INTRODUCTION
Ticks (Arachnida: Parasitiformes: Ixodida) are a well-known group of haematophagous ectoparasites with about 900 living species (Guglielmone et al. Reference Guglielmone, Robbins, Apanaskevich, Petney, Estrada-Peña and Horak2014). Several are of considerable economic importance as vectors for viral and bacterial infections, affecting both humans and domestic animals. A summary of tick biology can be found in Sonenshine and Roe (Reference Sonenshine and Roe2013) and a list of valid names in Guglielmone and Nava (Reference Guglielmone and Nava2014). Given that ticks are parasitic on terrestrial vertebrates, there are interesting questions about when (and where) they first evolved and who their original hosts were; see e.g. Black and Piesman (Reference Black and Piesman1994) or de la Fuente (Reference de la Fuente2003), as well as the Discussion section below. The tick fossil record is sparse. Dermacentor reticulatus (Fabricius, 1794) and Ixodes sigelos Keirans et al. 1976 are living species recorded as subfossils (Kulczyński in Schille, Reference Schille1916; Sanchez et al. Reference Sanchez, Nava, Lareschi, Ortiz and Guglielmone2010). Records of Amblyomma Koch, 1844 which are almost indistinguishable from the living species Amblyomma argentinae Neumann, 1905 and Amblyomma dissimile C. L. Koch, 1844 were found in Miocene (ca. 16 Ma) Dominican Republic amber (Lane and Poinar, Reference Lane and Poinar1986; Keirans et al. Reference Keirans, Lane and Cauble2002). The argasid tick Ornithodoros antiquus Poinar, Reference Poinar1995 also comes from Dominican amber. Ixodes succineus Weidner, Reference Weidner1964 from the Eocene (ca. 44–49 Ma) Baltic amber in northern Europe was re-described by Dunlop et al. (Reference Dunlop, Apanaskevich, Lehmann, Hoffmann, Fusseis, Ehlke, Zachow and Xiao2016) and assigned to the modern Ixodes subgenus Partipalpiger Hoogstraal et al. 1973, whose members are geographically restricted to Asia nowadays. The argasid tick Carios jerseyi Klompen and Grimaldi, Reference Klompen and Grimaldi2001 comes from Cretaceous (ca. 90–94 Ma) New Jersey amber from the USA. Finally, two tick species, Cornupalpatum burmanicum Poinar and Brown, Reference Poinar and Brown2003 and Compluriscutata vetulum Poinar and Buckley, Reference Poinar and Buckley2008, were formally described in Burmese amber from Myanmar, representing the thus far oldest records from the Cretaceous (ca. 99 Ma). Both were assigned to extinct genera.
Burmese amber also hosts a provisional record of the genus Amblyomma by Klompen in Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002). This is significant as it is the oldest putative example of a tick genus with species still living today. Here, we confirm the presence of an Amblyomma tick in Burmese amber based on a new specimen, which we formally describe as a new species and compare it to its living representatives and their patterns of distribution.
MATERIALS AND METHODS
The type and only known specimen comes from the Jörg Wunderlich collection and bears the inventory number F24671BN/CJW. His material will probably later be deposited in the Senckenberg Museum Frankfurt/Main (J. Wunderlich, personal communication). Most Burmese amber comes from the Hukawng Valley in northern Myanmar. It is currently dated to the Late Cretaceous (lowermost Cenomanian) with an absolute age of ca. 99 Ma (Shi et al. Reference Shi, Grimaldi, Harlow, Wang, Yang, Lei, Li and Li2012). In other words, it is very close to the boundary between the Early and Late Cretaceous.
Imaging
The images for focus stacking were taken using a Leica Z16 APO, driven and controlled by a Cognisys Stackshot macro rail system. The lighting system used was a 12 channel light-emitting diode (LED) system developed by one of the authors (Bruno Cancian de Araujo) and composed of 300 super bright LEDs. The system works with direct light diffused by a central tube made with a photographic filter (Lee filters, model 129). The stack images were combined using the software Helicon Focus 6·7·1. A Keyence VHX-5000 Digital Microscope with a tiltable stand and a combination of upper light and transmitted light for focus stacking was used. We partly used polarized light for more details.
