An estimated 1·6 billion people are anaemic worldwide, and anaemia is common during pregnancy(1). Approximately 50 % of pregnant women have anaemia (Hb <110 g/l) in resource-limited settings, compared with 12–25 % in developed regions(1). Several studies in Sub-Saharan Africa have identified a prevalence of 80 % or higher in HIV-infected pregnant women(Reference Antelman, Msamanga and Spiegelman2–Reference Mehta, Manji and Young5).
Fe deficiency is the leading cause of anaemia worldwide and in pregnancy(6). The consequences of anaemia and Fe deficiency in pregnancy have been well established(Reference Stoltzfus7), and include maternal and infant mortality(Reference Allen8) and low birth weight(Reference Brabin, Hakimi and Pelletier9). A substantial body of evidence also supports the relationships between Fe deficiency and poorer cognitive development in children(Reference Stivelman10) and reduced work capacity in adults(Reference Haas and Brownlie11). Fe supplementation (with folic acid) is standard prenatal care in most countries, based on its likely benefits in preventing maternal anaemia and related complications(Reference Mungen12).
The aetiology of anaemia in the context of HIV is particularly complex. In addition to Fe deficiency, anaemia of inflammation is a leading cause of anaemia in HIV-infected individuals(Reference Weiss and Goodnough13). Nutritional deficiencies of folate and vitamin B12(14) and concurrent infections may also contribute to the risk of anaemia. Studies in HIV-infected pregnant women have found an extremely high prevalence of anaemia, despite presumed availability of Fe supplementation(Reference Antelman, Msamanga and Spiegelman2–Reference Mehta, Manji and Young5); however, most have focused on cross-sectional assessments of anaemia prevalence during pregnancy. Risk factors for incident haematological outcomes need to be examined, to elucidate the aetiology of anaemia in HIV-infected pregnant women receiving Fe supplementation.
We conducted a prospective observational analysis of incident anaemia and Fe deficiency during pregnancy and the postpartum period in HIV-infected women who were pregnant at enrolment and followed throughout the postpartum period.
Methods
Study design
Participants were pregnant women between 12 and 27 weeks of gestation who were enrolled in the Trial of Vitamins (TOV), a double-blind, placebo-controlled randomized trial conducted in Dar es Salaam, Tanzania (1995–1997). That study was conducted to examine the effects of daily micronutrient supplementation to HIV-infected pregnant women on the risks of mother-to-child HIV transmission, HIV disease progression and adverse perinatal outcomes. The detailed design of the trial has been described previously(Reference Fawzi, Msamanga and Spiegelman15).
All women received 120 mg of ferrous Fe (as ferrous sulfate) and 5 mg of folate daily during pregnancy starting at their first antenatal clinic visit, and chloroquine (300 mg) weekly as malaria prophylaxis, as per the then current standard of care in Tanzania. Women received bottles of ninety tablets of Fe–folate supplements and were followed up at monthly visits. HIV/AIDS care was provided according to WHO guidelines; antiretroviral therapy (ART) was not available to most women in Tanzania at the time of the study, including participants in this trial.
Ethics
Informed consent was obtained from all participants. The research protocol was approved by the Research and Publications Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the Tanzanian National AIDS Control Program and the Institutional Review Board of the Harvard School of Public Health.
Assessment of baseline covariates
Structured interviews were conducted at the initial clinic visit (i.e. 12–27 weeks’ gestation, referred to as ‘baseline’), to collect information on demographic characteristics, including age, educational level and socio-economic status, symptoms and obstetric history. Gestational age was determined based on the date of the last menstrual period and assessment by a trained nurse. Physicians conducted a medical evaluation of HIV-related symptoms, disease stage and clinical morbidities; HIV disease stage was determined based on WHO guidelines(16). Blood, stool, urine and vaginal swab specimens were collected at enrolment to assess co-infections, including sexually transmitted infections, malaria and helminth infections. Anthropometric measurements, including height, weight and mid-upper arm circumference, were obtained using standardized procedures and calibrated instruments.
Follow-up
Of the 1078 women in the trial, three were not pregnant, six died before delivery and one was WHO HIV disease stage IV. Nine hundred and four women had a baseline Hb measurement and at least one measurement thereafter, and were included in the present analyses (Fig. 1). Women were followed for a median of 57 months (interquartile range (IQR): 29–67 months), with a mean of 8·6 (sd 4) Hb measurements (median: 9, IQR: 5–12).
