In recent years, various studies have investigated prenatal Fe status and its effects on maternal and fetal health. It is well established that Fe deficiency (ID) is highly prevalent during pregnancy (18–40 %) in both developing and developed countries( 1 ) and is related to adverse obstetric outcomes such as low birth weight, preterm delivery( Reference Aranda, Ribot and Garcia 2 – Reference Haider, Olofin and Wang 6 ) and behavioural, emotional and cognitive outcomes throughout infant development( Reference Oyemade, Cole and Johnson 7 – Reference Hernández-Martínez, Canals and Aranda 10 ). This is because, during fetal development, Fe is essential for Hb synthesis and is an important factor in neuronal synapse formation, myelination and the synthesis of certain neurotransmitters in the central nervous system (CNS)( Reference Georgieff and Rao 11 ).
To prevent ID during pregnancy, several countries have launched generalized and routine Fe supplementation programmes. Most of these are generally beneficial to maternal–fetal health but, in some cases, they may contribute to increased Fe and Hb levels and thus lead to haemoconcentration( Reference Casanueva and Viteri 12 ). Because this condition is less prevalent (13·3 %)( Reference Aranda, Ribot and Viteri 13 , Reference Arija, Ribot and Aranda 14 ), its effects are less studied.
High ferritin levels have been related to oxidative stress( Reference Aranda, Ribot and Garcia 2 , Reference Aisen, Cohen and Kang 15 – Reference Khambalia, Aimone and Nagubandi 18 ) and to reduced absorption of other important minerals( Reference Sandstrom 19 ), and haemoconcentration has been related to increased blood viscosity and blood pressure( Reference Ziaei, Norrozi and Faghihzadeh 20 ), which impedes the placental–fetal blood flow and interferes with the fetal supply of oxygen and nutrients( Reference Gaillard, Eilers and Yassine 21 , Reference Cordina, Bhatti and Fernandez 22 ). All of these conditions may interfere during the course of pregnancy with the general development of the fetus and the development of its CNS. In fact, high Fe levels and haemoconcentration have been related to premature birth and low birth weight( Reference Aranda, Ribot and Viteri 13 , Reference Arija, Ribot and Aranda 14 , Reference Ziaei, Norrozi and Faghihzadeh 20 , Reference Gaillard, Eilers and Yassine 21 , Reference Steer, Alam and Wadsworth 23 – Reference Cung, Paus and Aghbar 25 ) but there are few studies that have related these conditions with neurodevelopmental outcomes. The CNS is the most complex and vulnerable organ in the human body and its development is also correspondingly an extremely complex and vulnerable period( Reference Ellison 26 ). This process is largely dependent on the appropriate release of oxygen, protein, energy and micronutrients, and an inadequate supply of these may contribute to alterations in neurodevelopment( Reference Massaro, Rothbaum and Aly 27 ). Tamura et al.( Reference Tamura, Goldenberg and Hou 28 ) studied the relationship between cord ferritin concentrations and cognitive development and found that infants with cord ferritin levels in the highest quartile showed lower IQ (intelligence quotient) scores at 5 years old. Those authors attributed these results to possible maternal infections or neuronal damage caused by oxidative stress resulting from Fe overload. In relation to the amount of Fe supplementation, the study by Hanieh et al.( Reference Hanieh, Ha and Simpson 29 ) compared a daily Fe supplementation pattern with a twice weekly Fe supplementation pattern and found that children of mothers with a daily Fe supplementation pattern scored lower in a cognitive test at 6 months old; in fact, 40 % of women with this pattern of supplementation were found to have ferritin levels greater than 41 µg/l and normal Hb concentrations, results that were attributed to oxidative stress processes related to high Fe levels. There are certain aspects of these studies that deserve special attention. First, their main aims were to examine the effects on different levels of ID (serum cord ferritin, Hb concentrations), which meant that they paid little attention to results related to elevated ferritin or high Hb concentrations. Second, cognitive development was assessed at 4 months and 4–6 years old, ages at which other multiple factors can also affect infant cognitive development, including maternal education and psychopathology, obstetric conditions, mother–infant attachment, infant breast-feeding, educational programmes, genetic factors, nutritional status, etc.( Reference Hernández-Martínez, Arija and Balaguer 30 – Reference Victora, Horta and de Mola 35 ). Third, the infant’s own Fe status also affects her/his cognitive development( Reference Angulo-Barroso, Schapiro and Liang 36 ) and this variable was not taken into account. Consequently, to study the long-term effects of pregnancy Hb concentrations, several variables must be taken into account. However, neonatal behavioural characteristics shortly after birth are not influenced by these longer-term postnatal variables, although prenatal conditions must be also taken into account. Several studies have shown that prenatal maternal toxic habits, socio-economic status and anxiety, among other factors, are related to neonatal behaviour( Reference Hernández-Martínez, Arija and Balaguer 30 , Reference Hernández-Martínez, Arija and Escribano 31 , Reference Koutra, Chatzi and Roumeliotaki 33 , Reference Hernández-Martínez, Arija and Escribano 37 ), suggesting that relationships must be adjusted for them. To our knowledge, to date there are no data regarding the potentially harmful effect of high Hb levels on neurobehavioural development in newborns.
