Assessing trends in eating habits and dietary patterns is an essential component in the surveillance of population health and the development of dietary advice and interventions. Dietary monitoring requires a tool validated in the target population, and biomarkers of dietary exposure may assist in uncovering the errors associated with the method in use(Reference Bingham1). For a biomarker to be used for validation of a dietary instrument, it should have a strong direct and independent relationship with the nutrient or food group of interest. Recovery biomarkers, like doubly labelled water(Reference Livingstone and Black2) and urinary N(Reference Bingham3), are less influenced by the body’s homeostatic control than concentration biomarkers, which are extensively used in spite of their weaker association with consumption(Reference Hunter4).
Biomarkers that reflect the intake of a food group rather than a specific nutrient are scarce. Plasma carotenoids and urinary flavonoids have been used as biomarkers of fruit and vegetable groups(Reference van Kappel, Steghens, Zeleniuch-Jacquotte, Chajes, Toniolo and Riboli5, Reference Krogholm, Haraldsdottir, Knuthsen and Rasmussen6) and plasma or tissue n-3 PUFA, mainly EPA and DHA, have been used as biomarkers of both total fish and fatty fish intake(Reference Andersen, Solvoll and Drevon7, Reference Marckmann, Lassen, Haraldsdottir and Sandstrom8). However, as lean or semi-lean fish and seafood comprise nearly two-thirds of the total fish intake in Norway(9), a biomarker related to total fish/seafood intake rather than fatty fish and fish-oil supplements could contribute to better validation of this food group than EPA or DHA.
Dietary fish and seafood are rich sources of the essential trace elements iodine and Se, but are also a major source of methylmercury and arsenic(Reference Mahaffey, Clickner and Bodurow10, 11). The presence of methylmercury in human tissues has been shown to be directly related to intake of fish and seafood(Reference Mahaffey, Clickner and Bodurow10), whereas the relationship between blood arsenic, blood Se and intake of fish and seafood has been investigated less. Arsenic in fish and seafood is predominantly present in the organic form arsenobetaine. Organic forms of arsenic are considered non-toxic compared with inorganic arsenic from airborne industrial emissions and in drinking water(Reference Gomez-Caminero, Howe, Hughes, Kenyon, Lewis, Moore, Ng, Aitio and Becking12). In Norway, arsenic levels in drinking water are negligible (<0·2 μg/l). Fish and seafood are good natural sources of iodine, but other food groups have been identified as more important contributors to dietary iodine in some countries(Reference Brussaard, Brants, Hulshof, Kistemaker and Lowik13–Reference Hess, Zimmermann, Torresani, Burgi and Hurrell15).
Monitoring the dietary intake of foods like fish and seafood is complex in large population surveys and often relies on the use of an FFQ, whereas biological measures are possible only in smaller population groups. Fish and seafood intake during pregnancy is of particular interest owing to the potential positive impact of this food group on birth weight and neurodevelopment of the child(Reference Thorsdottir, Birgisdottir, Halldorsdottir and Geirsson16–Reference Hibbeln, Davis, Steer, Emmett, Rogers, Williams and Golding18).
The Norwegian Mother and Child Cohort Study (MoBa), conducted at the Norwegian Institute of Public Health, is a large pregnancy cohort aiming to include 110 000 pregnancies by the end of 2008(Reference Magnus, Irgens, Haug, Nystad, Skjaerven and Stoltenberg19). The aim of the present study was to compare fish and seafood intake assessed by the MoBa FFQ with intake assessed by a 4 d weighed food diary, and to explore how Se, iodine, Hg and arsenic compare with the traditionally used n-3 fatty acids EPA and DHA as biomarkers for total fish and seafood intake.
Experimental methods
Validation study subjects and design
The present study was part of a validation study of a new FFQ developed for use in the MoBa study(Reference Meltzer, Brantsæter, Ydersbond, Alexander and Haugen20). Other results of the validation study have been published elsewhere(Reference Brantsæter, Haugen, Alexander and Meltzer21). Healthy pregnant women in MoBa referred to Bærum Hospital (Norway) were invited to participate in the validation study when they came for routine ultrasound examination at 17–18 weeks of gestation. Exclusion criteria were hyperemesis and anorexia. Before inclusion, subjects had to have completed the MoBa FFQ. The inclusion period lasted from 15 January 2003 to 1 February 2004.