Micro CT
For X-ray microCT scanning the piece of amber containing the specimen was fixed on a glass stub with a clamp formed of Styrofoam. Scanning was performed with a Phoenix Nanotome m (GE Measurement and Control, phoenix|x-ray, Wunstorf, Germany). The 1440 projections (3 averaged, 1 skipped, total duration 48 min) were taken at a current of 100 kV and 100 mA using a molybdenum target. Voxel resolution of the resulting dataset was 3·42472 µm. Initially, the dataset was cropped and converted to 8 bit (by adjustment of the histogram) with the help of the software VG Studio Max 2.3 (volume Graphics, Heidelberg, Germany). The noise was reduced with VG Studio Max or Amira 6.0.1 (FEI, Hillsboro, OR, USA). The data were visualized by volume rendering with VG Studio Max (Phong Volume renderer), Drishti 2.6.2 (Limaye, Reference Limaye2012, https://github.com/nci/drishti).
SYSTEMATIC PALAEONTOLOGY
Ixodida Leach, 1815
Amblyomma C.L. Koch, 1844
Amblyomma birmitum sp. nov.
Etymology: From Burmite, the original name of this fossil resin used in the first description by Helm (Reference Helm1894).
Material: Holotype and only known specimen, Jörg Wunderlich collection no. F24671BN/CJW, Burmese amber, Myanmar, Late Cretaceous (Cenomanian).
Diagnosis: Body subcircular, scutum heart-shaped, the second article of palps is at least twice as long as third article, 11 festoons, eyes present, spiracle plates comma-shaped, coxae I–IV with no obvious spurs.
Description: Unengorged female (Fig. 1A–C). Idiosoma: Ornamentation indistinct; body subcircular; length (excluding capitulum) 1569 µm, greatest width 1591 µm; scutum 849 µm (measured in the middle of the scutum, and 732 µm from the scapula to the edge) in length; maximum width 1026 µm, heart-shaped, punctuations rare, deep and medium-sized, distributed irregularly, sides are straight and posterior angle broad; scapulae rounded and short; cervical grooves short and deep; eyes small and distinctly convex located around level of leg II; eleven festoons ranging in basal width from 153 to 156 µm and 144 to 149 µm length (Fig. 1B and C); anus located 456 µm from posterior border; anal groove Y-shaped with lateral branches reaching upper margin of anus and the Y-tail extending to the sixth festoon; genital aperture subcircular in form, situated between coxa III–IV; spiracle plates comma-shaped, medial and lateral margins parallel, posterior margin slightly convex, dorsal prolongation long, broad, perpendicular to the anterior–posterior axis, macula, round, situated subterminally.
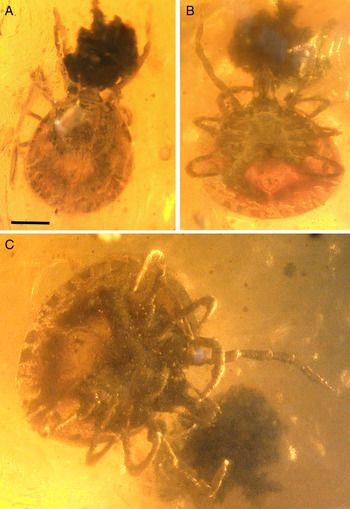
Fig. 1. Holotype of Amblyomma birmitum sp. nov., Jörg Wunderlich collection no. F24671BN/CJW, from Late Cretaceous (ca. 99 Ma) Burmese amber from Myanmar. Overview in (A) dorsal, (B) ventral and (C) ventral-oblique view.
Capitulum: Length from apices to the posterior margin of basis 671 µm; basis capituli subtriangular posterior margin almost straight, lateral margins round, cornua absent, ventrally posterior margin straight, length from palpal insertion to posterior margin of basis 428 µm, width 228 µm; palps cylindrical, with length of four segments as follows; segment 1, 46 µm; segment 2, 266 µm; segment 3, 139 µm; segment 4, 56 µm; visible dorsally and ventrally and no setae observed; hypostome length 537 µm, width at base 177 µm; columns of teeth on hypostome are 2 + 2 blunt-tipped teeth on the anterior half; apical corona not observed; porose areas oval, diameter of one area 122 µm length, 62 µm width, slightly depressed, length in horizontal position.