Another important issue is the moment during gestation at which these harmful conditions occur. As Georgieff suggested( Reference Georgieff 38 ), the effect of any nutrient deficiency or overabundance on brain development will be governed by the principle of timing, dose and duration. In this regard, Hernández-Martínez et al.( Reference Hernández-Martínez, Canals and Aranda 10 ) found that the effects of ID on CNS development varied depending on the trimester of pregnancy in which the ID occurred and depending on the level of ID. Although late pregnancy seems to be a vulnerable period for nutritional insults( Reference Thompson and Nelson 8 , Reference Dobbing and Sands 39 , Reference Rao and Georgieff 40 ), there are no studies that investigate whether the effect of elevated levels of Fe on neurodevelopment is different depending on the point during pregnancy when this condition occurs.
Given all this information, it is clear that further studies are needed on the possible effects of high Hb concentrations in pregnant women on newborn neurodevelopment. Consequently, the main aim of the present study was to assess the effects of haemoconcentration at the end of pregnancy on neonatal behaviour after controlling for confounding factors. We hypothesized that high Hb concentrations at the end of pregnancy will be related to poor neonatal development.
Methods
Sample
Participants were pregnant women followed from the first trimester of gestation to childbirth. These women were volunteers and the Unit of Obstetrics and Gynaecology of the Sant Joan University Hospital in Reus (Spain) carried the recruitment out over 4 years (from 2004 to 2008).
The inclusion criteria for the study were being a healthy pregnant Caucasian woman older than 18 years with a singleton pregnancy at gestational week ≤11. Exclusion criteria applied across the duration of the study were: having a chronic illness or a possible inflammation (serum ferritin (SF) >62 µg/l and transferrin saturation <16 %)( Reference Chen, Scholl and Stein 41 , Reference Rambod, Kovesdy and Kalantar-Zadeh 42 ); having a multiple pregnancy; not meeting the prescribed Fe supplementation during pregnancy; not complying with the scheduled clinical visits; not having blood drawn at each clinical visit; and giving birth at another hospital.
A total of 299 well-nourished Spanish pregnant women were recruited, of which 27·8 % were excluded for fetal loss, having incomplete biochemical parameters or having gone to another hospital to give birth. In addition, of the 216 women, six had a possible inflammation that could alter biochemical parameters and were excluded. Hence, the final sample was composed of 210 mothers aged from 18 to 43 years and their neonates (47·6 % boys and 52·4 % girls). There were no significant differences in psychosocial and sociodemographic characteristics between the excluded and the included women. The statistical power of the results in terms of sample size, standard deviation from the Neonatal Behavioral Assessment Scale (NBAS) scores and the number of women with and without haemoconcentration risk at the end of pregnancy and partum is 87 %.
Procedure and study design
The present longitudinal study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving pregnant women were approved by the Ethics Committee of the Sant Joan University Hospital in Reus. Women admitted to the study were recruited during their first prenatal care visit (at gestational week ≤11) by gynaecologists from the Sant Joan University Hospital in Reus and written informed consent was obtained from all of them.