Participants were asked to keep a 4 d weighed food diary (FD) and to provide one 24 h urine collection and a blood sample. Age, self-reported height and weight were recorded. Data pertaining to smoking and education were collected from a separate MoBa questionnaire in which participants answered questions related to lifestyle and demographic factors. Of 120 subjects included, one dropped out due to illness. The average time interval between completion of the FFQ and participation in the study was 24 (sd 12) d. The study protocol was approved by the Regional Ethics Committee of Southern Norway, and informed written consent was obtained from all participants in the validation study.
The FFQ
The MoBa FFQ (available at http://www.fhi.no/dav/22CA50E0D7.pdf) was mailed to all participants around the 15th week of gestation. It is a semi-quantitative questionnaire designed to capture dietary habits and intake of dietary supplements during the first four months of pregnancy. The FFQ includes questions about the intake of 255 food items including ten questions about cold cuts and spreads made of fish/shellfish, sixteen questions about fish/shellfish eaten for dinner, and four questions about cod-liver oil/fish oil/n-3 supplement use.
The questionnaires were optically read. Consumption frequencies were converted into food amounts (g/d) by the use of standard Norwegian portion sizes. FoodCalc(Reference Lauritsen22) and the Norwegian food composition table(Reference Rimestad, Borgejordet, Vesterhus, Sygnestveit, Løken, Trygg, Pollestad, Lund-Larsen and Omholt-Jensen23) were used for calculating nutrients from food. A database containing details of the declared content of supplements was developed for the calculation of nutrients from dietary supplements(Reference Brantsæter, Haugen, Hagve, Aksnes, Rasmussen, Julshamn, Alexander and Meltzer24).
4 d weighed food diary
Participants were asked to weigh and record all foods, beverages and dietary supplements consumed during three consecutive weekdays and one weekend day. Each participant was given an FD and a digital balance, and asked to continue with their normal food intake. Each completed FD was checked for completeness of description by the project nutritionist (A.L.B.).
Blood sampling
Non-fasting blood samples were drawn by venepuncture at the time of recruitment into Vacutainers intended for trace element measurement (Becton and Dickinson, Plymouth, UK). After collection, the blood tubes were inverted to ensure complete mixing of the anticoagulant. Samples were stored at −70°C within 2 h of venepuncture. Erythrocytes for lipid measurements were isolated by centrifugation for 10 min at 250g, washed with 0·9 % (w/v) NaCl, resuspended in NaCl and stored at −70°C until analysis.
24 h urinary collection
At the end of the FD period, each participant provided one 24 h urine collection taken on a weekday. On the first morning of the urine collection, participants were asked to discard their first urine specimen and to collect all specimens for the next 24 h. Participants were provided with a funnel and bottles. All urine was pooled for each participant and the samples stored at −20°C within 8 h of collection.
Analysis of biomarkers
Blood concentrations of arsenic, Se and Hg were determined by inductively coupled plasma–sector field mass spectrometry using an Element 2 mass spectrometer (Thermo Electron, Bremen, Germany) following previously described methods(Reference Ellingsen, Dubeikovskaya, Dahl, Chashchin, Chashchin, Zibarev and Thomassen25). In short, 1·5 ml of 65 % (w/v) ultrapure nitric acid was added to 1 ml of whole blood in a polypropylene digestion tube and then heated to 95°C for 1 h. After cooling, an internal standard solution (containing 205Tl for 200,201,202Hg; 72Ge for 75As and 77,78,82Se) was added to the digested blood sample before dilution to volume (14 ml). The instrument was calibrated with whole blood matrix matched standard solution and the accuracy was determined by use of Seronorm Trace Elements human whole blood quality control samples (Sero Ltd, Asker, Norway). The measured values for arsenic, Se and Hg were on average within ±5 % of the recommended values supported by the producer. The within-assay precision was typically 3–5 %.
Urinary concentration of iodine was determined by inductively coupled plasma–mass spectrometry (Agilent Quadrupole 7500c mass spectrometer; Agilent Technologies, Santa Clara, CA, USA)(Reference Julshamn, Dahl and Eckhoff26).