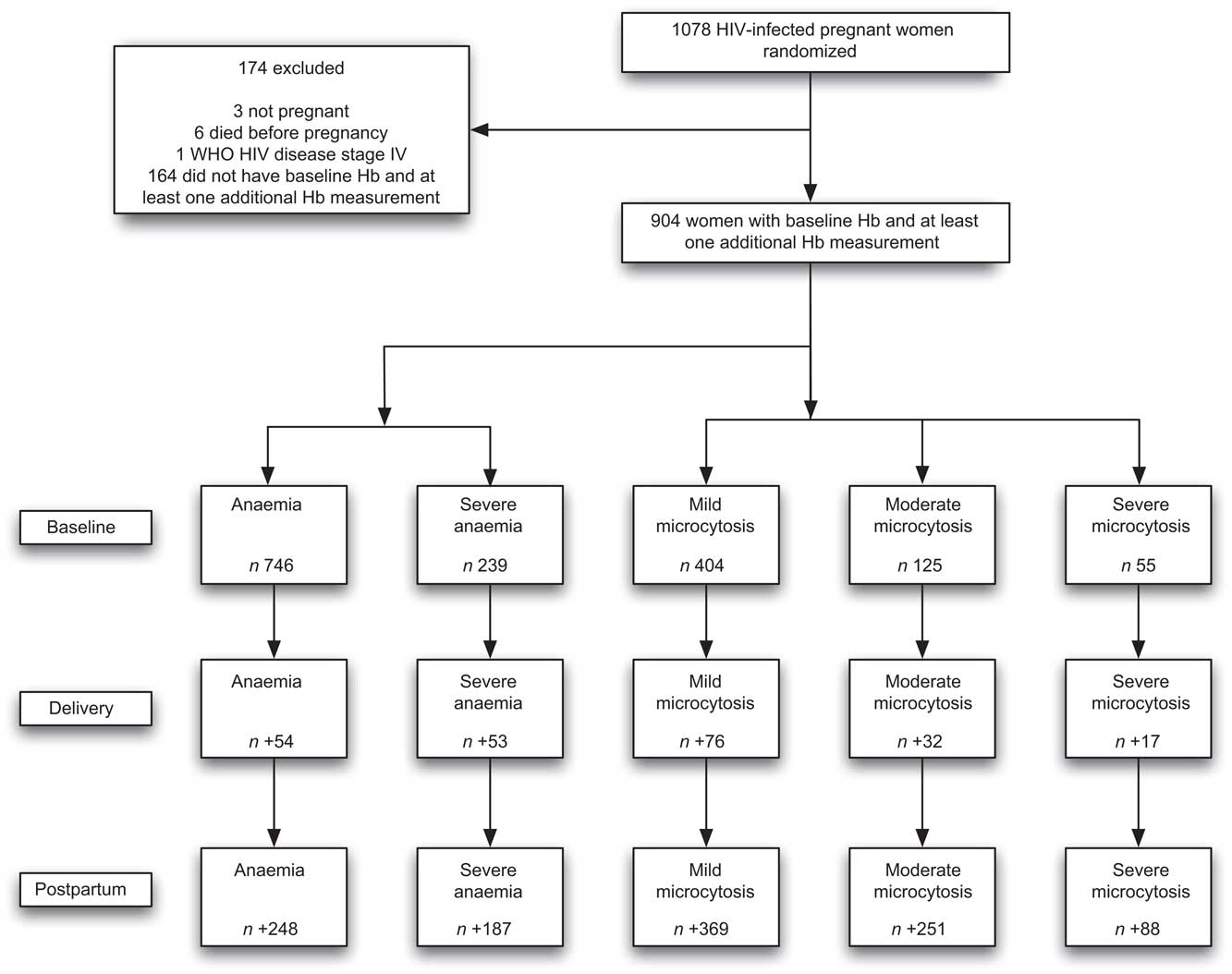
Fig. 1 Study profile of trial participants (n 1078) and women (n 904) included in the present analyses with available baseline and follow-up haematological measurements: HIV-infected pregnant women enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania. At baseline, n is the number of new cases; at delivery, n is the number of new cases among those who did not have the outcome at baseline; at postpartum, n is the number of new cases during follow-up among those who did not have the outcome at delivery
Clinical evaluations were performed monthly to assess HIV-related complications, disease stage and clinical morbidities(16). Women who missed a clinic visit or travelled outside Dar es Salaam were followed-up via home visits.