Study data were recorded during their pregnancy consultations. At week 10, consultation, clinical and obstetric histories were recorded, and blood samples were collected for laboratory analysis. At week 15, the obstetrician prescribed 40 mg Fe/d in accordance with the study protocol and the guidelines of the Spanish Ministry of Health and Consumption( 43 ). This dose was adapted in the following clinical visits according to maternal Hb and SF levels. At 24 and 34 weeks, the clinical and obstetric histories were revised, blood samples were collected, and a member of research team (in a separate room to encourage true responses) carried out a semi-structured interview on adherence to the Fe supplementation. At delivery, blood samples were collected and obstetric and neonatal variables were recorded (gestational age, neonatal birth weight and gender). In the immediate postpartum period (between 48 and 72 h after delivery) retrospective maternal anxiety and neonatal behaviour were assessed by two trained examiners (κ accordance 0·9) in a room at the hospital with optimal conditions. The examination of the newborn was completed in 20–25 min and was carried out midway between feedings. Not all newborns could be scored on the habituation items because they did not achieve the optimal state of consciousness (state 1, 2 or 3).
Measures
Hb concentrations in the blood were measured immediately at the clinical visits using a Coulter GENS analyser (Coulter, Hialeah, FL, USA). Serum and plasma were stored at −80°C in our biobank and thawed immediately prior to batch analyses to reduce inter-batch variation in analysis. SF was determined by turbidimetric immunoassay as described by Gomez et al.( Reference Gomez, Simo and Camps 44 ). According to the WHO recommendations( 45 , 46 ), Hb values were codified into three categories: (a) anaemia (Hb<105 g/l in the second trimester and <110 g/l in the third trimester and at delivery, and depleted Fe stores as SF<12 µg/l); (ii) normal (Hb between 105 and 130 g/l in the second trimester and between 110 and 130 g/l in the third trimester); and (iii) risk of haemoconcentration (Hb>130 g/l)( Reference Peña-Rosas and Viteri 24 ).
Neonatal behaviour was assessed at 48–72 h of age using the Spanish translation of the NBAS( Reference Brazelton and Nugent 47 ). The NBAS assesses the behaviour of the newborn within the dynamic context of the infant–caregiver relationship. The NBAS contains twenty-eight behavioural items scored for optimal performance on a 9-point scale (except for one item, ‘smiles’, for which the scoring criterion is to record the number of times a smile is observed during the exam); seven supplementary items, also scored on a 9-point scale; and eighteen reflex items that are designed to identify gross neurological abnormalities and which are scored on a 4-point scale. Behavioural items are classified into six clusters: (i) habituation, which evaluates the neonate’s ability to respond to and inhibit discrete stimuli while asleep (four items); (ii) social-interactive, which evaluates the neonate’s ability to attend to visual and auditory stimuli and the quality of overall alertness (seven items); (iii) the motor system, which evaluates neonate motor performance, quality of movement and muscular tone (five items); (iv) state organization, which evaluates infant arousal irritability and lability of states (four items); (v) state regulation, which evaluates the neonate’s ability to regulate her/his state when faced with increasing levels of stimulation (four items); and (vi) the autonomic system, which records signs of stress related to homeostatic adjustments in the CNS (four items, ‘smiles’ included). The following supplementary items assess several aspects of overall infant behaviour during assessment: quality of alertness (assesses the infant attentional state), cost of attention (assesses the effort that the infant makes to keep an optimal state of alertness), examiner facilitation (assesses the effort that the examiner makes to help the infant to keep a good state of alertness), general irritability (assesses the infant reactions of irritability), robustness and endurance (assesses the general physical resistance), state regulation (assesses the infant general self-regulation capabilities) and examiner’s emotional response (assesses the emotional response that the examiner feels during the examination).
Maternal anxiety was assessed using the Spanish version of the State Trait Anxiety Inventory (STAI)( Reference Spielberger, Gorsuch and Lushene 48 ). This self-report questionnaire contains forty items that assess state anxiety, which is the level of transient and situational anxiety; and trait anxiety, the level of dispositional and stable trait anxiety. For the present study, we used trait anxiety as a confounding variable.
The socio-economic level of the family was assessed using the Hollingshead Index( Reference Hollingshead 49 ). This index estimates the individual’s social status by grouping occupations into nine categories (from unskilled to highly skilled work) and by grouping the level of education into seven categories (from non-completed primary education to completed higher education). The status score is estimated by multiplying the occupation-scale value by 5 and the education-scale value by 3 and then combining the two scores.
Statistical analysis
All statistical analyses were carried out using the statistical software package IBM SPSS Statistics Version 22.0.
All variables were checked for normality of distribution. All variables were normally distributed, and are presented as percentages or as means and standard deviations.
The Bonferroni correction was applied to control for the increase in type I error due to multiple comparisons; the significance level was 0·025.