For analysis of the fatty acid pattern in erythrocytes, approximately 4 × 109 cells were used. Erythrocyte lipids were extracted by the method of Folch et al.(Reference Folch, Lees and Sloane Stanley27). The phospholipid fraction was separated from neutral lipids using bonded silica gel columns (Analytical International, Harbor City, CA, USA) by sequential elution with CHCl3 and MeOH(Reference Kaluzny, Duncan, Merritt and Epps28). It was then transmethylated(Reference Metcalfe and Schmitz29) and analysed by GC using an SP-2340 capillary column on a Hewlett-Packard 5880A gas chromatograph (Palo Alto, CA, USA) equipped with a flame-ionization detector, with Ar as the carrier gas. The oven temperature was maintained at 140°C for 10 min and then increased by 3°C/min up to 250°C. The fatty acids were identified by comparing retention times with authentic standards. The fatty acid pattern in erythrocyte phospholipids was not obtained in twenty-five subjects owing to technical problems in the fatty acid analysis and insufficient amounts of samples for re-analysis.
Statistical analysis
The differences between food intakes calculated by the FFQ and the FD were tested with the Wilcoxon signed rank test (paired data) and the differences between groups were tested using the Mann–Whitney U test (unpaired data). The linear trend in blood arsenic, Se and Hg, urinary iodine excretion, erythrocyte EPA and erythrocyte DHA with fish and seafood intake was assessed by regression.
The FFQ and the FD may be considered as two independent, unbiased methods for assessing dietary intake and therefore their estimation errors may be expected to partially cancel out, making their average a better estimate of true intake than either. As the averages provided better model fits, they were used in subsequent analyses.
For analysis of the association between blood and urine data and indicators of fish intake, fish spread and fatty fish were aggregated into one variable (as fish spreads are mainly fatty fish) and, likewise, lean fish and processed fish foods were aggregated.
The associations between the parameters were analysed by multiple regression. The dependent variables were transformed to the power giving best model fits and, in order to compare coefficients as easily as possible, they were ‘standardized’ to a mean of 1. When several explanatory variables were used in a model, they were transformed to make an independent set. The basic assumption of ordinary regression analysis – that for every combination of the explanatory variables, the dependent variable follows distributions with the same variance – was in no case shown to be false. Only covariates significantly different from zero were retained in the models.
Regression coefficients from standardized models are reported, as well as adjusted correlation coefficients describing total model fit.
The significance level was set at 5 % (two-tailed) and all analyses were performed using the SPSS statistical software package version 14 (SPSS Inc., Chicago, IL, USA).
Results
Among the 119 participants in the study, there was large dispersion with regard to age, BMI, education, parity and smoking status (Table 1). Mean daily total fish and seafood intake was 42 g (median 39 g) by the FFQ, 37 g (median 30 g) by the FD and 40 g (median 38 g) as an average of the two methods. Processed fish, i.e. fish in fish fingers, fish burgers, fish pudding and fish au gratin, was the largest contributor to total fish intake (Table 2). Intakes calculated by the two dietary methods correlated significantly for fish used for sandwich spread (fish spread), tuna, shellfish and total fish/seafood intake (Table 2). Three participants (2·5 %) reported no intake of fish or seafood in the FFQ and twenty-one participants (17·6 %) reported no intake of fish and seafood in the FD.
Table 1 Demographic and lifestyle information of validation study participants: subset of pregnant women (n 119) in the Norwegian Mother and Child Cohort Study (MoBa)

FD, 4 d weighed food diary.
†No. of weeks pregnant calculated based on days since date of last menstruation.
Table 2 Daily intake and Spearman correlations of fish and seafood intakes calculated by the FFQ and the 4 d weighed food diary (FD): subset of pregnant women (n 119) in the Norwegian Mother and Child Cohort Study (MoBa)

P5, 5th percentile; P95, 95th percentile.
*Difference between FFQ and FD: P < 0·05.
**Significance of correlation: P < 0·01.
†Fish used as sandwich spread: mackerel/sardines in tomato sauce, sardines in oil, herring, smoked salmon/trout/mackerel, caviar, paste made of fish liver and roe.
‡Canned tuna and tuna in products used as sandwich spread.
§Fish in fish fingers, fish burgers, fish pudding, fish au gratin and fish wok.
||Liver and roe as dinner.
¶Crab, shrimp and mussels.
One subject with extremely high blood arsenic concentration (>6 sd above the 95th percentile) was excluded from analyses regarding arsenic. The mean, median and range indicator values of biomarkers of the different nutrients in all participants and supplement non-users are presented in Table 3.