Laboratory methods
Whole blood samples were collected from participants at baseline, delivery, 6 weeks postpartum and every 6 months thereafter. Laboratory samples were tested in batch, and instruments were calibrated daily using standardized procedures.
HIV serostatus was determined by Enzygnost anti-HIV-1/2 Plus (Dade Behring, Marburg, Germany) followed by the Wellcozyme HIV-1 recombinant test (Murex Biotech Ltd, Dartford, UK). Discordant ELISA results were resolved by Western blot (Bio-Rad Laboratories Ltd, Hertfordshire, UK) assay(Reference Urassa, Matunda and Bredberg-Raden17).
Hb concentrations were assessed using a CBC5 Coulter Counter (Coulter Corporation, Miami, FL, USA) or the cyanmethaemoglobin method with a colorimeter (Corning Inc., Corning, NY, USA). Thin blood films with Leishman's stain were prepared and examined microscopically. Hypochromasia, microcytosis and macrocytosis were classified into four levels, coded as absent, 1+, 2+ or 3+.
Total leucocyte counts were evaluated with a CBC5 Coulter Counter (Coulter Corporation). Absolute CD3, CD4 and CD8 T-cell counts were determined with the FACSCount system (Becton-Dickinson, San Jose, CA, USA); differential white blood cell counts were evaluated manually. A complete blood count was obtained (Coulter Corporation), and erythrocyte sedimentation rate (ESR) was determined.
Measurements of infections, including malaria, intestinal parasites and genital infections, were conducted in all participants only at the baseline clinic visit. Additionally, if a participant presented with symptoms suggestive of malaria or other infections, they were treated as per the clinical guidelines of WHO and the Ministry of Health of Tanzania at that time. Malaria parasites were identified in thick-smear blood films stained with Giemsa. Malaria parasite density was calculated based on a leucocyte count of 8000 × 106/l blood(18). Urine samples were examined for the presence of Schistosoma haematobium. Sera and genital swabs were tested for vaginal candidiasis and sexually transmitted infections including syphilis, gonorrhoea and trichomoniasis. To identify intestinal helminths (hookworm, Ascaris lumbricoides, Trichuris trichura, Strongyloides stercoralis and Schistosoma mansoni) and pathogenic protozoan infections (Giardia lamblia, Entamoeba histolytica and Cryptosporidium parvum), stool specimens were first examined macroscopically for general characteristics (pus, mucus, blood) and worms. Stools were then examined microscopically using saline wet mount for detection of eggs, larvae protozoan trophozoites and cysts, followed by iodine wet mount to identify cysts. The formalin–ether concentration technique was used for further identification of eggs, larvae and cysts. Co-infections were treated at the time of diagnosis, in accordance with clinical guidelines of the Ministry of Health of Tanzania.
Serum 25-hydroxy vitamin D (25(OH)D) concentrations were measured using the fully automated chemiluminescence ADVANTAGE 25(OH)D assay (Nicholas Institute Diagnostics, San Juan Capistrano, CA, USA). Vitamin D insufficiency was dichotomized at 32 ng/ml, based on requirements for optimal Ca homeostasis(Reference Hollis19) and in accordance with previous studies in this trial(Reference Mehta, Hunter and Mugusi20). 25(OH)D concentrations were measured in all participants, using blood samples collected at the baseline clinic visit.
Assessment and definitions of outcomes
Anaemia and severe anaemia were defined as Hb less than 110 g/l and 85 g/l, respectively, in accordance with WHO criteria and clinical guidelines in Tanzania. We examined the morphology of peripheral erythrocytes as a proxy to identify Fe deficiency (presence of hypochromic and microcytic cells) and vitamin B12 and folate deficiency (presence of macrocytic cells)(Reference Dacie and Lewis21). Hypochromic microcytic anaemia was categorized as severe (hypochromasia ≥2+ and microcytic cells observed), moderate and above (hypochromasia ≥1+ and microcytic cells observed), and mild and above (hypochromasia ≥1+ with or without microcytosis). Participants diagnosed with severe anaemia received clinical management as per standard of care, including Fe supplementation.
Statistical analyses
We used Cox proportional hazard models to examine predictors of time to categorical haematological endpoints: anaemia, severe anaemia and hypochromic microcytosis(Reference Cox22). For each analysis, we examined time to the first occurrence of each haematological endpoint during follow-up; women who had the endpoint of interest at baseline were excluded. For those without the outcomes, follow-up ended on the date on which HIV disease stage was last assessed. We also investigated predictors of resolution of haematological endpoints during pregnancy, by conducting binomial regression analyses among individuals with the outcome of interest at baseline, to examine if these endpoints resolved at delivery.