ANOVA was performed to test the differences in neonatal behaviour between the different groups of Hb levels and a Bonferroni post hoc analysis was applied.
To study if the risk of haemoconcentration is a significant predictor of neonatal behaviour, unadjusted and adjusted multiple linear regression models were performed for each NBAS cluster and supplementary item, and for the third trimester and delivery. Prior to these analyses, collinearity between variables was checked and the collinear variables (maternal SF level, umbilical cord blood ferritin and gestational age at birth) were deleted from the analysis. The Hb level variable was transformed into two dummy variables (dummy 1: anaemia v. normal Hb levels; dummy 2: haemoconcentration risk v. normal Hb levels) for entry in the multiple linear regression. In the unadjusted multiple linear regression models, the entry method was used to introduce the Hb level variable, and in the adjusted models, the entry method was used to enter the Hb level variable and the stepwise method to enter the confounding variables. The candidate variables for entry in the models were: infant birth weight at delivery (grams); socio-economic status of the family (total score); prenatal tobacco smoke exposure (no, yes); infant gender (female, male); and prenatal anxiety maternal trait (total score).
Results
Descriptive data of the sample
The sample characteristics are shown in Table 1. In general, the socio-economic status of the families was medium–high (92 %) and the level of trait anxiety was moderate (mean 15·4 (sd 7·9)) in comparison to Spanish data. In relation to neonatal variables, the mean gestational age was 39·3 (sd 1·4) weeks and the mean birth weight was 3231·2 (sd 403·7) g.
Table 1 Characteristics of the sample of healthy and well-nourished pregnant women and their full-term, normal-weight newborns (n 210), Reus, Spain, 2004–2008
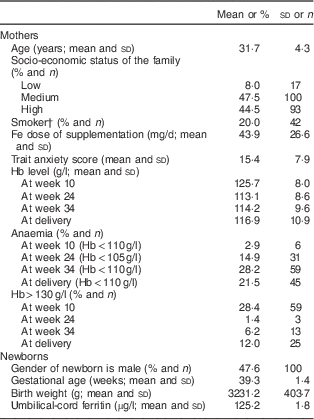
† Active or second-hand smoker.
All the mothers included in the analysis systematically received Fe supplements from about week 15 and the mean pharmacological Fe supplementation was 43·9 (sd 26·6) mg/d. The prevalence of haemoconcentration risk in the third trimester was 6·2 % and at delivery was 12·0 %. The prevalence of anaemia and low SF levels increased in the second and third trimesters. At delivery, 53·4 and 21·5 % of women had low Fe stores and anaemia, respectively.
Neonatal behaviour differences between Hb level groups at different stages of pregnancy
Table 2 shows differences in NBAS clusters between groups of Hb levels in the third trimester of pregnancy and at delivery. The first and second trimesters were not included in the analyses due to the low number of women in the haemoconcentration risk group.
Table 2 Descriptive data of neonatal behaviour depending on Hb levels of mothers at the end of pregnancy in the sample of healthy and well-nourished pregnant women and their full-term, normal-weight newborns (n 210), Reus, Spain, 2004–2008
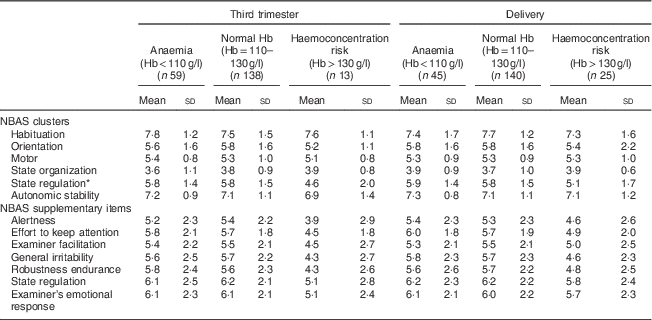
NBAS, Neonatal Behavioral Assessment Scale.
* Significant P values from Bonferroni post hoc analysis: normal Hb group v. haemoconcentration risk group (P=0·020).
Children of mothers with higher levels of Hb (>130 g/l) in the third trimester scored significantly lower in the state regulation cluster (mean 4·6 (sd 2·0)) than children of women with normal levels (mean 5·8 (sd 1·5); P=0·020). No significant differences were found between these conditions at birth in the bivariate analysis.
