There is growing awareness that a plant-based diet decreases morbidity and mortality associated with a range of chronic disease including hypertension, diabetes and coronary artery disease( Reference Fraser 1 ). While diet plays an obvious role in management of autoimmune disease like coeliac disease or type 1 diabetes, fewer studies have documented relationships between diet and autoimmune diseases( Reference McCarty 2 ). Isolated rural populations following traditional diets and lifestyles appear to be protected from autoimmune disease. Observations by Trowell five decades ago indicated that in rural sub-Saharan Africans consuming a near-vegan diet a number of autoimmune disorders were rare, or virtually unknown, including rheumatoid arthritis, type 1 diabetes, thyrotoxicosis and Hashimoto’s thyroiditis( Reference McCarty 2 ).
The effects of plant-based diets may be due to the exclusion of animal foods, the effects of plant foods, or both. Animal foods like meat, eggs and dairy products may contain high oestrogen concentrations, which have been linked to autoimmunity in cell and animal studies( Reference Chighizola and Meroni 3 ). Small studies have found that fasting followed by a vegetarian or a Mediterranean diet rich in vegetables and oils may ameliorate symptoms of rheumatoid arthritis; however, effects are still uncertain due to the small number of studies( Reference Hagen, Byfuglien and Falzon 4 ).
Hyperthyroidism is a prevalent condition with many causes, of which the most common is Graves’ disease( Reference Cooper 5 ). Graves’ disease is an autoimmune disorder caused by antibodies directed against the thyrotropin receptor, resulting in excess synthesis and secretion of thyroid hormone. Dietary causes of hyperthyroidism are rare, although excess dietary iodine has been linked to hyperthyroidism( Reference Golkowski, Buziak-Bereza and Trofimiuk 6 , Reference Stanbury, Ermans and Bourdoux 7 ). Rarely, contaminated meat products have been reported as a cause of thyrotoxicosis( Reference Hedberg, Fishbein and Janssen 8 , Reference Hendriks and Looij 9 ).
In the current study we hypothesized that vegetarian diets may protect against hyperthyroidism in a Western population. We compared the prevalence of hyperthyroidism according to dietary pattern in a population exhibiting a wide range of diets. These diets included a vegan diet that excluded all animal products, a lacto-ovo vegetarian diet that excluded meat but allowed milk and eggs, a pesco vegetarian diet that included fish but no meat and a non-vegetarian diet.
Methods
Participants and study design
The Adventist Health Study-2 (AHS-2) is a longitudinal study initiated to investigate the role of foods and their relationship to disease, particularly cancer. Participants were members of the Seventh-day Adventist church recruited through their respective churches in the USA and Canada. About 97 000 individuals joined between 2002 and 2006( Reference Butler, Fraser and Beeson 10 ). Participants were eligible if they were proficient in English and were aged 30 years or older. All instruments and procedures were approved by the Loma Linda University Institutional Review Board in June 2001; approval was renewed annually thereafter.
Questionnaire
Once enrolled, each participant received a previously validated questionnaire with the informed consent materials. The questionnaire was divided into sections on demographics including place of early residence, medical history, dietary patterns, physical activity and other lifestyle habits and self-reported height and weight. Questionnaires were returned by pre-paid envelopes and edited for missing data and stray marks. If any of the research staff felt data entries required confirmation, or demographic data were missing, a telephone call was made to verify these entries.
Race and ethnicity were elicited for the following categories: Black/African American, West Indian/Caribbean, African and other Black, Hispanic and non-Hispanic White. In the current study all Blacks were collapsed into one category with non-Blacks in the other category. Respondents reported annual personal and household income by checking one of eight income categories, ranging from <$US 10 000 to >$US 200 000. Responses were grouped into four categories: ≤$US 10 000, $US 11 000–20 000, $US 21 000–30 000 and >$US 30 000. BMI was calculated from respondents’ self-reported height and weight as kg/m2. These self-reports were validated( Reference Bes-Rastrollo, Sabate and Jaceldo-Siegl 11 ).
Prevalent cases of hyperthyroidism and other diseases
In the medical history section of the questionnaire, respondents were asked if they had hyperthyroidism diagnosed by a physician and if affirmative to indicate when they were first diagnosed. They were also asked if they have been treated for hyperthyroidism within the last 12 months. Prevalent CVD included all cases of reported angina, heart attack, stroke and transient ischaemic attack.
