively eluted with a stepwise gradient of the hexane–ethyl acetate system (100:0, 90:10, 80:20, 70:30, 60:40, 55:45, 50:50, 40:60, 30:70, 20:80, 10:90 and 0:100). For each gradient, three subfractions were obtained; thus, a total of thirty-six fractions were collected. Among the thirty-six fractions, second, fifteenth and seventeenth fractions were found to possess bioactivity and subjected to further structural elucidation. The percentage weight of these fractions was 0·70, 18·57 and 2·39 % (w/w), respectively, relative to that of the pet ether fraction.
GC–MS analysis
GC–MS analysis of active fractions was carried out using a GCD-1800 A model (Shimadzu). Typically, 0·2 μl of the sample were injected onto a HP-5 column with an initial temperature of 100°C and a hold time of 2 min at 150–160°C.
Fourier transform infrared spectroscopy studies
IR analysis was carried out using a Fourier transform infrared spectrometer in the range of 450–4000 per cm by the KBr pellet technique.
NMR analysis
1H NMR was carried out at the Department of Chemistry, Shivaji University, Kolhapur, using 2H-labelled chloroform (CDCl3) at 300 MHz on a Bruker spectrophotometer.
Determination of β-cell protective action through the alkaline comet assay
The β-cell protective action of fractions and isolated components was assessed through the single-cell gel electrophoresis alkaline comet assay(Reference Singh, McCoy and Tice16). Groups of twenty-five islets were dispersed into single cells through the trypsinisation method(Reference Choi, Choi and Yoon17). The dispersed cells were washed thrice with Hank's balanced salt solution, and used for further experimentation. The isolated cells were challenged with 5 mm-STZ in the presence or absence of the isolated components (25 μg/ml). After 24 h, the supernatant was collected and used for nitrite production in culture medium. The remaining cells were used for the comet assay. The comet slides were stained with the silver staining method and quantisation was carried out using image analysis software (TriTek CometScore™, version 1.5; TriTek Corp.). Each experiment was performed in triplicate with an average of twenty to thirty cells examined in each experiment. DNA damage was evaluated through the mean comet length(Reference Delaneya, Green and Lowe18).
Nitrite determination
Nitrite produced by the cells was determined using a colorimetric assay(Reference Green, Wagner and Glogowski19) by adding 100 μl of Greiss reagent to 100 μl of culture supernatant. Absorbance was measured at 540 nm, and nitrite concentrations were calculated using a linear standard curve obtained from serial dilutions of sodium nitrite in the working medium.
Statistical analysis
All data are expressed as means and standard deviations. Statistical analysis was performed using two-way ANOVA and unpaired Student's t test. P< 0·05 was considered as statistically significant.
Results
Oral glucose tolerance test for the solvent fractions
Among the four fractions isolated from the C. cyminum distillate, the pet ether fraction showed the most prominent anti-hyperglycaemic action in the oral glucose tolerance test (OGTT; Table 1); however, it was not as potent as the glibenclamide-treated group. The remaining three fractions, namely the chloroform, dichloromethane and aqueous fractions, did not reduce blood glucose levels and demonstrated a pattern similar to that of the diabetic control. Hence, the bioactive pet ether fraction was used for further in vivo studies.
Table 1 Effect of the Cuminum cyminum solvent fractions on blood glucose levels† (Mean values and standard deviations, n 6)
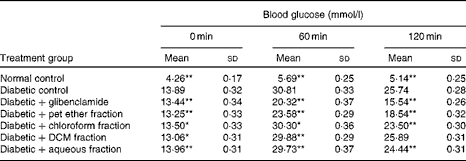
bw, Body weight; pet ether, petroleum ether; DCM, dichloromethane.
Mean values were significantly different with respect to the diabetic control: * P< 0·05, ** P< 0·001.
† Glibenclamide (2·5 mg/kg bw) and solvent fractions (10 mg/kg bw) were administered 10 min before the glucose load.