Covariates
Conventional cut-offs were used to categorize risk factors, where available; otherwise, medians were used to classify variables, as is consistent with previous publications from the TOV(Reference Antelman, Msamanga and Spiegelman2, Reference Antelman, Smith-Fawzi and Kaaya23). BMI was categorized as <18·5, 18·5–<25·0, 25·0–<30·0 and ≥30·0 kg/m2(24). CD4 T-cell counts were categorized as <200, 200–499 or ≥500 cells/μl, and CD3 and CD8 T-cell counts were evaluated based on their median concentrations. Malaria parasitaemia was categorized as light (1–999/μl), moderate (1000–9999/μl) or heavy (≥10 000/μl). Intestinal parasites were categorized by the presence of ova or larvae in stool specimens. Presumed adherence to prenatal Fe supplements was defined as the number of days for which supplements were available divided by the total number of days between enrolment and delivery, and was included as a covariate in all multivariate analyses.
Variables with univariate P values of less than 0·20 were included in each of the multivariate regression models and retained if their P values were less than 0·05. The missing indicator method was used to account for missing predictor data(Reference Miettinen25).
Potential predictors were also examined as continuous variables. We explored potential non-linearity of the relationships between covariates and outcomes non-parametrically, using stepwise restricted cubic splines(Reference Durrleman and Simon26, Reference Govindarajulu, Spiegelman and Thurston27). We used tests for non-linearity, using the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. If non-linear associations are not reported, they were not significant.
Multivitamin supplementation was shown to significantly improve haematological outcomes in a previous analysis(Reference Fawzi, Msamanga and Kupka28); therefore, micronutrient regimen (multivitamins, vitamin A, multivitamins and vitamin A, or placebo) assignment was included as a covariate in all multivariate analyses.
Additional analyses
We allowed covariates to vary with time in the analyses for each of the outcomes, including measurements at each assessment during the follow-up period. We considered both the ‘discrete’(Reference Cox22) and ‘Breslow’(Reference Breslow29) options for ties in analyses, since the Breslow method may give biased results when the number of ties is large and risk sets are small. All results reported use the Breslow method, since no differences were observed when the discrete method was used. We calculated population-attributable fractions for risk factors for each of the outcomes, in order to estimate the number of cases of outcomes that could be averted if all of the risk factors were prevented.
Pregnancy and postpartum periods
We also conducted the aforementioned analyses separately within the pregnancy and postpartum periods, to explore predictors of haematological outcomes. For analyses in the pregnancy period, binomial regression(Reference Wacholder30, Reference Spiegelman and Hertzmark31) was used to obtain risk ratio estimates for the predictors of each haematological endpoint at delivery. We also investigated predictors of resolution of haematological endpoints during pregnancy by running binomial regression analyses among individuals with the outcome of interest at baseline, to examine if these endpoints resolved at delivery. For analyses in the postpartum period, Cox proportional hazards models were used to examine predictors of incident haematological outcomes from delivery through the end of follow-up for all endpoints.
Statistical analyses were performed using the SAS statistical software package version 9·2 (SAS Institute Inc., Cary, NC, USA).
Results
The baseline characteristics of the 904 women included in the present analyses (Fig. 1) are presented in Table 1. At the baseline assessment, 83 % of women were anaemic (Hb <110 g/l) and 26 % were severely anaemic (Hb < 85 g/l), with a mean Hb level of 94 g/l; 45 % and 14 % of women had mild or moderate hypochromic microcytosis, respectively. In contrast, only 6 % of women had severe microcytosis. Tables 2 to 5 present results only for anaemia, severe anaemia and severe microcytosis, except for resolution of anaemia; for the resolution of anaemia analyses, results are presented for mild microcytosis, as all cases of severe microcytosis resolved by delivery.
Table 1 Characteristics of study population: HIV-infected pregnant women (n 904) enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania

ESR, erythrocyte sedimentation rate.
Data are presented as mean and sd for continuous variables or as n and % for categorical variables.
*$US 1 was equivalent to approximately 500 Tanzanian Shillings at the time of data collection.
†Giardia lamblia, Entamoeba histolytica or Cryptosporidium parvum.
‡Hookworm, Trichuris trichura, Ascaris lumbricoides, Strongyloides stercoralis or Schistosoma mansoni.