Prediction of neonatal behaviour by levels of Hb
Unadjusted and adjusted multiple linear regression models were performed to assess the effect of haemoconcentration risk during pregnancy on neonatal behaviour (Table 3). In the third trimester, the presence of risk of haemoconcentration was related to a decreased score in the state regulation cluster (B=−1·273, P=0·006) and to a lower alert quality score (B=−1·848, P=0·006). At delivery, the risk of haemoconcentration was also related to a decreased score in the state regulation cluster (B=−0·796, P=0·021) and a poor robustness and endurance supplementary item score (B=−0·921, P=0·005). No other variables in the analysis were significant.
Table 3 Prediction of neonatal behaviour for high levels of Hb at the end of pregnancy in the sample of healthy and well-nourished pregnant women and their full-term, normal-weight newborns (n 210), Reus, Spain, 2004–2008
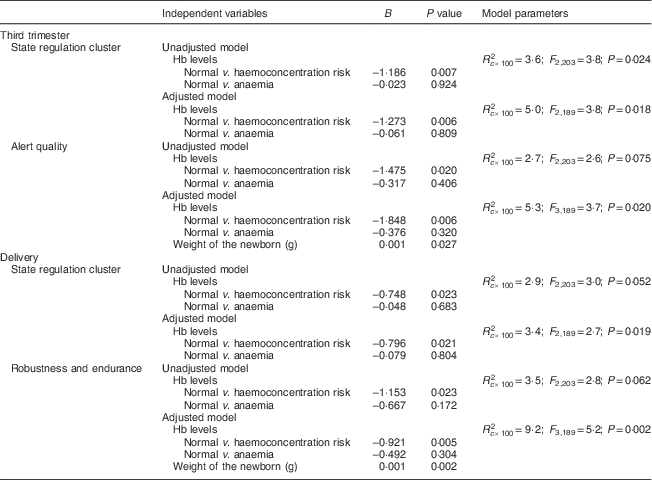
Candidate variables in the adjusted models: infant birth weight at delivery (grams); socio-economic status of the family; prenatal exposure to tobacco smoke (no, yes); infant gender (female, male); and maternal prenatal trait anxiety.
Discussion
We carried out a longitudinal study of a population of well-nourished pregnant women to analyse the effect of haemoconcentration at the end of pregnancy on neonatal behaviour. Our results show that, as we hypothesized, increased maternal Hb concentration at the end of the pregnancy is related to poor state regulation behaviour, alertness, robustness and endurance in the neonate.
Previous studies have described relationships between haemoconcentration during pregnancy and the risk of low birth weight, premature delivery and fetal loss( Reference Aranda, Ribot and Viteri 13 , Reference Gaillard, Eilers and Yassine 21 , Reference Cordina, Bhatti and Fernandez 22 , Reference Cung, Paus and Aghbar 25 ) and our results show for the first time relationships between pregnancy haemoconcentration and neonatal behaviour as a sign of fetal neurodevelopment.
The healthy pregnant women who participated in the study were Caucasian, with a medium–high socio-economic level, no known previous obstetric pathology, and typical general and obstetric characteristics in terms of gestational age and previous deliveries. In addition, the women took a moderate Fe supplementation with a mean of 43·9 mg/d in accordance with the study protocol and the guidelines of the Spanish Ministry of Health and Consumption( 43 ). Fe supplementation is recommended by international organizations in moderate doses (<60 mg/d)( 50 ) although these moderates doses could be excessive in women who start pregnancy with good Fe stores and with alterations in the haemochromatosis gene (HFE) that increase the intestinal absorption of Fe( Reference Aranda, Ribot and Viteri 13 , Reference Aranda, Viteri and Montserrat 51 ). Mutations in the HFE gene are common in our population, where 47 % of women carry one of the three major mutations( Reference Aranda, Viteri and Fernández-Ballart 52 ).