Legs: Coxae I–IV with no obvious spurs; tarsus I tapering distally, length 455 µm, clear, oval area on the dorsum of tarsi I is Haller's organ; claws paired, slender, simple, slightly curved; with distinct pulvillus on all legs. Chaetotaxy: Marginal setae observed. On the ventral side, small setae and two setae on each leg joint were observed.
DISCUSSION
The presence of eyes in the new fossil (Fig. 2A and B) excludes the previously described Burmese amber tick species. Both C. burmanicum and C. vetulum were described as eyeless (or at least eyes were not detected). Additionally, C. burmanicum differs from our new fossil in that it does not have an elongate second pedipalp article and has a unique extra claw on the penultimate (third) pedipalp article (Poinar and Brown, Reference Poinar and Brown2003: Figs 3 and 4). The pedipalp of C. vetulum is closer to that seen in our new fossil, but this species has 13 festoons along the posterior margin of the idiosoma (Poinar and Buckley, Reference Poinar and Buckley2008: Fig. 3). Our new specimen (and C. burmanicum) has only 11 festoons (Fig. 2A and B). We are therefore confident that at least three distinct species of hard tick were present in the Burmese amber forest.
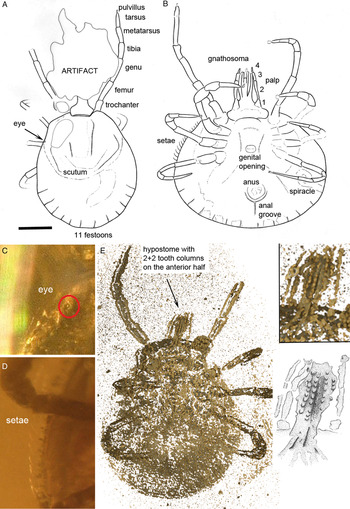
Fig. 2. Holotype of Amblyomma birmitum sp. nov., Jörg Wunderlich collection no. F24671BN/CJW, from Late Cretaceous (ca. 99 Ma) Burmese amber from Myanmar. (A) Drawing of the dorsal face showing the leg segments, eye, scutum and festoons. (B) Drawing of the ventral face showing gnathosoma with the palp segments, coxae, genital aperture, stigma, setae and anal groove. (C) Detail of eye (photos with Keyence 5000 Digital Microscope). (D) Focus on setae on the idiosoma side (photos with Keyence 5000 Digital Microscope). (E) Hypostome tooth columns (2 + 2 arrangement), μCT Drishti 2·6·2; including insets showing close up and interpretative drawing of the hypostome.
In the new fossil, the hypostome tooth columns have a 2/2 arrangement, albeit only in the anterior half (Fig. 2E). The hypostome teeth are not easy to resolve, but can be recognized as a total of four rows of sharp, triangular and backwards-pointing projections emerging along the length of the hypostome. All modern Asian, African and Neotropical Amblyomma adults have a 3/3 or 4/4 tooth arrangement, or even 5/5 in Amblyomma clypeolatum Neumann, 1899 females (Voltzit and Keirans, Reference Voltzit and Keirans2002, Reference Voltzit and Keirans2003; Voltzit, Reference Voltzit2007). In this respect, our new fossil resembles the Recent Australian species Bothriocroton auruginans (Schulze, 1936), although here the 2/2 teeth are distributed along the whole length of the hypostome. The new fossil differs from other Amblyomma species coming from the former Gondwana region in that the genital aperture is located between coxa III–IV (Fig. 1C). In the other species it is located between coxae II and III or between coxae II (Voltzit and Keirans, Reference Voltzit and Keirans2002, Reference Voltzit and Keirans2003), except in three Neotropical Amblyomma species which have the genital aperture between coxae III – namely Amblyomma cruciferum Neumann, 1901, Amblyomma darwini Hirst and Hirst, 1910 and Amblyomma humerale C.L. Koch, 1844 (Voltzit, Reference Voltzit2007) – and no coxal spurs. The position of the eyes is close to the condition seen in, e.g. Amblyomma variegatum Fabricius, 1794 or Amblyomma pomposum Dönitz, 1909. We should also note that the fourth segment of the palps is very long (Fig. 1B and C) compared with recent Amblyomma species and, in this sense, it is similar to the condition in one of the other amber species C. vetulum. Adults of most Amblyomma species are medium- or large-sized ticks in which the second article of the palp is at least twice as long as the third article. The scutum is usually ornamented with varying-colour iridescent patterns. Eyes are present and in most species are not positioned in sockets (Nicholson et al. Reference Nicholson, Sonenshine, Lane, Uilenberg, Mullen and Durden2009).