In vitro insulin secretion studies of the solvent fractions
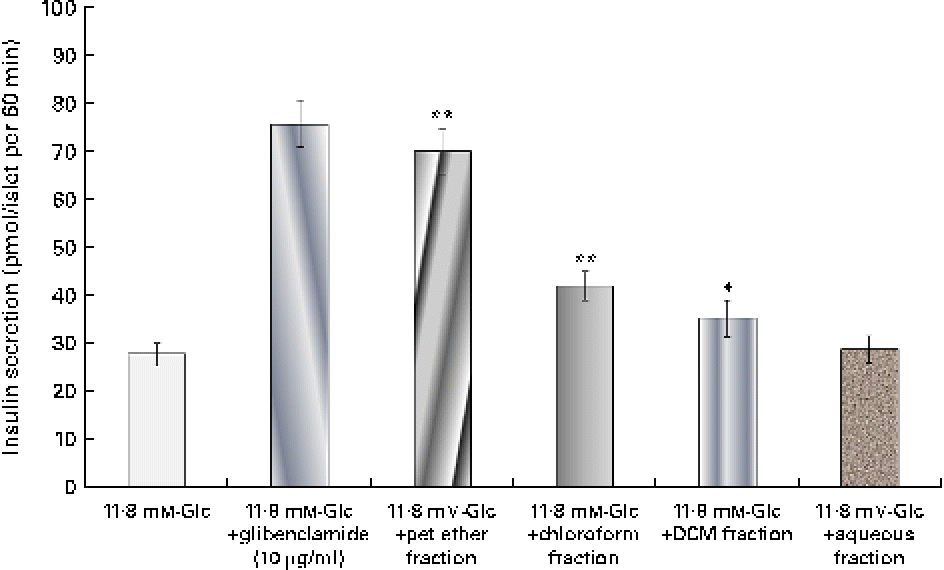
The insulin secretagogue action of the solvent fractions was assessed (Fig. 1) by the addition of the respective fraction to the isolated islets. Among all the four fractions, the pet ether fraction showed maximum insulin secretion (69·94 pmol/l). The chloroform fraction did show some stimulatory action but was not as pronounced as the pet ether fraction.

Fig. 1 Effect of the solvent fractions on insulin secretion. Insulin secretion induced by the petroleum ether (pet ether), chloroform, dichloromethane (DCM) and aqueous fractions (each 25 μg/ml). Glibenclamide (10 μg/ml) was considered as a positive control. Values are means, with standard deviations represented by vertical bars (n 6). Mean values were significantly different with respect to the 11·8 mm-glucose (Glc) control: * P< 0·05, ** P< 0·001. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Prolonged treatment experiments
The pet ether fraction with blood glucose lowering and insulin secretagogue activity was used for prolonged treatment at concentrations of 5·0 and 10 mg/kg bw for 45 d. After the treatment for 45 d, the effects of the treatment on blood glucose, GHb, serum insulin and lipid profile were evaluated (Table 2). It was observed that the pet ether fraction significantly reduced the fasting blood glucose levels. There was improvement in serum insulin levels and GHb values were very close to those of the normal control group. The fraction at 10 mg/kg bw reduced the blood glucose level as well as the GHb level below the standard control drug glibenclamide.
Table 2 In vivo long-term effect of the Cuminum cyminum petroleum ether (pet ether) fraction on blood glucose, glycated Hb (GHb), serum insulin and lipid profile (Mean values and standard deviations, n 6)

bw, Body weight; FBG, fasting blood glucose; TC, total cholesterol.
Mean values were significantly different with respect to the diabetic control: * P< 0·05, **P< 0·001.
The compounds showing good anti-lipidaemic action are beneficial in the treatment of diabetes. The treatment with the pet ether fraction of cumin improves the lipid profile. A lowering of total cholesterol, TAG, LDL, VLDL levels and improvement in HDL levels in the pet ether fraction-treated group were observed.
Isolation and characterisation of bioactive components
The GC–MS analysis of fraction 15 from the silica gel column revealed four peaks. The most prominent component was observed at the retention time of 15·6 min (Fig. S1, available online). The major ions observed from the electron ionisation-mass spectra of this peak included ions of m/z 148, 133, 119, 105, 103, 91, 79 and 77, which were matched with those of standard cuminaldehyde from the National Institute of Standards and Technology, Mass Spectrometry (NIST MS) Library. The fraction showed IR absorption bands at 2963, 1702, 1607 and 770 per cm, suggesting the presence of an aldehyde group, a C = O stretch, a C–C stretch in the aromatic ring and a para distribution in the molecule (Fig. S2, available online). 1H NMR data (300 MHz, CDCl3) of the compound were as follows: δ 9·957 (1H, s, H9), 7·782 (2H, d, H-3 and H-5), 7·356 (2H, d, H-2 and H-6), 3·015 (1H, m, H-7) and 1·244 (6H, m, 3H-8 and 3H-11) (Fig. S3, available online). Thus, data confirmed that the isolated compound fraction 15 was cuminaldehyde (synonym cuminal).