Table 3 Summary statistics for biomarkers of the different nutrients, in all participants and supplement non-users: subset of pregnant women (n 119) in the Norwegian Mother and Child Cohort Study (MoBa)

P5, 5th percentile; P95, 95th percentile.
†Erythrocyte fatty acid results available for ninety-four subjects only.
‡One outlier excluded.
Use of dietary supplements containing Se was reported by twenty-seven women in the FFQ and by thirty women in the FD. No statistical difference was found in blood Se between users and non-users of Se supplements. Iodine-containing supplement use was reported by twenty-three women in the FFQ and by twenty-five women in the FD. Urinary iodine excretion was twice as high in participants taking an iodine-containing dietary supplement (median at time of the FD: 220 μg/24 h) than in non-users of iodine supplements (median: 110 μg/24 h, P = 0·001).
Use of EPA- and DHA-containing supplements was reported by eighty-two women in the FFQ and by seventy-nine in the FD, whereas twenty-nine women had not taken any supplements containing n-3. The sum of erythrocyte n-3 fatty acids differed significantly between users and non-users of n-3 supplements (P < 0·001), but not erythrocyte DHA and EPA alone. Erythrocyte membrane EPA (% of fatty acids) did not correlate with dietary EPA (diet and supplements), whereas erythrocyte membrane DHA (% of fatty acids) correlated significantly with dietary DHA estimated by the FFQ (r = 0·25, P = 0·014), the FD (r = 0·24, P = 0·018) and the average (r = 0·28, P = 0·006).
Cod-liver oil is the most common vehicle for n-3 fatty acids in food supplements in Norway. Thus this oil might also serve as a vehicle for the non-essential elements under investigation in the present survey. We lack data for the arsenic and Hg content of cod-liver oil but personal communication from the main Norwegian producer (Mőller's) indicated that the concentrations are very low, and there was no difference in blood values between supplement users and non-users when it came to these two elements.
Women who were daily smokers in pregnancy (n 3) had lower blood Se concentration (median 77 μg/l) than non-smokers (median 106 μg/l, P = 0·001) and lower blood arsenic concentration (median 0·80 μg/l in smokers and 1·80 μg/l in non-smokers, P = 0·023). Blood Se was positively correlated with age (r = 0·31). Furthermore, there was a positive association between blood Se and Hg and education level. We found no differences in the intake of fish and seafood between the women with regard to smoking status or education, but the total intake of fish/seafood increased with increasing age (average of FFQ and FD v. age: r = 0·29, P = 0·003).
Three women who reported no intake of fish or seafood in the FFQ and twenty-one women who reported no intake of fish or seafood in the FD had significantly lower blood arsenic and Hg concentrations than women who reported intake of this food group (P FFQ = 0·003 for arsenic and P FFQ = 0·019 for Hg, P FD < 0·001 for arsenic and P FD = 0·008 for Hg). However, the only significant difference between fish-eaters and non-eaters for blood Se, urinary iodine excretion or erythrocyte EPA or DHA was in erythrocyte DHA when n-3 dietary supplement users were excluded. There was no significant positive correlation between fish/seafood intake and erythrocyte EPA.
Blood arsenic was correlated with blood Se (r = 0·25, P < 0·005) and blood Hg (r = 0·44, P < 1·0 × 10−5), and blood Se and Hg were correlated (r = 0·28, P < 0·003). However, the associations with Se lost significance when adjusting for intake of fish and seafood, whereas the arsenic–Hg relationship persisted (r = 0·34, P < 0·0002). No other factors were significantly associated with blood arsenic when entered into models together with fish/seafood intake and blood Hg.
There was no correlation between lean and fatty fish intake in the population studied. In multivariate analyses, total fish/seafood intake and blood Hg (adjusted for fish/seafood intake) emerged as the variables most closely associated with blood arsenic, together covering about 22 % of the variation in blood arsenic (Table 4). The association was non-linear in arsenic, having the equation:
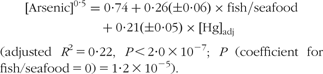
Table 4 Coefficients in regression models with all variables standardized to unit means, describing the association between the potential biomarkers and total fish/seafood intake and separate items of fish/seafood intake: subset of pregnant women (n 118 for arsenic, Hg and Se; n 94 for DHA) in the Norwegian Mother and Child Cohort Study (MoBa)

(*)0·05 < P < 0·06, *P < 0·05, **P < 0·01, ***P < 0·001.