Biology of Amblyomma
Following Klompen et al. (Reference Klompen, Dobson and Barker2002) the genus Aponomma Neumann, 1899 is now considered a synonym of Amblyomma, which is represented today by 132 valid, extant species (Guglielmone and Nava, Reference Guglielmone and Nava2014). In detail, Klompen et al. (Reference Klompen, Dobson and Barker2002) transferred some of the Aponomma species from the indigenous Australian Aponomma to Bothriocroton Keirans et al., 1994 and the remaining Aponomma species to Amblyomma. The genus Amblyomma is thus currently distributed in all six zoogeographic regions (Guglielmone et al. Reference Guglielmone, Robbins, Apanaskevich, Petney, Estrada-Peña and Horak2014), although we should stress that 98% of them occur exclusively or non-exclusively in territories that originally constituted Gondwana. By contrast, only three species are exclusively found in the Neartic region (Guglielmone et al. Reference Guglielmone, Robbins, Apanaskevich, Petney, Estrada-Peña and Horak2014), which was originally part of Laurasia. Typical hosts of Amblyomma today include lizards and snakes (Squamata) as well as tortoises (Testudines) which form the principal host for several species. In fact, Amblyomma as a group utilizes a wide range of hosts, which is different from the situation in other genera of Ixodidae (Guglielmone et al. Reference Guglielmone, Robbins, Apanaskevich, Petney, Estrada-Peña and Horak2014). Our new taxon in Burmese amber confirms the presence (see also Grimaldi et al. Reference Grimaldi, Engel and Nascimbene2002) of Amblyomma in the Cretaceous of Southeast Asia. It is only the third tick species to be described from this deposit and the first to be formally placed in an extant genus. Amblyomma can thus be traced back approximately 99 million years. Together with an opilioacarid mite from the same amber deposit (Dunlop and de Bernardi, Reference Dunlop and de Bernardi2014), these Cretaceous ticks also represent the oldest records of parasitiform acarids in general.
Phylogeny and divergence times
With respect to the phylogenetic position of Amblyomma, there is a basic division of hard ticks into the Prostriata group, restricted to the genus Ixodes, and the Metastriata group containing all the other genera (e.g. Black and Piesman, Reference Black and Piesman1994). In Prostriata the anal groove extends anterior to the anus, while in Metastriata the anal groove is located posterior to the anus (Beati and Keirans, Reference Beati and Keirans2001; Nicholson et al. Reference Nicholson, Sonenshine, Lane, Uilenberg, Mullen and Durden2009). We should note that for the genus Ixodes most authors now recognize a split between the Australian and non-Australian species, or have even questioned the monophyly of the genus (e.g. Klompen et al. Reference Klompen, Black, Keirans and Norris2000). In overview, Barker and Murrell (Reference Barker and Murrell2004, Fig. 1) proposed a working hypothesis for hard ticks of the form [Ixodes (Bothriocroton (Amblyomma (Haemaphysalis + Rhipicephalinae and Hyalommine genera)))]. This reflects the older, non-cladistic, scheme of Hoogstraal and Aeschlimann (Reference Hoogstraal and Aeschlimann1982) and the same basic pattern was also recovered in the molecular phylogeny of Mans et al. (Reference Mans, de Klerk, Pienaar, Castro and Latif2012, Fig. 2) using 18S rDNA; although other genes gave slightly different results (see their Fig. 3) such as the non-monophyly of Amblyomma.
