Micronutrient deficiencies, known as ‘hidden hunger’, affect more than two billion people worldwide( Reference Bailey, West and Black 1 ). Micronutrients are essential for human physiological functions. Micronutrient deficiencies thus promote developmental disorders in children and contribute to high morbidity and mortality( Reference Ruel-Bergeron, Stevens and Sugimoto 2 ). Deficiencies in vitamin A, Zn, Fe and iodine are the most frequent( Reference Bailey, West and Black 1 ) and are estimated to be responsible for 11–12 % of deaths in children under 5 years of age( Reference Ahmed, Hossain and Sanin 3 , Reference Bhutta 4 ).
Fe deficiency is the most common micronutrient deficiency, affecting 30 % of the world’s population( Reference Ruel-Bergeron, Stevens and Sugimoto 2 ). Fe deficiency in its advanced form leads to anaemia. Anaemia affects 293 million children worldwide( Reference Balarajan, Ramakrishnan and Ozaltin 5 ) and Fe deficiency of nutritional origin is its leading cause in developing countries( Reference DeLoughery 6 ). In Mali, the Demographic and Health Survey of 2013 estimated that 82 % of the children aged 6–59 months were suffering from anaemia( 7 ). Fe deficiency and anaemia have negative consequences on the development and growth of the children. They lead to psychomotor and cognitive impairments( Reference Bailey, West and Black 1 , Reference Jáuregui-Lobera 8 ) and learning disabilities( Reference Abbaspour, Hurrell and Kelishadi 9 ). They also promote immune deficiencies( Reference Hassan, Badr and Karam 10 ), therefore leading to infections.
Micronutrient deficiencies are the result of undernourishment, associated or not with micronutrient-absorption disorders due to infection, inflammation or disease( Reference Ruel-Bergeron, Stevens and Sugimoto 2 ). To reduce micronutrient deficiencies, different strategies are proposed, especially food fortification and supplementation. Several foods have been fortified with Fe, such as infant flours, dairy products, sugar and salt, but these products are often not accessible to the poorest. Routine Fe supplementation is recommended only in non-endemic areas of malaria or in controlled endemic areas because of the fear of feeding Plasmodium with Fe administration and therefore leading to the exacerbation of malaria episodes( 11 ). More recently, the strategy recommended by the WHO to fight Fe deficiency in regions where the prevalence of anaemia reaches 20 % in children under 2 years or 5 years of age is home fortification of foods (HFF) with multiple-micronutrient powders with low doses of Fe, to allow the use in endemic or non-endemic malaria areas( Reference Imdad and Bhutta 12 , 13 ). Its superiority to the administration of Fe alone has been proved in the fight against Fe deficiency and anaemia in infants under 2 years( Reference De-Regil, Suchdev and Vist 14 ).
In Mali, in the Sahel Nioro Circle, an HFF programme was initiated in a community setting by the Malian Red Cross and the Belgian Red Cross. The poor economic and food situation in the area explains the intervention of the Red Cross. The Circle of Nioro is indeed an arid zone, where economic activities are limited mainly to livestock and extensive subsistence agriculture. However, these activities are insufficient to cover the needs of the landlocked agro-pastoral population that is poorly served in health, social and educational services, because of the big size of the rural areas and large distances between villages and towns.
The populations in the Nioro Circle live in recurrent food insecurity characterized by low food availability, poor access to essential foodstuffs, instability of food availability, inadequate use of food and poor access to care, all together with a poor health system and with a health coverage needing improvement (twenty-five functional health centres out of twenty-eight), outdated infrastructures and insufficient qualified staff. The poverty index in the Nioro is 50 %. Many rural roads are often impassable in rainy periods, isolating entire villages from access to health services.
The HFF programme in Mali is a pilot and it is necessary to evaluate its effectiveness before making a recommendation for extension at the country level. The objectives of the present study were to evaluate the effectiveness of HFF of children aged 6–23 months on Hb change, anaemia and weight under real community settings with little monitoring.