Dietary information
The FFQ used in AHS-2 includes over 200 hard-coded foods and space for fifty write-ins covering the diet during the past 12 months( Reference Rizzo, Jaceldo-Siegl and Sabate 12 ). Vegetarian status was categorized by defining: vegans as participants who reported consuming no animal products (red meat, poultry, fish, eggs, milk and dairy products <1 time/month); lacto-ovo vegetarians as those who consumed dairy products and/or eggs but no fish or meat (red meat, poultry and fish <1 time/month); pesco vegetarians as those who consumed fish ≥1 time/month and dairy products and/or eggs but no red meat or poultry (red meat and poultry <1 time/month); semi-vegetarians as those who consumed dairy products and/or eggs and meat (red meat and poultry ≥1 time/month and <1 time/week); and omnivores as those who consumed animal products (red meat, poultry, fish, eggs, milk and dairy products >1 time/week). The frequency of adding salt to food was queried as ≤1 time/week, 2–6 times/week and ≥1 time/d. Iodine intake was not specified in the questionnaire. A previous report described the dietary methods and nutrient profiles of the vegetarian and non-vegetarian dietary patterns in the study in more detail( Reference Rizzo, Jaceldo-Siegl and Sabate 12 ). In summary, lacto-ovo vegetarians had lower intakes of dairy fat and animal protein than non-vegetarians while vegans had the lowest intakes of saturated and trans-fat and arachidonic acid, and the highest intakes of fibre, soya protein, β-carotene and Mg. Lacto-ovo and pesco vegetarians had intermediate intakes of saturated fat, fibre, soya protein, β-carotene and Mg.
To validate the nutrient intakes, a calibration study was conducted among participants randomly selected from the AHS-2 cohort( Reference Knutsen, Fraser and Linsted 13 , Reference Jaceldo-Siegl, Knutsen and Sabate 14 ). The result showed that the validity coefficients were moderate to high for macronutrients, fatty acids, vitamins, minerals and fibre.
Statistical analyses
Participants who reported treatment for hyperthyroidism in the past 12 months were a priori considered prevalent cases in the primary statistical analyses (n 603). We conducted a sensitivity analysis that included participants who reported treated hyperthyroidism, but not in the past 12 months.
Descriptive characteristics of the population were compared between participants with and without hyperthyroidism and between dietary patterns (vegan, lacto-ovo vegetarian, pesco vegetarian, semi-vegetarian or non-vegetarian) using the χ 2 test, the two-sample independent t test or ANOVA, as appropriate. Logistic regression was used to examine factors associated with the outcome (prevalence of hyperthyroidism). The logistic regression model included gender, ethnicity, age (as a continuous variable), BMI (categorized as <25·0, 25·0–29·9 and ≥30·0 kg/m2), income, education, report of CVD, frequency of salt use and dietary pattern. Results were presented as odds ratios (for the logistic model) and 95 % confidence intervals. All analyses were done using the statistical software package SAS version 9·3.
Results
There were 65 981 participants with complete data for all variables. Those who were included were younger (mean age 56·7 years) than those who were excluded (mean age 62·6 years), were more likely to be male (36·7 % v. 31·4 %) and had a higher proportion with some college education or longer education (81·3 % v. 69·1 %). Among the 65 981 participants, 603 (0·9 %) reported prevalent hyperthyroidism treated within the past 12 months. As shown in Table 1, participants with hyperthyroidism were older, had a higher BMI and reported less income and education compared with participants without hyperthyroidism. More women than men reported hyperthyroidism. Prevalence of hyperthyroidism did not differ according to early residence and this variable was not studied further. Those who reported hyperthyroidism were less likely to follow vegan, lacto-ovo, pesco and semi-vegetarian diets compared with those without hyperthyroidism, and had a higher prevalence of an omnivorous diet. As shown in Table 2, sociodemographic characteristics, BMI and prevalence of hyperthyroidism differed significantly according to dietary group. Vegetarians were older, had lower BMI, were less likely to be female, had a higher education and less CVD and hyperthyroidism compared with non-vegetarians.
Table 1 Demographic characteristics and diet according to prevalence of hyperthyroidism (n 65 981), Adventist Health Study-2
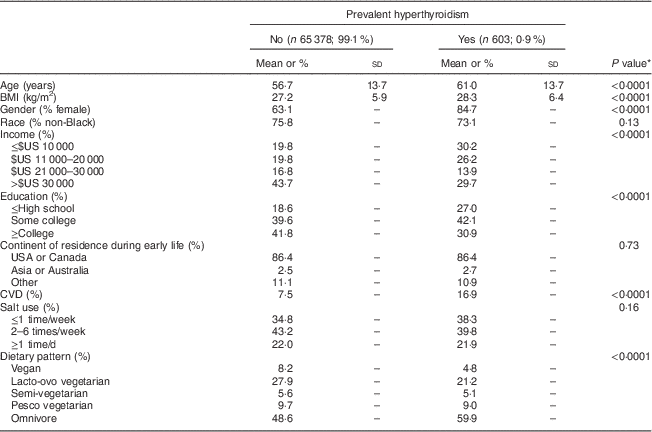
* Compared by the independent t test (continuous variables) or the χ 2 test (categorical variables).