Table 2 PredictorsFootnote * of haematological outcomes during the overall follow-up period (including the delivery and postpartum periods): HIV-infected pregnant women enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania
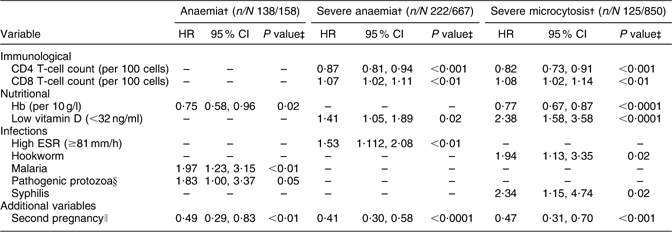
HR, hazard ratio; ESR, erythrocyte sedimentation rate.
* All multivariate analyses were adjusted for multivitamin regimen and adherence to prenatal Fe supplementation.
† Anaemia: Hb <110 g/l, severe anaemia: Hb < 85 g/l, severe microcytosis: hypochromasia ≥2+ and microcytic cells on peripheral smear.
‡ P values were obtained from Cox proportional hazards analyses; women who had the endpoint of interest at baseline were excluded, to examine time to first occurrence of outcome during follow-up.
§ Giardia lamblia, Entamoeba histolytica or Cryptosporidium parvum.
∥ Second pregnancy after enrolment into the trial.
Table 3 PredictorsFootnote * of haematological outcomes during pregnancy (incident cases at delivery): HIV-infected pregnant women enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania

RR, relative risk.
* All multivariate analyses were adjusted for multivitamin regimen and adherence to prenatal Fe supplementation.
† Anaemia: Hb <110 g/l, severe anaemia: Hb <85 g/l, severe microcytosis: hypochromasia ≥2+ and microcytic cells on peripheral smear.
‡ P values were obtained from binomial regression analyses; women who had the endpoint of interest at baseline were excluded, to examine the occurrence of each outcome at delivery.
Table 4 PredictorsFootnote * of resolution of haematological outcomes during pregnancy (resolution of cases at delivery): HIV-infected pregnant women enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania

RR, relative risk.
Note: all baseline severe microcytosis cases and all but five moderate microcytosis cases resolved at delivery.
* All multivariate analyses were adjusted for multivitamin regimen and adherence to prenatal Fe supplementation.
† Anaemia: Hb <110 g/l, severe anaemia: Hb <85 g/l, mild microcytosis: hypochromasia ≥1+ with or without microcytosis on peripheral smear.
‡ P values were obtained from binomial regression analyses; analyses were conducted among women with the outcome of interest at baseline, to examine resolution of outcome at delivery.
Table 5 PredictorsFootnote * of postpartum haematological outcomes: HIV-infected pregnant women enrolled in a randomized trial of vitamins (1995–1997), Dar es Salaam, Tanzania
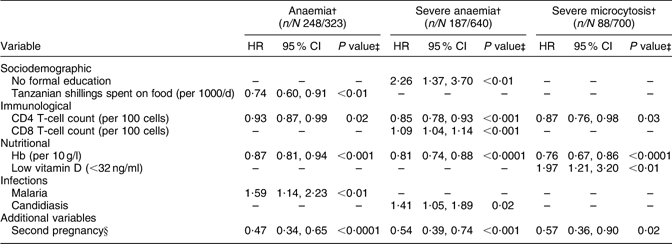
HR, hazard ratio.
* All multivariate analyses were adjusted for multivitamin regimen and adherence to prenatal Fe supplementation.
† Anaemia: Hb <110 g/l, severe anaemia: Hb <85 g/l, severe microcytosis: hypochromasia ≥2+ and microcytic cells on peripheral smear.
‡ P values were obtained from Cox proportional hazards analyses; women who had the endpoint of interest at baseline (delivery) were excluded, to examine time to the first occurrence of each outcome during follow-up.
§ Second pregnancy after enrolment into the trial.