Methodology
Design and participants
A pragmatic randomized controlled community trial was conducted in children aged 6–23 months. The trial took place in two municipalities and forty villages. The two municipalities were chosen randomly among five municipalities of the programme intervention area in the Circle of Nioro in Mali. The chosen municipalities were Korera-Kore and Sandaré.
Inclusion and exclusion criteria
In each municipality, all villages and village hamlets (with at least twenty children aged 6–23 months) were included. In each selected village, all children aged 6–23 months were eligible to take part in the study. The age of the children was documented through the birth books and/or important local events of the calendar after interview with the mother. All eligible children had their weight, length and mid-upper arm circumference (MUAC) measured before inclusion. All children diagnosed with severe acute malnutrition were excluded and referred to the health centre for treatment. Children who were receiving Fe supplements at the time of the study were also excluded.
Interventions
In the villages randomized into the supplementation group, the children received packets of micronutrient powder to be given daily by the mother during 90 d. The nurses of the Red Cross programme brought the micronutrient packets to the villages and they were distributed monthly to mothers by the community volunteers. Mothers were asked to put all the contents of the packet into a small amount of meal (lukewarm or cold) that the child could eat and mix it with the meal in a homogeneous way.
In the intervention group, mothers also received group counselling on optimal infant and young child feeding. The counselling was realized every 15 d by community volunteers trained during 3 d. In each village, the communities designated between four and six community volunteers who made up the Village Health Committee, of whom half were women. Women were selected from those who adequately observed the recommended child feeding practices and whose child had good nutritional status. Men chosen were either the community health workers or designated by the community among people who could read and write.
Pictograms were made available to community volunteers to facilitate group counselling sessions. The sessions were given in groups of about twenty women. The messages delivered on optimal infant and young child feeding were in accordance with the following recommendations of the WHO( Reference Dewey 15 ):
1. early breast-feeding within an hour after birth;
2. exclusive breast-feeding during the first 6 months of life;
3. the introduction of solid supplemental, healthy and nutritionally satisfactory foods at the age of 6 months in addition to continuation of breast-feeding up to the age of 2 years and beyond.
Recipes based on local foods were discussed with the participants.
The non-supplemented group did not receive any supplementation, but mothers received group counselling as described above.
The micronutrient powder used for supplementation contained, per packet: 400 µg vitamin A (retinol equivalents), 150 µg folic acid, 5 µg cholecalciferol (vitamin D3), 90 µg iodine, 17 µg Se, 0·9 µg vitamin B12, 6 mg niacin, 10 mg Fe, 4·1 mg Zn, 0·56 mg Cu, 0·5 mg thiamin, 0·5 mg riboflavin, 30 mg vitamin C, 0·5 mg vitamin B6 and 5 mg vitamin E. This composition is close to the WHO recommendation of 12·5 mg Fe, 300 μg vitamin A and 5 mg Zn per packet( 13 ).
Randomization and concealment
The Circle of the Nioro covers an area of 11 060 km2 and counts sixteen municipalities with 290 532 inhabitants. The municipality of Korera-Kore with 19 598 inhabitants has sixteen villages and twenty-one hamlets. The municipality of Sandaré counts nineteen villages and hamlets with 25 591 inhabitants. All villages (with at least twenty children aged 6–23 months) in each municipality were included.
The random selection of villages in the intervention or control group was done in a computer-aided way using Microsoft® Excel 2010 software. In each included village, every child aged 6–23 months whose parents consented to participate was included. Given the absence of a placebo, it was not possible to conceal the intervention to children and parents. In each of the two municipalities (Korera-Kore and Sandaré), ten villages were randomized in the intervention group and ten others in the control group.
Follow-up
Each child included was monitored and re-evaluated at endline (after 3 months).
Evaluations
Primary and secondary endpoints
The primary endpoints were change in weight, Hb concentration and anaemia after 3 months. Secondary endpoints were change in length and change in MUAC.
Anthropometric measurements
The measurements realized were weight, MUAC and length, each at baseline and endline (3 months). Children’s weight was assessed using a calibrated balance allowing double weighing (mother–child) and automatic deduction of the child’s weight. The balance used was a SECA® brand with an accuracy of 100 g. Children’s length was measured using a locally made gauge with an accuracy of 0·1 cm. MUAC was evaluated using a standard colour-coded tape. The measure was done on the left upper arm at the mid-point between the tips of the shoulder and the elbow with an accuracy of 1 mm.