†Model includes fish item and blood Hg adjusted for total fish/seafood intake.
‡Model includes fish item and categories of maternal education.
In the regression models Hg and Se were significantly correlated with tuna and lean/processed fish, DHA was significantly correlated with spread/fatty fish and lean/processed fish, whereas arsenic was significantly (or nearly significantly) correlated with all items (Table 4).
Figure 1 demonstrates how the daily intakes of the separate fish/seafood items as well as total fish/seafood (average of the FFQ and FD) are related to blood arsenic (n 118). Figure 2 illustrates the differences between n-3 supplement users and non-users regarding the association between erythrocyte DHA and fish/seafood intake: the association, as measured by the slopes of the regression lines, is attenuated by the use of supplements (P = 0·002, P = 0·368 and P = 0·005 for zero slope in supplement non-users, supplement users and the whole group, respectively).

Fig. 1 Median and 75th percentile (P75) daily intake (g/d) of tuna (□), shellfish (▪), fish spread/fatty fish (![]() ), lean/processed fish (
), lean/processed fish (![]() ) and total fish/seafood (
) and total fish/seafood (![]() ) by quintile of blood arsenic concentration. Intake estimates are calculated as the average of FFQ and food diary consumption data among a subset of pregnant women (n 118) in the Norwegian Mother and Child Cohort Study (MoBa)
) by quintile of blood arsenic concentration. Intake estimates are calculated as the average of FFQ and food diary consumption data among a subset of pregnant women (n 118) in the Norwegian Mother and Child Cohort Study (MoBa)
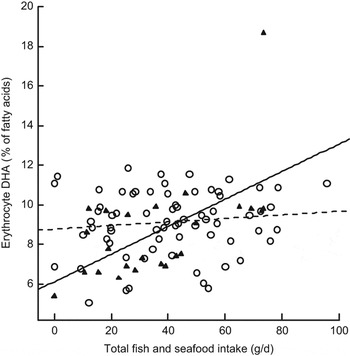
Fig. 2 Impact of fish and seafood intake on erythrocyte DHA in n-3 supplement users (– –○– –; r 2 linear = 0·014) and non-users (—▴—; r 2 linear = 0·342) among a subset of pregnant women (n 118) in the Norwegian Mother and Child Cohort Study (MoBa)
Discussion
In the current study we first compared the intakes of fish and seafood calculated by a new FFQ with intakes calculated by a weighed food diary, and then examined the association between the FFQ and FD intakes of fish and seafood with potential biomarkers in blood and urine. The intakes of the different items of fish and seafood calculated with the new FFQ were confirmed by the intakes recorded in the food diary. Within the methods’ limits of precision, the results may be considered a validation of the FFQ for fish and seafood intake. Our main finding was the positive association between the intake of fish and seafood and blood arsenic concentration in all fish/seafood categories and irrespective of the fatty acid content of the fish (Table 4). This indicates that blood arsenic may be a useful biomarker for short- and medium-term fish and seafood intake.
The average fish consumption in our study population (40 g/d, Table 2) was larger than that reported in pregnant women in Denmark (27 g/d)(Reference Halldorsson, Meltzer, Thorsdottir, Knudsen and Olsen30) and Mexico (32 g/d)(Reference Parra, Schnaas, Meydani, Perroni, Martinez and Romieu31), but lower than that reported in pregnant Icelandic women (47 g/d)(Reference Thorsdottir, Birgisdottir, Halldorsdottir and Geirsson16). In general, young women have the lowest fish consumption in Norway and, in agreement with previous studies, we found that approximately two-thirds of the ingested fish and seafood was lean or semi-lean species(9).
Biomarkers reflect actual rather than reported intakes and are thus unaffected by respondents’ reporting bias, typical of diet records and recalls(Reference Margetts and Nelson32). The most frequently used biomarkers for intake of fish are the marine n-3 fatty acids EPA (20 : 5n-3) and DHA (22 : 6n-3) in adipose tissue, plasma or erythrocytes(Reference Marckmann, Lassen, Haraldsdottir and Sandstrom8, Reference Fuhrman, Barba and Krogh33, Reference Mina, Fritschi and Knuiman34). They reflect fatty fish ingestion and intake of fish-liver oil and other n-3-containing supplements(Reference Mina, Fritschi and Knuiman34). In Norway, there is a high frequency of cod-liver oil/fish-oil supplement use(Reference Brustad, Braaten and Lund35, Reference Haugen, Brantsæter, Alexander and Meltzer36). Most fish consumers eat a mixture of lean and fatty fish, making EPA/DHA fairly good biomarkers in subjects not taking n-3 supplements; however, for the reasons mentioned, they cannot be considered very robust indicators.