A total of 87 % of women developed incident anaemia over the follow-up period. Infection with malaria and pathogenic protozoa were associated with significant twofold increases in the risk of developing anaemia during follow-up (Table 2). In contrast, higher Hb concentrations were associated with lower risk of anaemia during follow-up, with a significant 25 % lower risk per 10 unit (g/l) increase in baseline Hb concentrations. A total of 222 women out of 667 at risk developed severe anaemia during follow-up. Women with higher ESR (≥81 mm/h) and higher CD8 T-cell counts had significantly increased risk of developing severe anaemia; low vitamin D status (<32 ng/ml) was associated with a 41 % significant increase in the risk of severe anaemia during follow-up. In contrast, higher CD4 T-cell counts predicted lower risk of severe anaemia, with a 13 % lower risk per 100-cell increase in baseline CD4 T-cells. A significant non-linear relationship was observed between baseline CD4 T-cell counts and Hb concentrations (P < 0·01). Hb concentrations increased with CD4 T-cell counts until approximately CD4 count of 700 cells/μl, and then decreased after this point. A second pregnancy after enrolment in the trial was associated with 51 % reduction in the risk of anaemia during the follow-up period.
A total of 69 %, 38 % and 15 % women developed incident mild, moderate and severe hypochromic microcytosis during follow-up, respectively. The majority of microcytosis cases developed during the postpartum period. Increased CD8 T-cell, lower CD4 T-cell and lower Hb concentrations at baseline significantly predicted elevated risk of severe hypochromic microcytosis. Hookworm and syphilis infections were associated with a significant 1·9- and 2·3-fold increase in the risk of severe microcytosis during follow-up. Low vitamin D status was associated with a significant 2·4-fold increase in the risk of severe microcytosis.
A total of 125 women who were not anaemic at baseline also had Hb measures available at delivery; out of these, fifty-four became anaemic during pregnancy. In the pregnancy period, higher Hb concentrations were associated with significantly reduced risk of incident anaemia and severe anaemia at delivery (Table 3). Women with low vitamin D status at baseline also had a higher risk of developing severe anaemia or hypochromic microcytosis at delivery. Further, candidiasis was associated with increased risk of severe hypochromic microcytosis.
In analyses of population-attributable fractions, we estimated the number of cases of outcomes that could be averted if the selected risk factor was prevented. For anaemia, the population-attributable fraction for malaria was 8·6 %; approximately 9 % of anaemia cases would be averted if all of the malaria cases were prevented. For severe hypochromic microcytosis, the population-attributable fraction for hookworm was 6·2 %; approximately 6 % of anaemia cases would be averted if all of the hookworm cases were prevented through antihelminthic treatment.
Resolution of anaemia at delivery occurred in 251 women out of the 590 women who had anaemia at baseline (Table 4). Low vitamin D concentrations and candidiasis were associated with significantly reduced likelihood of resolution of anaemia. For severe anaemia and microcytosis, primiparity was associated with greater likelihood of resolution.
Among the 323 women who did not have anaemia at delivery, 248 became anaemic during postpartum follow-up (Table 5). Higher baseline Hb concentrations, increased money spent on food and higher CD4 T-cell counts were associated with a significant independent lower risk of anaemia during follow-up, whereas infection with malaria was associated with a significant 1·6 times greater risk of anaemia during the postpartum period. No formal education, higher CD8 T-cell counts and candidiasis were associated with significantly increased risk of severe anaemia during follow-up, whereas higher baseline Hb concentrations and CD4 T-cell counts were associated with significantly lower risk of severe anaemia during the postpartum period. Low vitamin D status was associated with a significant two-fold increase in the risk of severe microcytosis; higher baseline Hb concentrations and CD4 T-cell counts were associated with significantly reduced risk of severe microcytosis during the postpartum period. A second pregnancy after enrolment in the trial was also associated with decreased risk of severe microcytosis during the postpartum period.
Discussion
In the present study, parasitic infections, low CD4 T-cell counts, high ESR and vitamin D insufficiency were the main predictors of anaemia and Fe deficiency in HIV-infected pregnant women in Tanzania.
Our analysis is distinct from previous studies due to its longitudinal prospective evaluation of incident haematological outcomes, extensive assessment of potential risk factors, and investigation of predictors of resolution of haematological outcomes in a cohort of HIV-infected pregnant women receiving Fe–folate supplementation. Women were followed for a long duration through the postpartum period, with a median of 57 months, with an average of 8·6 Hb measurements per participant during follow-up.
The prevalence of anaemia and hypochromic microcytosis in this population was high and similar to other studies in HIV-infected pregnant women in Sub-Saharan Africa(Reference Meda, Dao and Ouangre3–Reference Mehta, Manji and Young5, Reference Friis, Gomo and Kastel32, Reference Semba, Kumwenda and Hoover33). For example, several studies have reported an extremely high prevalence of anaemia (Hb <110 g/l) in HIV-infected pregnant women: 78 % in Burkina Faso(Reference Meda, Dao and Ouangre3), 83 % in Côte d'Ivoire(Reference Ramon, Sawadogo and Koko4) and 73 % in an analysis of a multi-centre trial in Tanzania, Zambia and Malawi(Reference Mehta, Manji and Young5).