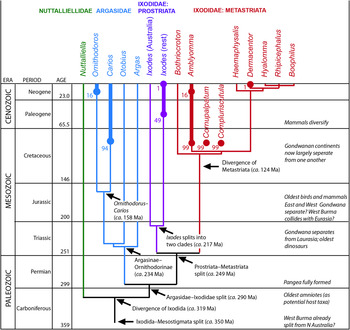
Fig. 3. Phylogenetic tree largely based on Mans et al. (Reference Mans, de Klerk, Pienaar, Castro and Latif2012) with the fossil record of the main genera superimposed. Circles indicate the known fossil occurrences with their estimated dates in millions of years. The inferred origination dates of the major clades are based on Mans et al. although we should caution that all of them had error bars of about ±20 million years. Colour scheme: green (Nuttalliellidae), blue (Argasidae), purple (Ixodidae: Prostriata), red (Ixodidae: Metastriata). Poinar's extinct genera are assumed to be metastriates too, possibly close to Amblyomma (see text for details).
There are numerous proposals, summarized by de la Fuente (Reference de la Fuente2003, Fig. 8), for when ticks – or specifically hard ticks – are thought to have originated. Most were derived, in part, from the fossil record of their typical modern host species. For example, Hoogstraal (Reference Hoogstraal1985) inferred late Palaeozoic or early Mesozoic origins for argasid-like ticks in general, with amblyommid ticks present on reptile hosts as early as the Permian and hyalommine and rhipicephaline tick lineages evolving later on mammalian hosts in the Cretaceous and Cenozoic, respectively. In fact, published inferences on tick origins range from as old as the Devonian (Oliver, Reference Oliver1989; Dobson and Barker, Reference Dobson and Barker1999), with amphibians as putative hosts, to as young as the Cretaceous (Black and Piesman, Reference Black and Piesman1994; Nava et al. Reference Nava, Guglielmone and Mangold2009): a difference of almost 300 million years! Klompen et al. (Reference Klompen, Black, Keirans and Oliver1996) also criticized the assumption that tick phylogeny is determined by the phylogeny of their hosts; i.e. that the early derivative ticks must be those that live on reptiles today. Mans et al. (Reference Mans, de Klerk, Pienaar, Castro and Latif2012: Table 2) estimated divergence times directly using molecular clock methods. Their results include origins for the entire Ixodida in the late Carboniferous (319 ± 23 Ma), the family Ixodidae around the Permian–Triassic boundary (249 ± 23 Ma), and the Metastriata radiating in the Early Cretaceous (124 ± 17 Ma). Thus the Late Cretaceous Burmese amber records of Amblyomma are not much younger than Mans et al.’s estimated divergence time for metastriate ticks in general. Fossils also constrain the split between Amblyomma and its putative sister-group (i.e. Haemaphysalis + rhipicephalines + hyalommines) to at least 99 Ma.
The extinct Burmese amber genera have not been formally integrated into the phylogeny of living taxa. The position of the anal groove is unhelpful here as both C. burmanicum and C. vetulum were described without an anal groove (Poinar and Brown, Reference Poinar and Brown2003; Poinar and Buckley, Reference Poinar and Buckley2008); or at least none could be resolved in the material available. A better guide is the presence of marginal festoons in these Cretaceous fossils. These structures are absent in the prostriate Ixodes and strongly suggest that the two extinct Burmese genera are also metastriate ticks such as Amblyomma. We should also mention de la Fuente's (Reference de la Fuente2003) observation that C. burmanicum resembles Aponomma (= either Bothriocroton or Amblyomma) and comments in Mans et al. (Reference Mans, de Klerk, Pienaar, Castro and Latif2012) that these extinct species are probably close to Amblyomma. Transposing the (albeit limited) fossil record of the ticks onto the current phylogenetic hypothesis (Fig. 3, see also Mans et al. Fig. 4) yields a framework evolutionary scenario. Nuttalliellidae lacks a fossil record. Argasidae is known since the Cretaceous but is estimated to have split from Ixodidae in the Permian. Ixodes is known from the Eocene but is predicted to have split from the metastriate lineage in the early Triassic. The modern metastriate genera are inferred to have radiated in the Cretaceous.