In our subjects, erythrocyte DHA, but not EPA, concentration was significantly associated with the total intake of fish/seafood. The association was significant in the whole group, but was far stronger in the subjects not taking n-3-containing supplements (Fig. 2). We have previously reported a significant positive correlation between the erythrocyte membrane phospholipid n-6:n-3 fatty acid ratio and dietary n-6:n-3 fatty acid ratio(Reference Brantsæter, Haugen, Hagve, Aksnes, Rasmussen, Julshamn, Alexander and Meltzer24). Williams et al. found a strong positive correlation for frequency of fish consumption in early pregnancy with both erythrocyte DHA and EPA(Reference Williams, Frederick, Qiu, Meryman, King, Walsh and Sorensen37), whereas Parra et al., like us, found a significant positive association between dietary intake and erythrocyte DHA, but not EPA(Reference Parra, Schnaas, Meydani, Perroni, Martinez and Romieu31). Matorras et al. found no association between dietary intake and erythrocyte membrane fatty acids(Reference Matorras, Perteagudo, Sanjurjo and Ruiz38). During pregnancy, there is a faster turnover of fat storage that may modify the profile of erythrocyte n-3 fatty acids and lower the correlation between dietary intake and erythrocyte n-3 fatty acids(Reference Parra, Schnaas, Meydani, Perroni, Martinez and Romieu31). It has been suggested that EPA is under different homeostatic and metabolic control than DHA(Reference Mina, Fritschi and Knuiman34). The percentage of DHA in erythrocyte membranes is quantitatively much larger than EPA, and during pregnancy the conversion of EPA to DHA is given priority(Reference Williams and Burdge39). The present study is in accordance with previous studies in that the relationship between fish consumption and serum/tissue DHA applies primarily to intake of fatty fish and n-3 supplements(Reference Mina, Fritschi and Knuiman34, Reference Hjartaker and Lund40, Reference Philibert, Vanier, Abdelouahab, Chan and Mergler41).
When total intake of milk and other dairy products, containing iodine from mandatory fodder fortification(Reference Dahl, Johansson, Julshamn and Meltzer14, Reference Frey, Rosenlund, Try and Theodorsen42), was included as a covariate, no fish variables were significantly associated with urinary iodine excretion(Reference Brantsæter, Haugen, Julshamn, Alexander and Meltzer43). Urinary iodine excretion is therefore not suitable as a biomarker for fish and seafood intake in Norwegians.
While fish and shellfish is the main dietary source of Hg, dietary sources of Se also include eggs, cereals and meat(44). Several investigators have evaluated self-reports of fish intake with blood concentrations of Hg and Se in women of childbearing age(Reference Bjornberg, Vahter, Grawe and Berglund45–Reference Bjornberg, Vahter and Petersson-Grawe48). Bates et al. studied the contribution of fish and other foods to variance of Se and Hg status in British adults and concluded that dietary fish, especially oily fish, was a strong predictor of blood Hg and Se in this population(Reference Bates, Prentice, Birch and Delves47). Bjornberg et al. found an increase in blood Hg, but not in Se, with increasing fish consumption in pregnant Swedish women(Reference Bjornberg, Vahter and Petersson-Grawe48).
Pregnant women have been shown to have lower consumption of certain fish species owing to concerns about Hg contamination(Reference Oken, Kleinman, Berland, Simon, Rich-Edwards and Gillman49). In Norway, pregnant women are advised to limit the intake of large perch, wild trout and halibut and to not eat pike, fish liver or exotic predatory fish like fresh tuna. Canned tuna is considered safe(50), although reports show that Hg concentrations here may also vary considerably(Reference Burger and Gochfeld51, Reference Rasmussen and Morrissey52). In spite of the relatively low intake of tuna (Table 2), this food item was significantly associated with blood Hg (Table 4). All tuna reported in the present study was in the form of canned tuna or tuna products used as sandwich spread. The overall low blood Hg levels in our subjects indicate that pregnant Norwegian women do not, generally, need to be more careful about their Hg intake than they are already.