Similar to our findings, malaria(Reference Fawzi, Msamanga and Hunter34), hookworm(Reference Stoltzfus, Chwaya and Tielsch35) and other infections, such as schistosomiasis and trichuriasis(6), have been identified as important contributors to the high prevalence of anaemia and Fe deficiency in many resource-limited settings. In the current analysis, malaria infection at baseline predicted a two-fold increase in the risk of anaemia during follow-up and a 1·6 times greater risk of anaemia during the postpartum period. In a previous cross-sectional analysis, women with high malaria parasite density (≥1000 parasites/μl) had 2·7-fold greater odds of severe anaemia (95 % CI 1·6, 4·6; P = 0·0003) at baseline, compared with uninfected women(Reference Antelman, Msamanga and Spiegelman2).
The relationship between parasitic worm infections and anaemia has not been evaluated in HIV-infected pregnant women. However, the relationship between parasitic worm infections, particularly hookworm infection, and anaemia has been well established in children(Reference Brooker, Akhwale and Pullan36). Further, in a meta-analysis by Brooker et al. of thirteen studies among presumably HIV-uninfected pregnant women, hookworm infection was significantly associated with anaemia and infection intensity was significantly inversely related to Hb concentrations(Reference Brooker, Hotez and Bundy37). These infections, along with malaria, may contribute to Fe deficiency via destruction of erythrocytes, occult blood loss and nutrient malabsorption(Reference Brooker, Akhwale and Pullan36).
Higher CD8 T-cell counts and ESR, and lower CD4 T-cell concentrations, predicted increased risk of anaemia and Fe deficiency in the present study, possibly due to associated increase in inflammation. Although the conventional role of CD8 T-cells is as cytotoxic killer cells, they may also be effector cells in inflammation(Reference Meehan and DeLuca38–Reference Monteiro, Hingorani and Peroglizzi42) and lead to anaemia. HIV infection and advanced HIV disease may itself contribute to the aetiology of anaemia and Fe deficiency through a number of mechanisms, such as infection of marrow stromal cells(Reference Koka, Jamieson and Brooks43), impaired haematopoietic progenitor cell growth(Reference Moses, Williams and Heneveld44), bone marrow pathologies, autoimmune haemolysis and intestinal blood loss(Reference Coyle45–Reference Kreuzer and Rockstroh47). Anaemia of inflammation(Reference Schilling48) is a leading cause of anaemia in HIV infection(Reference Weiss and Goodnough13, Reference Schilling48) and is characterized by a distinctive haematological profile and a lack of response to Fe supplementation(Reference Fauci, Braunwald and Kasper49).
The relationship between vitamin D and Fe status has not previously been evaluated in the context of pregnancy or HIV infection. However, findings are consistent with a previous analysis in the TOV in which low baseline vitamin D status was associated with decreased risk of anaemia during overall follow-up(Reference Mehta, Giovannucci and Mugusi50). In the present analysis we demonstrated that low baseline vitamin D concentrations predicted risk of anaemia during the pregnancy and postpartum periods, and adequate vitamin D status was an important predictor of resolution of anaemia and Fe deficiency. There are several plausible biological mechanisms by which vitamin D could modulate risk of anaemia, such as through decreasing inflammation. Vitamin D deficiency has also been associated with marrow myelofibrosis, which is a known cause of anaemia(Reference Yetgin, Ozsoylu and Ruacan51). An association between low vitamin D status and Fe deficiency has been observed in earlier studies in individuals with renal disease in the third National Health and Nutrition Examination Survey(Reference Kendrick, Targher and Smits52) and among children in Bangladeshi, Pakistani and Indian(Reference Lawson and Thomas53), and Asian(Reference Grindulis, Scott and Belton54) communities in Britain. However, there is a lack of evidence regarding aetiological mechanisms and the relationship between vitamin D and Fe status needs to be further explored in pregnant women and in resource-limited settings.