Corrections to the tick fossil record
Origins of the most recently diverged hard tick genera – namely Haemaphysalis, Dermacentor, Hyalomma, Rhipicephalus and Boophilus – were previously constrained to an Eocene divergence date of at least 49 Ma by de la Fuente's (Reference de la Fuente2003: Fig. 6) report of a Hyalomma sp. from Baltic amber. However, during the preparation of the present manuscript, it was pointed out to us (Ekaterina Sidorchuk, personal communication) that de la Fuente's amber fossil is not a tick. In fact, it is a rake-legged mite (Acariformes: Caeculidae), a fact clearly indicated by the large inward-facing spines on the forelegs which are very typical for this group of predatory mites and not seen in Hyalomma or any other tick genus. de la Fuente's (Reference de la Fuente2003) paper contains a further misidentification (Ekaterina Sidorchuk, personal communication). The soft ticks figured from Miocene Dominican amber (Fig. 7) are large parasitengonids. It may also be necessary to further check the identity of the larval Ixodes from Baltic amber (de la Fuente, Reference de la Fuente2003: Fig. 1) and larval ticks from Miocene Dominican amber (his Fig. 4), but this is difficult from the photographs and would require restudy of the original material. Removal of the amber Hyalomma record means that the oldest fossil constraint for the five derived metastriate tick genera listed above is the subfossil D. reticulatus mentioned by Kulczyński in Schille (Reference Schille1916) from the ear of an extinct rhinoceros. Its ca. 1 Ma age is clearly much too young for realistically dating when the genus evolved and means that we must currently rely on molecular clock methods to estimate cladogenesis dates for Haemaphysalis, Dermacentor, Hyalomma, Rhipicephalus and Boophilus.
Palaeobiogeography
A last point of interest is the fact that Southeast Asia has a complex geological history (e.g. Heine and Müller, Reference Heine and Müller2005; Metcalfe, Reference Metcalfe, Hall, Cottam and Wilson2011, Reference Metcalfe2013; Seton et al. Reference Seton, Müller, Zahirovic, Gaina, Torsvik, Shephard, Talsma, Gurnis, Turner, Maus and Chandler2012). The region containing modern Myanmar was originally interpreted as having been part of the so-called Sibumasu terrane, which is thought to have rifted from the northern (i.e. Australian) part of Gondwana in the late Palaeozoic/early Mesozoic and collided with the Indochina plate by the Late Cretaceous. This led to a hypothesis that Burmese amber arthropods could have arrived in Asia by rafting across the Sibumasu terrane from Australia; see e.g. Dunlop and de Bernardi (Reference Dunlop and de Bernardi2014) for a scenario along these lines involving an opilioacarid mite. A challenge to this hypothesis is the fact that back when the Sibumasu terrane is thought to have rifted from Gondwana (creating the Meso-Tethys Ocean), the landmass may actually have been covered by shallow seas. Also, much of Australia at this time was thought to have been covered in ice, and Sibumasu was still linked to Gondwana via the Lhasa block, Argoland and South West Borneo blocks (see e.g. Metcalfe, Reference Metcalfe, Hall, Cottam and Wilson2011). If this model is correct, the survival of terrestrial arthropods on the Sibumasu terrane after separation from Australia seems unlikely.
More recently, the area containing the source of Burmese amber has been interpreted as having been on the West Burma terrane instead; see Broly et al. (Reference Broly, Maillet and Ross2015: Fig. 1) for a map of how the various elements may have accreted together to form modern Myanmar. As discussed for a Burmese amber spider by Selden et al. (Reference Selden, Zhang and Ren2016), one hypothesis is that the West Burma terrane rifted from northern Australia in the Late Jurassic at ca. 156 Ma, and collided with the Sibumasu terrain the Late Cretaceous at about 80 Ma. In this scenario, at the time of amber deposition (ca. 99 Ma) the fauna would have been on an island which had been separated from Australia for about 75 million years. However, Metcalfe (Reference Metcalfe2013) provided evidence for a much earlier (Devonian) separation from Australia and an earlier (Jurassic) collision of the West Burma terrane with Eurasia. In this model the Burmese amber fauna could potentially have arrived from Eurasia at some time between the Jurassic and the mid-Cretaceous.