In the GC–MS analysis of fraction 17, a major peak was observed at the retention time of 17·0 min. The major ions observed for this peak included ions of m/z 150, 135, 119, 105, 91, 79 and 77, which were matched with those of standard cuminol from the NIST MS Library (Fig. S4, available online). The IR spectra of the compound showed absorption bands at 3413, 2924, 1607, 1019 and 770 per cm, suggesting the presence of an alcoholic group, an aromatic C–H and C–C stretch, a C–O stretch and a para distribution in the molecule (Fig. S5, available online). 1H NMR data of this compound were as follows: 1H NMR (300 MHz, CDCl3); δ 7·290 (2H, m, H-3 and H-5), 4·648 (2H, s, H-7a and b), 2·914 (1H, m, H-8), 1·228 (6H, m, 3H-10 and 3H-11) (Fig. S6, available online). Thus, it was confirmed that the major component from fraction 17 was cuminol (synonym cuminic alcohol, p-cymen-7-ol, etc.). The structure of cuminaldehyde and cuminol is shown in Fig. 2.
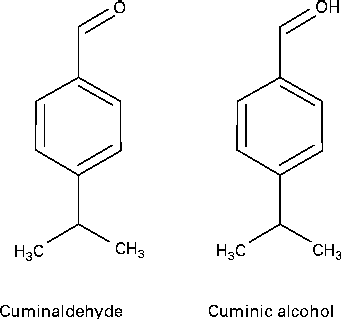
Fig. 2 Structure of cuminaldehyde and cuminol.
The in vivo activity of cuminaldehyde and cuminol was verified through the OGTT (Table 3). It can be observed that cuminaldehyde and cuminol identified as bioactive components reduced the blood glucose level at the concentration of 5·0 mg/kg bw and activity was on a par with the glibenclamide-treated rats.
Table 3 Effect of cuminaldehyde and cuminol on blood glucose levels† (Mean values and standard deviations, n 6)
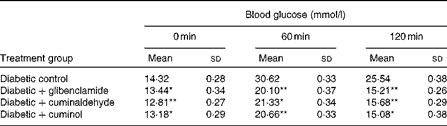
bw, Body weight.
Mean values were significantly different with respect to the diabetic control: * P< 0·05, **P< 0·001.
† Glibenclamide (2·5 mg/kg bw), cuminaldehyde and cuminol (5·0 mg/kg bw) were administered 10 min before the glucose load.
In vitro insulin secretion studies of the isolated components
Insulin secretion by cuminaldehyde and cuminol
Both cuminaldehyde and cuminol demonstrated dose-dependent insulin secretion. As further insulin stimulatory activity was not observed at a concentration more than 25 μg/ml, the same concentration was used for further studies (results not shown).
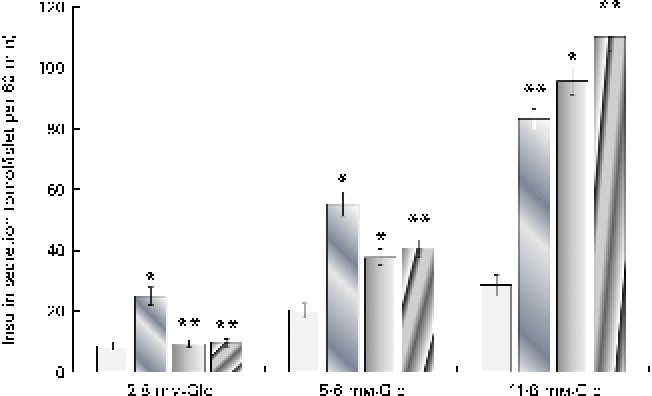
It can be observed from Fig. 3 that both cuminaldehyde and cuminol demonstrated a glucose-dependent insulin secretion and were not able to stimulate insulin secretion at the 2·8 mm-glucose concentration. An elevation in glucose concentration to 5·6 and 11·8 mm potentiated the stimulatory action of cuminaldehyde and cuminol. At a reaction mixture concentration of 25 μg/ml, cuminaldehyde and cuminol showed higher insulin secretion by 3·34- and 3·85-fold than the 11·8 mm-glucose challenge. Glibenclamide, on the other hand, demonstrated a glucose-independent mode of insulin secretion that was 2·89-fold higher than the 2·8 mm-glucose control.