To define groups of Hb levels and depletion of Fe stores we used the limits established by international organizations( 45 ). SF is considered the best biochemical parameter for monitoring a deficient Fe status in pregnancy in the absence of infection or inflammation because it correctly identifies the women without Fe stores( Reference Walsh, O’Broin and Cooley 53 ). However, a known limitation of SF is that it also increases with acute or chronic inflammation, malignancy or liver disease. As transferrin saturation does not increase in the presence of inflammation, we used both parameters to detect inconsistent values (high SF and low transferrin saturation) that may hide a possible inflammation with ID, as suggested by some authors( Reference Rambod, Kovesdy and Kalantar-Zadeh 42 ), and we excluded six women in this situation from the analysis. To define haemoconcentration, we considered high levels of Hb without taking into account the total plasma volume. This is a limitation because the total plasma volume helps to differentiate the haemodilution status of normal pregnancy from haemoconcentration. However, measuring blood viscosity is laborious and expensive, and therefore, in everyday clinical practice and in many studies, Hb concentration is reported as a measure of haemoconcentration because its relationship with blood viscosity is linear when haematocrit is <0·5, which is usually the case in pregnancy( Reference Yip 54 ). Considering all this, we were not able to state that the women with Hb>130 g/l had haemoconcentration; therefore, we defined them as being at risk of haemoconcentration( Reference Peña-Rosas and Viteri 24 ). Our results show that as pregnancy progresses, the percentage of women with depleted Fe stores and anaemia increases. Furthermore, 6·2 % of pregnant women are at risk of haemoconcentration in the third trimester and 12·0 % at delivery. These data are similar to those published by the WHO in its global database on anaemia( 1 ) and also to the results from other European studies( Reference Gaillard, Eilers and Yassine 21 , Reference Cung, Paus and Aghbar 25 , Reference Scholl 55 ), confirming that ID is elevated even in industrialized countries and that a high percentage of women are at risk of haemoconcentration.
Neonatal behaviour is a phenotypical manifestation of the CNS development and is biologically determined by an interaction between genetics and environmental intra-uterine variables( Reference Brazelton and Nugent 56 ). In addition to prenatal nutritional status, other environmental variables can affect prenatal neurodevelopment such as maternal prenatal infections, prenatal toxic habits, prenatal emotional states, etc. and the NBAS is a good and sensitive instrument for detecting differences between exposed groups( Reference Cucó, Fernandez-Ballart and Arija 9 , Reference Hernández-Martínez, Arija and Balaguer 30 , Reference Hernández-Martínez, Arija and Escribano 31 , Reference Koutra, Chatzi and Roumeliotaki 33 , Reference Hernández-Martínez, Arija and Escribano 37 , Reference Debnath, Venkatasubramanian and Berk 57 – Reference O’Donnell, Glover and Barker 60 ). These and other variables affect prenatal neurodevelopment by interacting and even modifying gene expression( Reference Ellison 26 ). Therefore, our analysis has taken these aspects into account to disentangle the compound variables that can affect neurodevelopment. Our results show that, after controlling for confounding variables, the risk of haemoconcentration in the third trimester and at delivery is related to poor state regulation cluster scores and infant alert quality and robustness and endurance; that is to say, in general, these infants show more problems with self-regulation, being more excitable and showing more irritability during the examination, and the examiner perceives them to be weaker and to have more attention problems. These results suggest that the third trimester of pregnancy is a critical period for neurodevelopment, when the CNS is more vulnerable to environmental insults possibly due to the speed of several neurological changes, including synapse formation and myelination, and the rapid increase in brain volume (up to 260 % in the third trimester and more than double in the first year of life)( Reference Thompson and Nelson 8 , Reference Dobbing and Sands 39 , Reference Rao and Georgieff 40 ).