With respect to ticks, Klompen et al. (Reference Klompen, Black, Keirans and Oliver1996) argued that basal taxa among both prostriate and metastriate ticks predominantly come from Australia today, which could imply that at least the Ixodidae have their origins here. Given that modern Amblyomma are strongly associated with a Gondwanan distribution (see above) it would be instructive to compare estimated dates of cladogenesis with some of these dates for major events in palaeobiogeography (see also Mans et al. Reference Mans, de Klerk, Pienaar, Castro and Latif2012: Fig. 4). Caution is needed in dating ancient geographical splits between continents, and complete physical separation of landmasses may postdate the start of a split by a considerable period of time. Upchurch (Reference Upchurch2008) reviewed several competing models for Gondwana – albeit with a focus on fossil vertebrates – and recognized four potential scenarios. In the Samafrica model, West Gondwana (South America + Africa) separated from East Gondwana (Antarctica, Australia and Indo-Madagascar) during the mid-Jurassic (perhaps ca. 180 Ma). This seems to be the most widely accepted scenario; see also Gibbons et al. (Reference Gibbons, Whittaker and Müller2013) for example. In the Africa-first model, Africa separated from the rest of Gondwana during the Cretaceous while South America was still in contact with East Gondwana until the Late Cretaceous. In the multistage scenario elements of the previous hypotheses are combined, and in the Pan-Gondwana hypothesis the southern continents were still connected, at least at their southern tips, until the Late Cretaceous; perhaps as late as 80 Ma.
If the Samafrica model is correct, current molecular estimates for the Cretaceous radiation of the metastriate ticks (Fig. 3) postdate the Jurassic dissolution of Gondwana into an eastern and western province. Genera such as Amblyomma may show a Gondwanan distribution today, but current estimates of their origination dates correspond to a time in which Gondwana may have already separated into what would become the modern continents. In this hypothesis, either the molecular dates are too young, or modern patterns of biogeography have to be explained by alternative mechanisms. Furthermore, we still have to explain the presence of Amblyomma in the Cretaceous of Southeast-Asia. If its origins are Gondwanan, then the northern route was potentially closed by the breakup of Pangaea and the separation of Gondwana from Laurasia as early as ca. 220 Ma. If its ancestors rafted from Australia on the West Burma terrane, then in Metcalfe's (Reference Metcalfe2013) model the rafting lineage must have separated from the Australian tick fauna around 360–420 million years ago. This seems highly unlikely as it would require these animals to have radiated into modern genera back in the Devonian, which significantly predates the Late Carboniferous molecular estimates (see above) of tick origins.
A final possibility is that Amblyomma actually originated in Eurasia – again perhaps during the Cretaceous as part of this metastriate radiation – and arrived (and was fossilized) in the West Burma region via a northern route long after it had collided with Asia. As noted above, Upchurch's (Reference Upchurch2008) scenarios for the dissolution of Gondwana are largely based on fossil vertebrate data. However, there are numerous examples of plants and animals distributed both in former Gondwanan regions and (often as fossils) in the northern hemisphere. Examples include palpimanoid spiders (Wood et al. Reference Wood, Matzke, Gillespie and Griswold2013, and references therein), ants (Zryanin, Reference Zryanin2015), hemipterans (Szwedo et al. Reference Szwedo, Stroiński and Lin2015), neuropterans (Wedmann and Makarkin, Reference Wedmann and Makarkin2007) and branchiopod crustaceans (Korovchinsky, Reference Korovchinsky2006; Van Damme and Sinev, Reference Van Damme and Sinev2013). The implication here is that these groups were once globally distributed across both the northern and southern continents, but at some stage (perhaps in response to glaciation) either became extinct in the north and/or migrated south. This has been referred to as the ousted relicts (Eskov and Golovatch, Reference Eskov and Golovatch1986) or ejected relicts (Korovchinsky, Reference Korovchinsky2006) hypothesis. Such a scenario should also be borne in mind when considering how Amblyomma came to be so widespread in the former Gondwana region today: did it really originate here, or is it a relict of a once much wider distribution? A final thought is that several species of Amblyomma parasitize birds today, in which case trans-oceanic dispersal through flight remains a possibility.
ACKNOWLEDGEMENTS
We thank Jörg Wunderlich (Hirschberg) for making this material available for study, Lorenza Beati Ziegler (Georgia Southern University) for initial comments on the specimen, Ekaterina Sidorchuk (Borissiak Paleontological Institute) for drawing our attention to misidentifications in the literature and Martin Pfeffer and two anonymous reviewers for helpful comments on the manuscript.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.