Fig. 3 Effect of increasing glucose (Glc) concentrations on the insulin secretagogue action of cuminaldehyde and cuminol. Values are means, with standard deviations represented by vertical bars (n 6). Mean values were significantly different from the respective glucose control: * P< 0·05, ** P< 0·001. ![]() , Control;
, Control; ![]() , glibenclamide (10 μg/ml);
, glibenclamide (10 μg/ml); ![]() , cuminaldehyde (25 μg/ml);
, cuminaldehyde (25 μg/ml); ![]() , cuminol (25 μg/ml). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, cuminol (25 μg/ml). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Evaluation of the mechanism of insulin secretagogue action
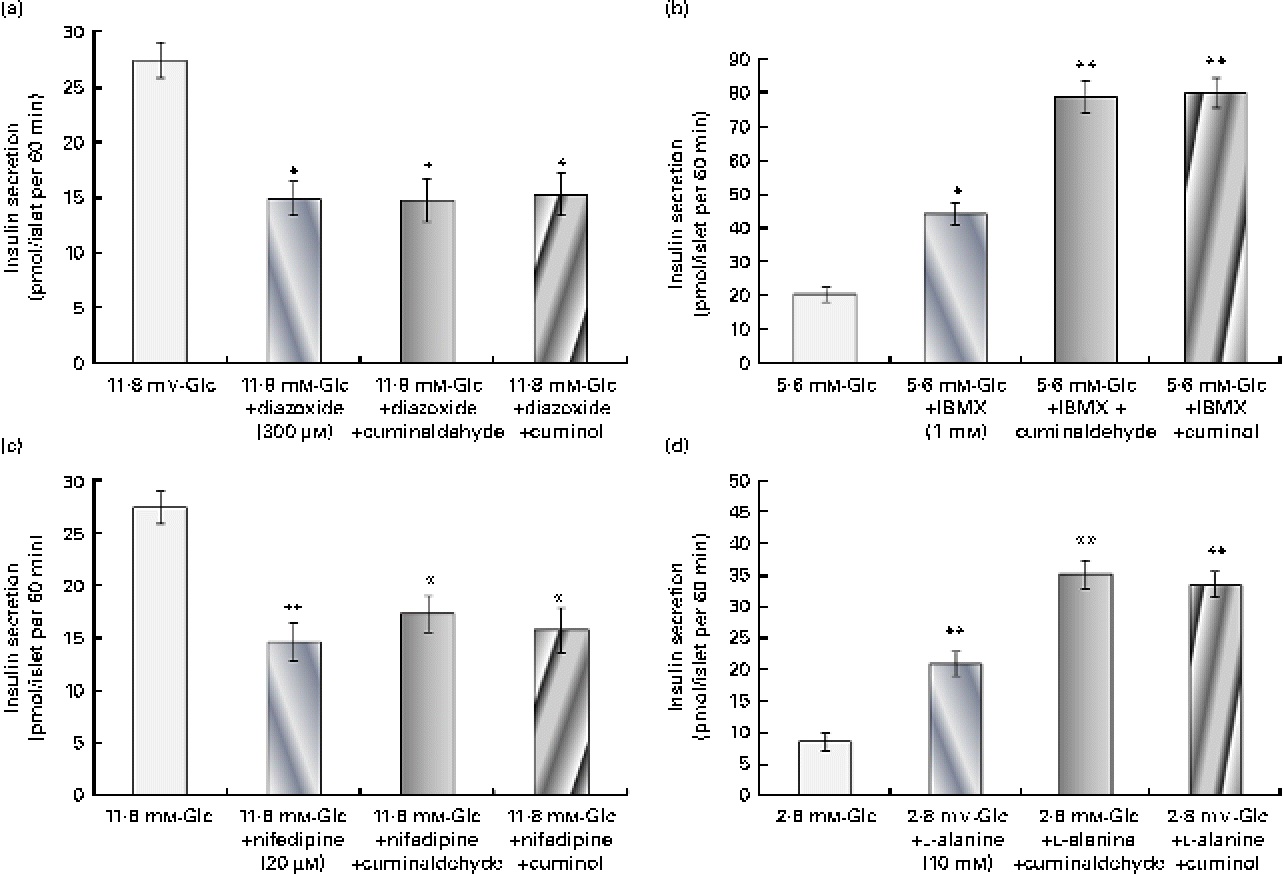
The probable pathway for the insulin secretagogue action of cuminaldehyde and cuminol was assessed (Fig. 4). In the presence of 300 μm-diazoxide (K+-ATP channel opener), insulin secretion declined from 27·44 to 14·98 pmol/l when compared with the control. Both cuminaldehyde and cuminol did not increase further insulin secretion in the presence of diazoxide (Fig. 4(a)). The addition of 20 μm-nifedipine (the L-type Ca2+ channel blocker) inhibited the secretion of insulin when compared with the elevated glucose control. Cuminaldehyde or cuminol did not overcome inhibition mediated by nifedipine (Fig. 4(b)).