Hb concentration measurement
Children’s Hb concentration was measured using the HemoCue® Hb 301 automatic analyser. The equipment is portable, allowing an extemporaneous measurement of Hb concentration in the villages by capillary sampling. The HemoCue Hb 301 analyser performs two wavelength measurements (506 and 880 nm) to compensate for turbidity. The system is calibrated according to the method of cyanmethaemoglobin (HiCN), the international reference method for the determination of Hb concentrations in blood( 16 ). The calibration is done in the factory and does not need to be remade. The HemoCue Hb 301 is equipped with an internal self-quality control system. Each time it is switched on, the analyser automatically checks the measuring performance.
Adverse effects monitoring
Each month, during the delivery of supplements, mothers were asked if their child had developed an illness (fever or diarrhoea) during the last month. The data collected each month were analysed to determine whether the child had at least one episode of diarrhoea or fever within the 3 months of follow-up.
Sample size
For evaluation of weight gain
The sample size per group was determined using a recommended formula for cluster randomized trials( Reference Rutterford, Copas and Eldridge 17 ). The size was computed to detect a significant mean weight difference of 0·30 kg between the two groups( Reference Imdad, Yakoob and Bhutta 18 ) with an sd of 1·1 kg( Reference Barth-Jaeggi, Moretti and Kvalsvig 19 ) at the significance level α of 0·05 with a power of 80 %. The intra-cluster correlation coefficient used was estimated to be 0·02 from a preliminary study in Burkina Faso (YE Somassè, I Sawadogo, A Compaoré et al., unpublished results), a similar context in Mali. The average size of the clusters was thirty-five children aged 6–23 months. The calculated sample size n was 351 children per group, which means 702 children for both groups. Based on this information, it was estimated that twenty villages (with an average of thirty-five children per village) was the minimum sample size of required villages per group.
For Hb concentration analysis
The mean difference in Hb change to be detected was estimated to be 0·5 g/dl with an sd of 1·05 g/dl, with intra-cluster correlation coefficient being 0·016( Reference Barth-Jaeggi, Moretti and Kvalsvig 19 , Reference Jack, Ou and Chea 20 ). To detect this difference at significance level of 0·05 with a power of 80 %, the estimated sample size per group was 107 subjects. The formula used was the same as that for weight gain. It was planned to randomly choose twenty children per village to reach a sample of 400 children, which would remain sufficient taking account of a loss to follow-up of 20 %. The sample size required to show a decrease in the prevalence of anaemia of 31 %( Reference De-Regil, Suchdev and Vist 14 ) (from 85 to 59 %) in the intervention group was 159 subjects in each group, taking an intra-cluster correlation of 0·19 into account( Reference Ngnie-Teta, Receveur and Kuate-Defo 21 ).
Ethical aspects
The study was approved by the Ethics Committee of the National Institute of Public Health Research of Mali. Prior to inclusion, each mother gave her oral consent after being informed of the study objectives. All children diagnosed with severe acute malnutrition were referred for treatment in the health centre.
Statistical analyses
The usual descriptive statistics were presented: mean and sd for quantitative variables when the distribution was normal, or median and 25th–75th percentile if not. For the categorical variables, proportions were presented.
Comparison of the groups of children (intervention and control) for socio-economic and anthropometric characteristics at baseline was made, to ensure their comparability. To analyse the HFF effect, we performed an intention-to-treat analysis using regression techniques with se taking account of the cluster design. Linear regression was applied to analyse weight gain, the gain in MUAC and the change in Hb concentration from baseline to endline. Logistic regression was used to analyse the dichotomous criteria: adverse effects. McNemar’s χ 2 test was used to test the change in the proportion of severe anaemia in each group. For gain in length (from baseline to endline), for which the distribution was asymmetric, we performed a quantile (median) regression using a bootstrap procedure, taking account of the clusters with 500 re-samplings.