Table 2 Demographic characteristics and prevalent hyperthyroidism according to dietary pattern (n 65 981), Adventist Health Study-2
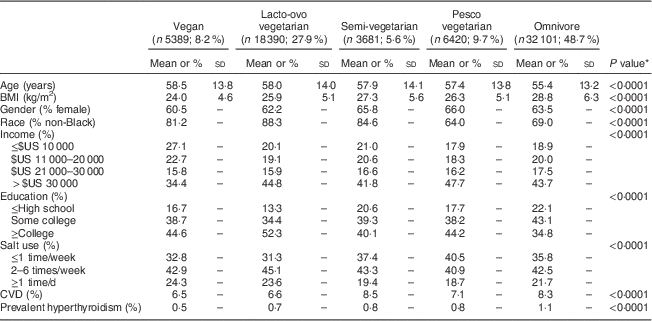
* Compared by ANOVA (continuous variables) or the χ 2 test (categorical variables).
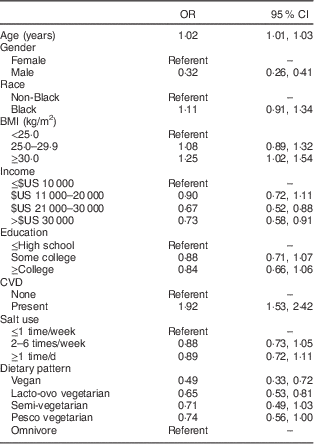
Table 3 shows multivariate odds ratios for factors associated with a diagnosis of hyperthyroidism. Age, female gender and overweight or obesity were associated with increased risk, while increasing education and income were protective against a diagnosis of hyperthyroidism. Vegan, lacto-ovo vegetarian and pesco vegetarian diets were associated with a lower prevalence of hyperthyroidism than omnivorous diets, while semi-vegetarian diets were not associated with less hyperthyroidism.
Table 3 Odds ratios and 95 % confidence intervals for prevalence of hyperthyroidism treated within the past 12 months (603 cases, total n 65 981), Adventist Health Study-2. Odds ratios are adjusted for all of the variables shown
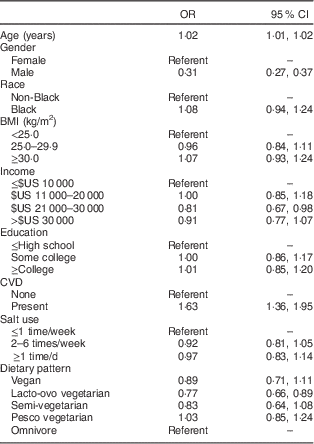
Results of the sensitivity analysis showed little change in the odds ratio estimates and significance levels (Table 4). While lacto-ovo vegetarians had lower odds of hyperthyroidism than omnivores, the relationship was no longer seen for vegans and pesco vegetarians.
Table 4 Odds ratios and 95 % confidence intervals for prevalence of hyperthyroidism treated or not treated within the past 12 months (1191 cases, total n 65 981), Adventist Health Study-2. Odds ratios are adjusted for all of the variables shown
Discussion
Our findings indicate that participants consuming a vegan diet had 52 % lower odds of prevalent hyperthyroidism compared with omnivores. Lacto-ovo vegetarians and pesco vegetarians also carried lower odds of hyperthyroidism, but the reduction appeared smaller than that in the vegan group. Semi-vegetarian diets were not protective against hyperthyroidism. The strengths of the study include the sizeable cohort following a range of diets who belonged to a church that discourages use of animal foods, smoking and use of alcohol. Thus, cigarette smoking, a common confounder of diet and disease relationships, was extremely rare (<2 %).
Prevalent cases of hyperthyroidism composed about 0·9 % of the population, which is somewhat lower than national estimates. In comparison, the third National Health and Nutrition Examination Survey (NHANES III) showed a prevalence of hyperthyroidism of 1·3 %( Reference Hollowell, Staehling and Flanders 15 ) while in another source the prevalence was stated as 1·2 %( Reference Bahn Chair, Burch and Cooper 16 ). The demographic predictors were as expected, with age, female gender and overweight and obesity identified as predictors of hyperthyroidism( Reference Cooper 5 ).