The poor alertness and self-regulation behaviours shown by infants prenatally exposed to high Hb concentration levels may indicate that they are more prone to developing the cognitive problems observed by several authors( Reference Tamura, Goldenberg and Hou 28 , Reference Hanieh, Ha and Simpson 29 , Reference Yang, Ren and Liu 61 ). In this regard, previous studies have shown that lower scores in the NBAS clusters that assess infant irritability were good predictors of verbal and total IQ at 6 years old( Reference Canals, Hernández-Martínez and Esparó 62 ) and infant psychopathological symptoms( Reference Canals, Esparó and Fernández-Ballart 63 ). The neurobehavioural characteristics shown by neonates of mothers with high Hb concentrations may be explained in several ways. One explanation is that high Hb concentrations are related to high blood viscosity which can directly reduce the perfusion and oxygenation of the placental and fetal tissues by reducing the blood flow and the efficiency of the nutrient exchange processes, thus decreasing the oxygen and nutrients available to the fetus( Reference Ziaei, Norrozi and Faghihzadeh 20 – Reference Cordina, Bhatti and Fernandez 22 ). Furthermore, the reduction in nutrient transfer can reduce the availability of the substrate substances needed by the placental hormones that stimulate fetal growth( Reference Barker 64 ) and can increase plasma cortisol in response to hypoxia( Reference Iwamoto, Murray and Chernausek 65 ). Other authors suggest that an alternative explanation for the inverse association between Hb levels and fetal growth (and probably fetal CNS development) may be provided by the direct role of Hb in nitric oxide regulation and endothelial function. Hb binds and inactivates nitric oxide (by endothelium-derived smooth-muscle relaxation factor), which leads to vasoconstriction and consequently hypertension and placental ischaemia( Reference Kim-Shapiro, Schechter and Gladwin 66 ). A recent study shows a direct link between reduced cerebral oxygenation and impaired brain growth in fetuses with congenital heart disease and raises the possibility that in utero brain development could be improved with maternal oxygen therapy( Reference Sun, Macgowan and Sled 67 ). On the other hand, among the causes of haemoconcentration are excessive Fe supplementation and pregnancy is a state where there is an increased propensity to produce free radicals, particularly in the presence of Fe( Reference Casanueva and Viteri 12 ), which suggests that both Fe deficiency and excess could produce excessive mitochondrial damage and oxidative stress( Reference Walter, Knutson and Paler-Martinez 68 ) that could also affect the development of the fetus and its CNS( Reference Sun, Macgowan and Sled 67 ). Several authors have observed the negative influence of high ferritin levels during pregnancy on maternal and infant health( Reference Aranda, Ribot and Garcia 2 , Reference Casanueva, Viteri and Mares-Galindo 16 – Reference Khambalia, Aimone and Nagubandi 18 , Reference Peña-Rosas and Viteri 24 , Reference Viteri, Casanueva and Tolentino 69 , Reference Peña-Rosas, De-Regil and Gomez Malave 70 ) and cognitive development( Reference Tamura, Goldenberg and Hou 28 ), both of which could be due to increased oxidative stress caused by excess Fe. In this regard, oligodendrocyte injury in the developing brain( Reference Shen, Yu and Yuan 71 ) has been related to prenatal oxidative stress and an excessive generation of free radicals in the intra-uterine environment, which increases the risk of neurodevelopment disorders such as schizophrenia in adulthood( Reference Debnath, Venkatasubramanian and Berk 57 , Reference Sun, Macgowan and Sled 67 ). Fe is essential for Hb synthesis and is important to the CNS in that it contributes to neuronal synapse formation, myelination and the synthesis of certain neurotransmitters( Reference Georgieff and Rao 11 ). However, as in other studies( Reference Cung, Paus and Aghbar 25 , Reference Ribot, Aranda and Giralt 72 ), we did not observe a negative effect from anaemia. It is likely that the physiological haemodilution that normally occurs in pregnancy can mask these results and that the Hb cut-off of <110 g/l for anaemia in pregnancy endorsed by the WHO is set too high for the present study population( Reference Viteri, Casanueva and Tolentino 69 ).
Our study has some strengths and limitations. While the prospective longitudinal data are methodologically strong, our final sample is modest, and we cannot rule out the possibility of residual confounding. We have included several confounders in the analysis, but it is possible that other confounders affect the relationships studied.
Taking all this information into account, our data allow us to conclude that high levels of Hb at the end of pregnancy are related to neonatal problems in self-regulation (excitability and irritability), alertness and with a poor robustness in terms of the neonatal behavioural development indicators measured. We suggest and support the practice of tailoring the pattern of Fe supplementation during pregnancy to the individual characteristics of each pregnant woman in order to improve fetal development.
Acknowledgements
Financial support: This study was financially supported by a grant from the Instituto de Salud Carlos III, Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Madrid, Spain (grant number PI052462). The funder had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that they have no conflict of interest. Authorship: N.A. and C.H.-M. are equal contributors. N.A. and C.H.-M. performed the literature search and statistical analyses and drafted the manuscript. N.A., C.H.-M., B.R. and J.C. participated in the fieldwork and in the design of the study. V.A. and J.C. conceived and participated in the design of the study, coordinated the fieldwork and supervised the statistical analyses. All authors contributed to the interpretation of the results and critically revised and approved the manuscript. Ethics of human subject participation: This longitudinal study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving pregnant women were approved by the Ethics Committee of the St Joan Hospital in Reus. Women admitted to the study were recruited during their first prenatal care visit (at gestational week ≤11) by gynaecologists from the St. Joan University Hospital of Reus and written informed consent was obtained from all of them.