All tests were two-tailed and performed with the statistical software package STATA version 12.1.
To determine the prevalence of different levels of anaemia, the WHO-recommended thresholds were used( 16 ). Thus, for children aged between 6 and 59 months, the thresholds defining anaemia were: ≥11 g/dl, non-anaemia; 10–10·9 g/dl, mild anaemia; 7–9·9 g/dl, moderate anaemia; and <7 g/dl, severe anaemia.
Results
Figure 1 shows the flow diagram of study participants. Out of 722 children initially included, 542 were reviewed and evaluated for anthropometric measurements at endline (3 months), which means a loss to follow-up rate of 24·9 %. For Hb concentration, 440 children were sampled at baseline and 359 were reviewed at endline (3 months), which means a loss to follow-up rate of 18·4 %. No death was reported in both groups (intervention and control).
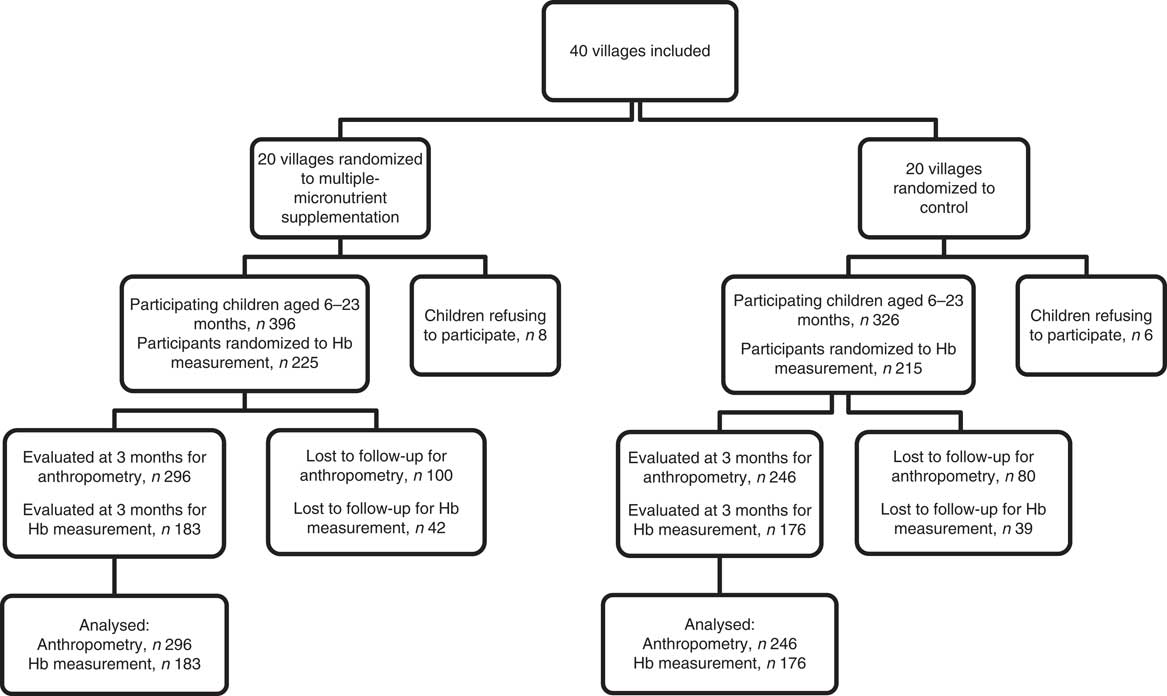
Fig. 1 Diagram of participants in the cluster-randomized controlled trial, Nioro Circle, Mali, January 2016
Characteristics of children in the two groups
Table 1 shows that intervention and control groups of children were comparable in terms of age, sex, anthropometric measurements, education level of mother and Hb concentration at baseline. The mean Hb concentration at baseline was 9·1 g/dl in each group.
Table 1 Characteristics of participants at baseline, by study group: children aged 6–23 months (n 722), Nioro Circle, Mali, January 2016
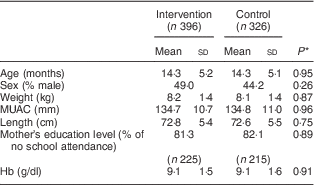
MUAC, mid-upper arm circumference.