Risk of microcytosis was inversely associated with the daily amount of household funds allocated to food and educational level. This is consistent with previous studies that identified poverty as a risk factor for Fe deficiency and the increased prevalence of anaemia in resource-limited settings. This may be related to the relatively higher cost of Fe-rich food sources, such as animal products, nuts and green leafy vegetables, compared with grain-based staples. In developing settings, bioavailable haem Fe may comprise as little as 5 % of the diet, compared with 18 to 25 % in adults consuming typically Western diets(55).
Of note is the high number of baseline cases of anaemia (42·5 %), severe anaemia (86·7 %) and microcytosis (73·0 %) that resolved at delivery, with relatively high numbers of new cases of anaemia (76·8 %), severe anaemia (29·2 %) and mild (66·5 %), moderate (36·9 %) and severe (12·6 %) Fe deficiency developing during the postpartum period, after discontinuation of prenatal Fe supplementation. These results suggest that Fe supplementation only during pregnancy may not be sufficient to prevent anaemia and Fe deficiency during the postpartum period.
Interestingly, a second pregnancy after enrolment was associated with marked reductions in risk of haematological outcomes during overall follow-up and the postpartum period. This finding may be attributable to reverse causation, i.e. women with better haematological or nutritional status were more likely to become pregnant a second time during the follow-up period. Although a second pregnancy may be associated with adverse pregnancy outcomes in resource-limited settings due to depletion of maternal reserves, it also presents an opportunity for greater contact with the health-care system. In previous publications, we have noted a significantly lower incidence of anaemia in women who received multivitamin (vitamins B, C, E) supplementation. The combination of multivitamins received during the trial, plus Fe and folate supplementation during pregnancy and access to health care, may synergistically reduce the risks of adverse haematological outcomes in this setting.
Our study has a few limitations. The use of Hb and hypochromic microcytosis as the only indicators of Fe status is a limitation. Although Hb is the most common indicator for anaemia worldwide, it does not reflect Fe stores and has low sensitivity. Similarly, hypochromic microcytosis does not reflect available body Fe stores. Physiological changes in pregnancy, particularly haemodilution, also affect the concentrations and interpretation of Fe indicators. The lack of recording of alternative indicators of Fe status (such as serum ferritin and soluble transferrin receptor) and inflammation (such as C-reactive protein or α-1 acid glycoprotein) are limitations in our analysis. According to WHO, where possible, concentrations of Hb, serum ferritin and transferrin receptor, and at least one acute-phase protein should be measured(Reference Beard56). For example, acute-phase reactants such as C-reactive protein and α-1 acid glycoprotein would further improve Fe assessment in the context of inflammation and infection; recent research has also identified hepcidin as an important regulator of Fe metabolism in anaemia of inflammation.
The present analysis was also conducted to examine predictors of anaemia among HIV-infected women who were not on ART; as such, findings may not be generalizable to pregnant women receiving ART. Scale-up of ART and prenatal services in many developing settings may have reduced the prevalence of anaemia in HIV-infected populations. However, using baseline characteristics may inform prevention and clinical management of anaemia during pregnancy and the follow-up period among women who have not yet been initiated on ART. Further research is also needed to examine novel biomarkers, such as hepcidin, and to explore the relationships of these predictors with anaemia in HIV-infected individuals on ART.
Fe supplementation alone, and particularly only during pregnancy, may not be adequate to prevent anaemia and associated morbidities in HIV-infected individuals. Consideration of other aetiological factors, namely HIV, malaria, hookworm and other endemic infectious diseases, and underlying nutritional deficiencies; infectious disease control; and micronutrient supplementation, are needed in the prevention, screening and clinical management of anaemia and Fe deficiency in HIV-infected women during the pre- and postpartum periods. Further research is also needed to examine the interactions of these risk factors among individuals on ART.
Acknowledgements
The study was supported by the National Institute of Child Health and Human Development (NICHD R01 32257; and K24HD058795) and the Harvard School of Public Health. None of the authors had a personal or financial conflict of interest. J.L.F., S.M., C.P.D., D.S. and W.W.F. contributed to the plans for data analysis. J.L.F. analysed and interpreted the data and wrote the initial draft of the manuscript. S.M. assisted with the interpretation of data. D.S. provided statistical guidance and helped interpret data analyses. G.I.M., S.A., D.S. and W.W.F. were investigators of the trial and contributed to the study design and implementation. All co-authors participated in manuscript preparation. The authors are grateful to the mothers and children, also the field teams, including physicians, nurses, midwives, supervisors, laboratory staff and the administrative staff, who made this study possible; and Muhimbili Medical Centre, Muhimbili University College of Health Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support.