Fig. 4 Study of the mechanism for insulin secretagogue action. (a) Effect of 300 μm-diazoxide, (b) 20 μm-nifedipine, (c) 1 mm-3-isobutyl-1-methylxanthine (IBMX) and (d) 10 mm-l-alanine on the insulin secretagogue activity of cuminaldehyde (25 μg/ml) and cuminol (25 μg/ml) was assessed. Values are means, with standard deviations represented by vertical bars (n 6). Mean values were significantly different with respect to the 11·8 mm-glucose (Glc) control: *P< 0·05, **P< 0·001. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
IBMX (a phosphodiesterase inhibitor) at a concentration of 1 mm potentiated insulin secretion almost 2·18 times higher than the 5·6 mm-glucose control (Fig. 4(c)). Both cuminaldehyde and cuminol increased the stimulatory effect of IBMX. Insulin secretion in the presence of cuminaldehyde and cuminol was 78·88 and 80·08 pmol/l, respectively, when compared with the IBMX control (44·33 pmol/l).
l-Alanine (10 mm) stimulated insulin secretion almost 2·42 times higher than the 2·8 mm-glucose control (Fig. 4(d)). Insulin secretion in the presence of l-alanine and cuminaldehyde was 35·24 pmol/l, and of cuminol was 33·66 pmol/l when compared with the l-alanine control alone (20·99 pmol/l).
Viability of islets by 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan conversion
The viability of islets after each test was assessed by the MTT conversion test. Cell viability in each treatment group was found to be higher than 90 %. Thus, it was confirmed that there was no toxic or detrimental cellular effects by both the components.
Inhibitory effect of an unknown compound on insulin secretion
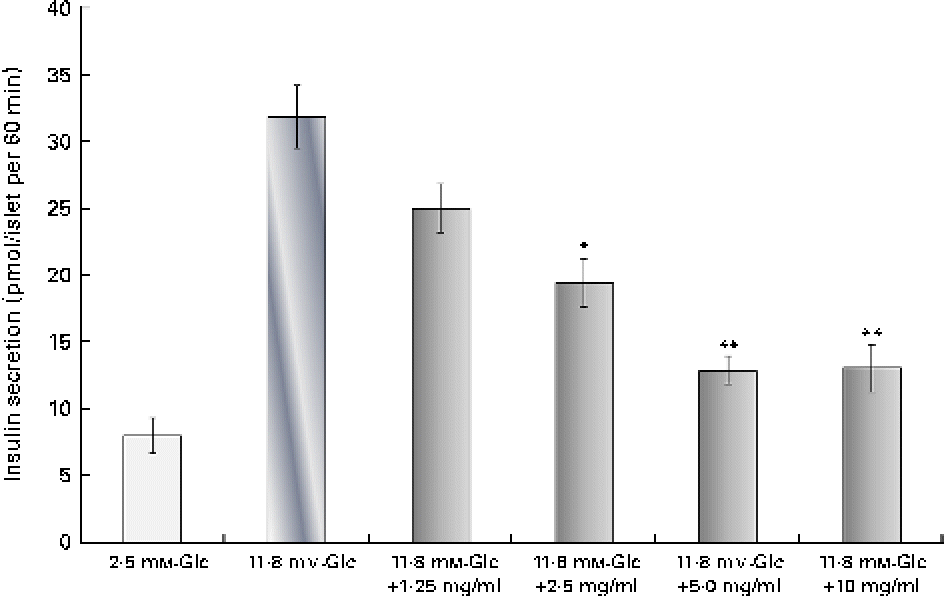
The analysis of silica gel column chromatography fractions revealed that fraction 2 had an inhibitory action on insulin secretion. The dose-dependent insulin secretagogue inhibitory activity of an unknown compound is shown in Fig. 5. At a concentration of 1·25, 2·5 and 5 μg/ml, the compound showed a dose-dependent decrease in insulin secretion. The maximum inhibition was observed at the 5 μg/ml concentration, which reduced insulin secretion by 60 % when compared with the 11·8 mm-glucose control. This was even higher than diazoxide (46 % inhibition) at the 300 μm concentration. At an increased concentration (10 μg/ml), no further inhibition was observed. The percentage of viability at concentrations of 1·25, 2·5, 5 and 10 μg/ml was found to be 114, 112, 124 and 117 %, respectively, when compared with the control group. The GC–MS analysis of fraction 2 (100:0 of silica gel column chromatography) revealed only a single major peak at a retention time of 27·199 min. The precise structure of this compound could not be elucidated at present.