* P values were computed taking account of se adjusted for the cluster effect.
We compared the characteristics of children lost to follow-up with those who completed the follow-up and no significant difference was found.
Effect of supplementation on anthropometric measurements
Table 2 shows that supplementation had no significant effect on growth (anthropometric measurements). Indeed, the mean weight gain was similar in the intervention and control groups (0·76 and 0·74 kg, respectively), as was the MUAC gain (3·4 and 3·8 mm, respectively). However, there was a median gain of 3·0 cm in length in the intervention group compared with 2·0 cm in the control group, but the difference was not statistically significant.
Table 2 Effect of the intervention on anthropometric and haematological characteristics, by study group, among children aged 6–23 months (n 542), Nioro Circle, Mali, January 2016
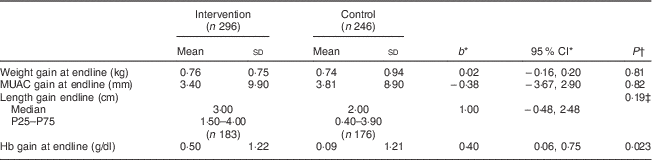
* Regression coefficient (intervention/control) with the 95 % CI.
† P values were computed taking account of se adjusted for the cluster effect.
‡ Computed from a regression on medians with a bootstrap technique and adjustment for the cluster effect.
Effect of supplementation on Hb concentration and anaemia prevalence
Hb concentration and anaemia prevalence at baseline
As shown in Table 1, the mean Hb concentration of children at baseline was 9·1 g/dl, with no difference between control and intervention groups. The prevalence of anaemia was 89·9 % and was also comparable between the two groups at baseline (90·9 % in the intervention group v. 88·9 % in the control group).
Figure 2 shows the distribution of children according to the degree of anaemia at baseline. Only one child out of ten (10·1 %) had no anaemia and about the same proportion had severe anaemia (8·7 %).
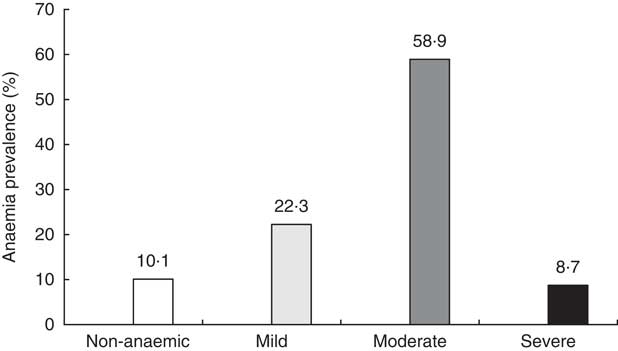
Fig. 2 Prevalence of anaemia at baseline among study children aged 6–23 months with Hb concentration measurement (n 440), Nioro Circle, Mali, January 2016.
Gain in Hb concentration and anaemia prevalence at endline
The mean gain in Hb concentration at endline (3 months) was 0·50 g/dl in the intervention group v. 0·09 g/dl in the control group. The difference was statistically significant (Table 2). Thus, the gain in Hb concentration was slightly better if the children were supplemented. Figure 3 illustrates the distribution of Hb concentration gain.