Few studies have examined the association between fish consumption and blood arsenic concentration(Reference Lehmann, Ebeling and Alsen-Hinrichs53–Reference Brown, Newton, Pickford and Sherlock55). We found a positive association between blood arsenic concentration and intake of fish and seafood, but it may be asked whether this could be due to interactions with other elements, such as Hg and Se, as these are also associated with fish/seafood intake. Our data indicate an independent role of arsenic for fish and seafood intake. Arsenobetaine in fish is absorbed and rapidly eliminated via the urine(Reference Gomez-Caminero, Howe, Hughes, Kenyon, Lewis, Moore, Ng, Aitio and Becking12). Volunteers who ingested arsenobetaine through seafood eliminated 70 % of the arsenic within the first two days and 77 % within 8 d of ingestion, and in volunteers who ingested radioactively labelled arsenobetaine with fish, <1 % of the ingested activity remained in the body after 24 d(Reference Brown, Newton, Pickford and Sherlock55). The effect of fish arsenic on blood arsenic has, to our knowledge, been reported in only one study. In a semi-controlled feeding study, Meltzer et al. found a strong positive correlation (r = 0·85, P < 0·001) between dietary arsenic from fish (estimated by analysis) and blood arsenic concentration and between total grams of fish and blood arsenic concentration (r = 0·41, P < 0·02)(Reference Meltzer, Maage, Ydersbond, Haug, Glattre and Holm54).
The rapid excretion might make blood arsenic a good biomarker for short-term fish intake only, but our results strongly indicate that arsenic might also be associated with medium-term fish/seafood intake. Furthermore, the independent association with Hg might indicate a similar association with long-term intake not reflected in the dietary assessment. Fish and seafood may be assumed to be the most important contributor to the Hg load in these women, with blood Hg partially reflecting historical fish/seafood ingestion. To our knowledge, no specific Hg–arsenic interactions have been described to account for the observed association, so it might indicate historical intake of a common source of Hg and arsenic. For blood arsenic to be established as a biomarker for fish and seafood intake, detailed short- and medium-term studies are necessary.
The associations between the potential biomarkers and various subgroups of fish and seafood intake in the present study were generally not very strong, but probably close to what might be expected. The physiological and metabolic changes related to pregnancy may have attenuated some associations, but apart from possibly the fatty acids, we have no indications that the results would have been substantially different with non-pregnant women. As opposed to Se, iodine and EPA/DHA, blood arsenic will normally be minimally influenced by the use of dietary supplements. Another advantage of blood arsenic over the n-3 fatty acids as a biomarker for intake of seafood is the ability to reflect total fish and seafood intake independent of the fat content of ingested fish. Blood arsenic is, however, not applicable as a biomarker for fish intake in areas with high arsenic load from the drinking water.
Exploration of Se, iodine, Hg, n-3 fatty acids and arsenic as potential biomarkers of fish and seafood intake showed that blood arsenic concentration appears to be a robust potential biomarker for fish and seafood intake. Blood arsenic concentration reflected the intake of both fatty and lean fish and seafood in this population of pregnant women, and it is not influenced by dietary supplement use and fat content of the ingested fish, as the n-3 fatty acids are. More studies need to be carried out to confirm the results obtained.
Acknowledgements
Sources of funding: The study was financially supported by research grants from the Research Council of Norway and by the European Commission, 6th Framework Programme, Priority 5 on Food Quality and Safety (FOOD Contract No. 016320 Integrated Project), ‘Newborns and genotoxic exposure risk: development and application of biomarkers of dietary exposure to genotoxic chemicals and of biomarkers of early effects, using mother–child birth cohorts and biobanks (NewGeneris)’. Conflict of interest declaration: None of the authors had a conflict of interest. Authorship responsibilities: A.L.B., M.H., J.A. and H.M.M. contributed to the study design and planning. A.L.B. conducted the study. Y.T. and D.G.E. performed the analysis of blood arsenic, Hg and Se, and T.-A.H. performed the analysis of erythrocyte fatty acids. T.A.Y assisted with the statistical modelling. All authors took part in the interpretation of the data and the preparation of the manuscript. Acknowledgements: The authors would like to thank all the women who participated in the study.