Fig. 5 Dose-dependent inhibition of insulin secretion by the inhibitor. Dose-dependent insulin secretion inhibitory action of an unknown compound from the second fraction was evaluated at concentrations of 1·25, 2·5, 5 and 10 μg/ml. The results are compared with the 2·8 mm- and 11·8 mm-glucose (Glc) controls. Values are means, with standard deviations represented by vertical bars (n 6). Mean values were significantly different with respect to the 2·8 mm-Glc control: *P< 0·05, **P< 0·001. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
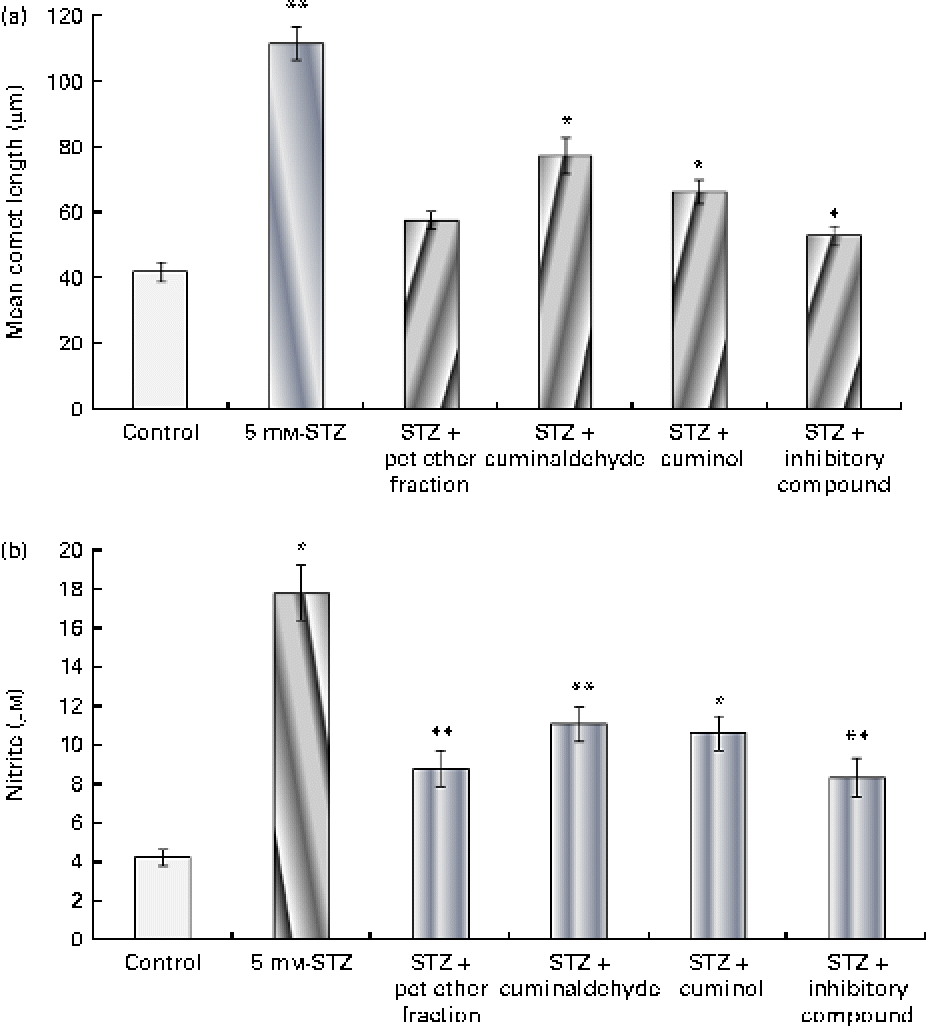
β-Cell protective action of the isolated components
The effect of the isolated components on β-cells is shown in Fig. 6(a). Cells treated with 5 mm-STZ showed increased DNA fragmentation and had the highest comet length. The pet ether fraction and its isolated components, namely cuminaldehyde, cuminol and the insulin secretion inhibitory compound from the second fraction, showed β-cell protection as evidenced by the decreased comet length. Cells treated with the unknown inhibitory fraction showed the most prominent action when compared with the other isolated components.