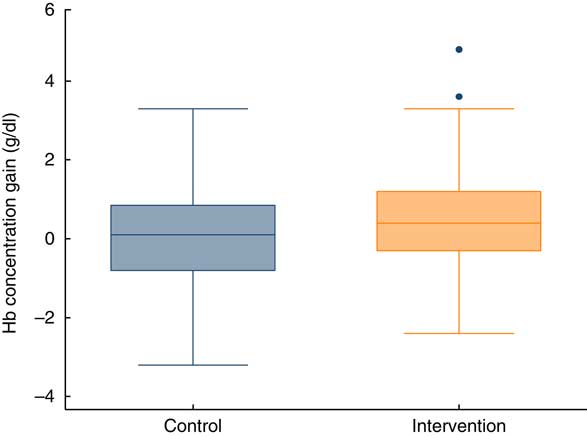
Fig. 3 (colour online) Box-and-whisker plots showing gain in Hb concentration at endline (after 3 months), by study group, among children aged 6–23 months (n 359), Nioro Circle, Mali, January 2016. The bottom and top edge of the box represent the 25th and 75th percentile (interquartile range); the line within the box represents the median; the ends of the bottom and top whiskers represent the minimum and maximum values; and the dots represent outliers
Among the children who completed the follow-up in the control group, the prevalence of anaemia was almost unchanged, and we even observed an increase of severe anaemia from 8·5 to 10·8 % (P=0·48). Conversely, in the intervention group, the prevalence of severe anaemia decreased from 9·8 to 1·6 % (P<0·001). Concurrently, the proportion of children with normal Hb concentration increased in the intervention group from 8·7 to 14·2 %, whereas it barely increased in the control group (11·9 to 12·5 %).
Episodes of diarrhoea and fever
Table 3 shows that the proportion of children who had at least one episode of diarrhoea during the follow-up was similar between the intervention and control groups (12·6 and 11·7 %, respectively). The difference was not statistically significant.
Table 3 Proportion reporting diarrhoea during the intervention, by study group, among children aged 6–23 months (n 542), Nioro Circle, Mali, January 2016
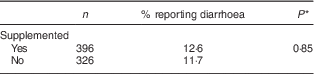
* Adjustment of se according to cluster effect.
The proportion of children who had at least one episode of fever during the follow-up (Table 4) was twice as high in the control group than the intervention group (2·3 v. 5·2 %, respectively), but the difference was not statistically significant.
Table 4 Proportion reporting fever during the intervention, by study group, among children aged 6–23 months (n 542), Nioro Circle, Mali, January 2016
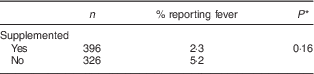
* Adjustment of se according to cluster effect.
Per-protocol analysis
The adherence to supplementation was measured in 235 mothers who returned the empty packets of supplements as recommended. The proportion of micronutrient packets administered to children varied from 55 to 100 %, with an average of 93 %. One-quarter of the children (27 %) consumed 100 % of the packets. There was no association between Hb concentration gain and the number of micronutrient packets administered (P=0·54). The Hb concentration gain for children who consumed 100 % of the packets was even lower than that of other children (0·34 v. 0·58 g/dl), but the difference was not statistically significant.
Discussion
We noticed that the multiple-micronutrient supplements given over 3 months had no effect on the growth of the children (gain in weight, length and MUAC) compared with the control group. This is in line with several studies such as that of Jack et al., who supplemented children daily from the age of 6 months over a period of 6 months( Reference Jack, Ou and Chea 20 ). Similarly, a Peruvian study concluded that there was no effect on growth in children aged 6–12 months supplemented for 6 months( Reference López de Romaña, Cusirramos and López de Romaña 22 ). Systematic reviews of clinical trials have come to the same conclusion that multiple-micronutrient supplementation does not improve growth either in weight or in length( Reference De-Regil, Suchdev and Vist 14 , Reference Salam, MacPhail and Das 23 ). In our study, the absence of an effect on growth and weight could be due to the short duration of the supplementation (3 months).
The multiple-micronutrient supplements given for 3 months significantly improved Hb concentration and reduced the proportion of severe anaemia in our children, while the prevalence of severe anaemia increased in the control group. However, the overall prevalence of anaemia did not decrease significantly with the intervention. According to a Cochrane review, multiple-micronutrient supplementation reduces the prevalence of anaemia by 31 % on average in children aged 6–23 months and the prevalence of Fe deficiency by 51 % on average( Reference De-Regil, Suchdev and Vist 14 ). The small reduction (of 5 %) in anaemia prevalence in our study could be related to the conditions of supplement use. Indeed, there was no close monitoring to reinforce compliance as is usually done in clinical trials. We were assessing the effectiveness of home supplementation under real conditions of use in the community. Despite the explanations provided to them, some mothers did not always follow the recommendations, putting the micronutrient powder in a hot meal, which could decapsulate the Fe and change the taste or colour of the meal with the risk that the child does not consume it. Sometimes, some mothers put the powder into too large quantities of meals that the child could not eat entirely. The adherence rate varied from 55 to 100 %, but we did not demonstrate a relationship between adherence and gain in Hb concentration. This observation might be due to lack of statistical power because adherence was measured in only 235 mothers who returned the empty packets of micronutrient powder.