Putative mechanisms to explain these findings include the lower BMI of vegans and other vegetarians. Although the relationship between diet and hyperthyroidism was found after controlling for BMI, residual confounding may be present. Obesity is associated with autoimmune disease and studies have linked elevated leptin concentrations to autoimmunity( Reference Procaccini, Carbone and Galgani 17 ). Vegetarian diets have been linked to lower body weight and BMI specifically in this cohort( Reference Tonstad, Butler and Yan 18 ). Other suggestions include the hypothesis that vegan diets may downregulate systemic insulin-like growth factor-1 activity, a modulator of lymphocyte proliferation and apoptosis( Reference McCarty 2 ). Polyphenolic phytochemicals such as flavonoids found in plant foods are thought to protect cells against autoimmune processes( Reference Ardestani and Yazdanparast 19 ). Could type of diet or environmental toxins in food affect the microbiome or directly trigger autoimmune disease? Some data support this notion( Reference Duntas 20 , Reference Kverka and Tlaskalova-Hogenova 21 ). It has been speculated that the rise in autoimmune diseases in recent years may be linked to environmental oestrogens, found in a variety of foods, but particularly in meat, eggs and dairy products given exogenous hormones( Reference Chighizola and Meroni 3 ). However, further study is needed regarding any causal effects of plant diets or elimination of animal foods on autoimmune disease. The sensitivity analyses that included all participants regardless of treatment in the last year found robust protection associated with the lacto-ovo vegetarian diet, suggesting that the meat content of omnivorous diets may be risky rather than dairy products and eggs. Notably, a recent review found that high consumption of meat was associated with risk of thyroid cancer while dairy products showed no significant association( Reference Choi and Kim 22 ). The vegetarian diets contained high amounts of antioxidants, possible protective factors against autoimmune disease( Reference Duntas 20 ).
Limitations
The present data are hypothesis generating, as there are considerable biases in cross-sectional studies. Unmeasured confounding may be present, although we controlled for a number of confounders of the relationship between diet and thyroid disease, including a range of sociodemographic characteristics. The cause of hyperthyroidism was not specified and a number of aetiologies may have been present in the cases reported, although in iodine-replete populations most thyroid disease is autoimmune. We were only able to confirm the diagnosis of hyperthyroidism by direct contact with a small number of respondents (data not shown) due to the elapse of time after inclusion and medical records were not examined. Vegans may be less likely to visit their physician for physical examination as compared with other dietary groups. Individuals may report thyroid problems because of other medical symptoms, like weight loss. However, in our primary analyses only participants who reported treatment in the last 12 months were considered prevalent cases, to reduce over-reporting. The sensitivity analysis, which included all participants who reported hyperthyroidism regardless of treatment or not in the last 12 months, showed similar results for lacto-ovo vegetarians, but not for vegans and pesco vegetarians. A distant history of hyperthyroidism may be less likely to associate with current diet than a current condition. A study suggests high sensitivity of self-report questionnaires for thyroid disorders( Reference Brix, Kyvik and Hegedus 23 ); however, a major limitation of the current study is the self-reported nature of the data. In a cross-sectional study such as the present one, causality cannot be assumed; further study is required, for example a cross-sectional study comparing diets of individuals with and without hyperthyroidism. This church-going population differed from the general population in several aspects, primarily lack of smoking (only 1–2 % were current smokers) and lesser use of alcohol and stimulants, which is discouraged by the church.
While the dietary questionnaires were validated, data on iodine intake were not measured, so we used added salt as a proxy measure. However, the questionnaire did not specify whether it was iodized. We did not record the family history of thyroid disease, which plays a part in the pathogenesis of autoimmune thyroid disease.
Conclusion
Vegan and other vegetarian diets were associated with protection against prevalent hyperthyroidism.
Acknowledgements
Financial support: The work was supported in part by the National Institutes of Health/National Cancer Institute (grant number 5U01CA152939). The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: G.E.F. is the primary investigator of AHS-2 and participated in study design, analysis and writing of the paper. S.T. initiated the study and participated in analysis, design and writing. E.N. participated in design and writing. K.O. performed the statistical analyses. All the authors have seen and approved the final manuscript. Ethics of human subject participation: All instruments and procedures were approved by the Loma Linda University Institutional Review Board in June 2001; approval was renewed annually thereafter.