Fig. 6 Effect of the isolated components on β-cell protection through (a) the alkaline comet assay and (b) subsequent nitrite production. The isolated cells were challenged with 5 mm-streptozotocin (STZ) with or without the test components (25 μg/ml). (a) DNA damage was evaluated through the mean comet length. (b) Nitrite produced by the cells in the medium is shown. The results are compared with the 11·8 mm-glucose control. Values are means, with standard deviations represented by vertical bars (n 6). Mean values were significantly different with respect to the 11·8 mm-glucose control: * P< 0·05, ** P< 0·001. pet ether, Petroleum ether. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Nitrite production
The effect of the isolated components on nitrite production is shown in Fig. 6(b). Incubation of β-cells with 5 mm-STZ for 24 h resulted in significant nitrite formation (a stable oxidised product of NO) by these cells. The treatment of cells with the pet ether fraction and its isolated components (25 μg/ml) showed a significant reduction in STZ-mediated NO production. The pet ether fraction-treated cells showed minimal production of nitrite when compared with the other treatment groups. The data correlated with β-cell protective action (through the comet assay) and decreased cytotoxicity (through the MTT assay) due to the pet ether fraction and its isolated components. The unknown inhibitory compound from the second fraction showed the most prominent β-cell protective action and reduced nitrite formation.
Discussion
The use of insulin secretagogues in the treatment of diabetes is very common owing to their easy availability, efficacy in lowering blood glucose and ability to be administered orally. It has been observed in the UK Prospective Diabetes Study that patients demonstrated 50 % normal β-cell functionality at the time of diagnosis, and then, surprisingly, it was revived to 78 % in the first-year treatment with secretagogues but later reduced to 28 % in 6 years(Reference Turner and Holman20). This necessitates a shift to insulin therapy(21, Reference Matthews, Cull and Stratton22), and given the status of β-cells, spikes of hyperglycaemia and hypoglycaemia are common, making it difficult to maintain a rational blood glucose level and leading to rapid progression of secondary complications. Thus, maintaining the health of β-cells and preventing them from burn out would be beneficial in the long-term management of diabetes with insulin secretagogue therapy(Reference Donath, Ehses and Maedler23).
In the present study, though glibenclamide led to a better lowering of blood glucose in the OGTT as against the pet ether fraction (Table 1), in prolonged experiments, we have observed that the blood glucose levels of the pet ether fraction-treated animals were close to those of the normal control group and lower than those of the glibenclamide-treated group. The treated animals showed improved GHb, better insulin level and improved lipid profile. C. cyminum has earlier been reported to possess good hypolipidaemic action in alloxan-induced diabetic rats(Reference Dhandapani, Subramanian and Rajagopal24). The present results confirm the hypolipidaemic action of C. cyminum to be present in the pet ether fraction of the seed distillate.
While glibenclamide demonstrated glucose-independent insulin secretion, both cuminaldehyde and cuminol isolated from the pet ether fraction in the present study demonstrated glucose-dependent insulin secretagogue action. This is desirable as glucose-independent secretagogues can cause bouts of hypoglycaemia that are most dreaded by diabetic patients(Reference Bakatselos25). The closure of the K+-ATP channel and the increase in intracellular Ca2+ concentration, whether by the influx of extracellular Ca2+ or by the release of Ca2+ from intracellular stores, plays a pivotal role in insulin secretion as both diazoxide (K+-ATP channel opener) or nifedipine (L-type Ca2+ channel blocker) were found to abolish glucose-mediated insulin secretion. However, the components increased insulin secretion in the presence of IBMX. Recently, saponins have been purified from Momordica charantia, demonstrating good insulin secretagogue action(Reference Keller, Ma and Kavalier26). Both cuminaldehyde and cuminol have not shown any adverse effect on viability or functionality of the β-cell. No toxicity has been reported towards these compounds. In fact, both components demonstrated β-cell protective action in STZ-challenged islets, as evidenced by the comet assay, and reduced nitrite production. Earlier, repaglinide and nateglinide, both glucose-dependent insulin secretagogues, have also demonstrated anti-apoptotic action in in vitro studies(Reference Donath, Ehses and Maedler23). Cuminaldehyde, isolated from C. cyminum seed oil, has been reported earlier to possess significant inhibitory activity against aldose reductase and α-glucosidase(Reference Lee4), adding to the efficacy of the component as an excellent anti-diabetic agent.