These findings lead us to believe that the reduction in the prevalence of anaemia was modest because of its multifactorial origin in these communities. Fe deficiency is only one of the observed causes. Also, we did not measure the Fe status of children to assess whether the supplements given helped to improve it. In our sample, almost nine out of ten children were anaemic and it is unlikely that the only reason is micronutrient deficiency. Other common causes of anaemia in African regions are malaria, Schistosoma infections and hookworms. A Kenyan study found that 70 % of pre-school children were infected with at least one or more of these parasites( Reference Kinung’hi, Magnussen and Kaatano 24 ). Some studies even claimed that Plasmodium was the leading cause of anaemia in children under 5 years of age in malaria-endemic areas( Reference Njua-Yafi, Achidi and Anchang-Kimbi 25 ). Deworming of the children is one of the strategies to reduce the prevalence of anaemia among children aged 6–59 months in Africa( Reference Kinung’hi, Magnussen and Kaatano 24 ). Apart from these causes, haemoglobinopathies represent also a cause of anaemia in that setting( Reference Kadima, Gini Ehungu and Ngiyulu 26 ).
The high anaemia prevalence observed in the present study is challenging. It requires the implementation of a strategy aiming at multifactorial causes because micronutrient deficiency, namely Fe deficiency, is only one factor upon which supplementation had only a modest effect. Anaemia is a real public health problem in developing countries, which is maintained (like other micronutrient deficiencies) by an intergenerational cycle( Reference Ruel-Bergeron, Stevens and Sugimoto 2 ) and in fact must be a priority in development agendas. Deficient children develop delays in growth and psychomotor development and a higher mortality risk. These effects persist in adolescence with learning disabilities which make them less able to become prosperous adults; or, more precisely, adults without the possibility to perform adequately or to compete on the employment market and lacking the capacity for personal planning and family achievement. Anaemic adult women will have pregnancy complications and will give birth to children with stunted growth.
Routine Fe supplementation has long been controversial in malaria-endemic areas because of the risk of malaria incidence increase( Reference Diallo, Sie and Sirima 27 ). Studies such as ours that show the interest and safety of multiple-micronutrient supplementation containing a low dose of Fe should lead to the generalization of this strategy in developing countries as recommended by the WHO( 13 ), but as part of a more complete package of interventions, given the multifactorial origin of anaemia. This intervention package should include improvement in the quality of infant feeding from 6 to 23 months using local foods for sustainability.
In regard to the safety of the supplementation, some studies have highlighted a high risk of diarrhoea in children supplemented with multiple micronutrients( Reference Soofi, Cousens and Igbal 28 ). We did not find this effect in our study.
Mali is an endemic country for malaria. Our study showed that episodes of fever were not more frequent in supplemented children. Malaria is the most frequent cause of fever in endemic countries for malaria( Reference Soofi, Cousens and Igbal 28 – Reference Wanja, Kuya and Moranga 30 ). No specific intervention in relation to malaria was implemented in the intervention villages in addition to the usual awareness measures regarding the use of impregnated mosquito nets done by the health workers and the community health workers. During the study period, these activities did not increase in intensity and thereby explain a possible reduction of malaria episodes. Clinical trials and systematic reviews show that in endemic contexts where minimal protection measures against malaria are implemented, daily supplements of Fe or micronutrients containing Fe do not increase episodes of malaria( Reference Zlotkin, Newton and Aimone 31 , Reference Neuberger, Okebe and Yahav 32 ).