Initially, when we evaluated the secretagogue action of the column fractions so as to isolate and identify the bioactive components, it was surprising to find a component that demonstrated the inhibition of insulin secretion. A repeated testing demonstrated a dose-dependent inhibitory activity. This finding could then explain the lowered action of the pet ether fraction in the OGTT as against the purified components. While cuminaldehyde and cuminol demonstrated a lowering of blood glucose on a par with glibenclamide in the OGTT (Table 3), the pet ether fraction showed higher values at the end of 2 h (Table 1). It was also observed that while the pet ether fraction demonstrated an increase in insulin secretion by 2·52-fold (Fig. 1), cuminol and cuminaldehyde demonstrated enhancement by 3·34- and 3·85-fold, respectively, when compared with the 11·8 mm-glucose control (Fig. 3). In contrast, the inhibitory component revealed a 59·6 % reduction in insulin secretion when compared with the 11·8 mm-glucose control (Fig. 5). Several other compounds, such as ghrelin(Reference Peng, Xiaolei and Al-Sanaban27) and Y-26 763(Reference Cosgrove, Straub and Barnes28), have been reported to inhibit insulin secretion. However, only a few plant compounds such as resveratrol have been reported to inhibit insulin secretion(Reference Szkudelski29). In attempting to study the role of this inhibitor, we identified that it possesses β-cell protective action, as evidenced by the comet assay, and showed reduced nitrite production (Fig. 6). It is proposed that a combinatorial therapy involving a secretagogue and a β-cell protective agent should be more beneficial in prolonged treatment. We noted that the long-term treatment with the pet ether fraction containing the combination revealed better lowering of blood glucose and improved serum insulin levels when compared with the glibenclamide-treated group. We also observed that the treated animals appeared more healthy and active. Although the exact mechanism of action of the inhibitor needs to be studied in detail, its β-cell protective action was evident in the comet assay.
Preventing the burn out of β-cells would be beneficial in the long-term management of diabetes. In 1976, for the first time, Greenwood et al. (Reference Greenwood, Mahler and Hales12) reported improvement in insulin secretion after the administration of diazoxide (K+-ATP channel opener) to diabetic subjects for 7 d. Similar protective effects were observed in patients classified with type 1 and type 2 diabetes who were given diazoxide(Reference Guldstrand, Grill and Bjorklund30).
Hence, we propose that the problem of β-cell damage due to repeated stimulation of islet cells, which poses as a major drawback for insulin secretagogue action, can be overcome by combining the therapy with β-cell protective action that prevents excess overt stimulation of β-cells. It appears that nature suggests a way out in having both the activities in the same plant, namely C. cyminum, and demonstrates better outcome on prolonged treatment.
Thus, in conclusion, drawing from the wisdom of ancient traditional medicines, we report here for the first time two components, namely cuminaldehyde and cuminol, as major insulin secretagogues and the presence of an inhibitor which also functions as a β-cell protective agent in C. cyminum that can lower blood glucose without fear of hypoglycaemia or β-cell burn out and paving the way for a new rationale for oral anti-diabetic therapy in diabetes mellitus. The crucial advantage of these findings is that cumin is a routinely edible spice consumed all over the world with no reported toxicity.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513000627
Acknowledgements
S. B. P. acknowledges the University Grant Commission, New Delhi, India for a fellowship under the Special Assistance Program for Basic Scientific Research. No special funding was received for the study. S. B. P. carried out the majority of the experimental analysis, designed the experiment and contributed to the writing of the manuscript. S. S. T. and M. M. J. contributed to the experimental study. V. S. H. and A. U. A. supervised the experimental work. A. U. A. contributed to the planning of the experimental work, the interpretation of the results and the writing and drafting of the manuscript. All authors read and approved the final manuscript. The authors have no conflicts of interest.