It is important to stress the inherent potential biases in any study. First, the study was not blinded. Participants were aware that their children received micronutrient powders. However, we did a cluster randomization which would reduce the risk of contamination. Moreover, even if the inhabitants of the control village came to know about the powders distributed in other villages, the risk of giving them to their children was low because the powders were not accessible on the market or in pharmacies. We did not have information either that the packets of powders were shared between parents of different villages. The agents who collected the anthropometric measurements and those who performed the Hb concentration measurements were not blind to the children supplemented and those not supplemented. This may have induced a possible bias in the measurements made.
Selection biases were to be feared given the non-negligible rates of loss to follow-up of 25 % for anthropometric measurements at 3 months and 18 % for the Hb concentration measurements. It was mostly due to children whose parents travelled or moved to other regions (nomadism). We compared the sociodemographic, anthropometric and haematological characteristics of the children lost to follow-up at baseline with those of other children and no significant difference was observed.
We did not exclude the children based on premature birth, HIV infection or malaria. These conditions may influence the effect of multiple-micronutrient supplementation. However, the randomization made is supposed to equally distribute these conditions in the different groups. Therefore, this should not have influenced the effect of the intervention.
The device used for Hb concentration measurement was the HemoCue Hb 301. The HemoCue system is based on the cyanmethaemoglobin method and has been shown to be stable and durable under field conditions, reasons why the WHO recommends it as a technique for measuring Hb in field surveys( 16 ). The Hemocue 201 is described as slightly underestimating the real value of Hb concentration( Reference Gwetu and Chhagan 33 , Reference Shamah, Méndez-Gómez-Humarán and Morales Ruán 34 ). With HemoCue Hb 301, which we used, the values obtained were evaluated as being less biased, therefore close to the standard values( Reference Jaggernath, Naicker and Madurai 35 , Reference Rappaport, Karakochuk and Whitfield 36 ).
Conclusion
The present study revealed a high prevalence of anaemia in children aged 6–23 months in the Sahel Nioro Circle in Mali. Anaemia affects nine out of ten children. One in ten children (8·7 %) suffers from severe anaemia with Hb concentration <7 g/dl.
We carried out a pragmatic cluster-randomized controlled trial of HFF with multiple-micronutrient powder for 3 months. Among the children assessed for Hb at 3 months, there was a reduction in the prevalence of severe anaemia in the intervention group from 9·8 to 1·6 %, while it increased from 8·5 to 10·5 % in the control group. Overall, under the real community settings, the HFF resulted in a modest but statistically significant gain in Hb concentration of 0·50 g/dl v. 0·09 g/dl in the control group. HHF did not provide any significant effect on growth. The proportion of children having diarrhoea or fever was not significantly higher when the child was supplemented, which shows a good tolerance of the micronutrient powder. Average adherence to the supplements was high (93 %), showing a good acceptability of the multiple-micronutrient powder.
The present study shows the interest and safety of the WHO strategy of home multiple-micronutrient supplementation containing a low dose of Fe in fighting anaemia, but this strategy should be combined with other interventions for better effectiveness given the high prevalence and the multifactorial origin of anaemia in this setting.
Acknowledgements
Acknowledgements: The authors thank the laboratory technicians of the National Institute of Public Health of Mali for the analyses of Hb concentrations. Financial support: The study was financially supported by the Red Cross of Belgium for Hb concentration assessments only. The Red Cross of Belgium had no role in the design, analysis or writing of this article. No researcher was paid for his/her participation in the study. Conflict of interest: All authors declared that they have no conflict of interest. Authorship: Y.E.S. designed the study, conducted the research, analysed the data and wrote the paper. M.Dr. co-designed the study, performed the statistical analysis and co-wrote the paper. B.T. coordinated the field research, collected data and contributed to the analyses. I.N. co-designed the study, co-analysed the data and co-wrote the paper. O.T. co-designed the study and co-wrote the paper. M.K. co-designed the study and co-wrote the paper. M.Di. co-designed the study and co-wrote the paper. P.D. coordinated the whole study, co-designed the study, co-analysed the data and co-wrote the paper. All authors have read and approved the final manuscript. Ethics of human subject participation: The Ethics Committee of the National Institute of Public Health Research of Mali approved the study. Each mother gave oral consent to participate after being informed of the study objectives.










